Abstract
Drug pricing in Canada is a divided responsibility between the federal and provincial governments. The price of patented medicines is largely controlled at the federal level through the Patented Medicine Prices Review Board that sets a maximum introductory price for new medicines and then limits the rate of rise of those prices to the rate of inflation. The provinces and territories have a minimal role in pricing of this group of medicines through product listing agreements. Generic drug prices, on-the-other hand, are solely the responsibility of the provinces and territories that set the prices for these products at a certain percentage of the price of the originator product. Separate from these bodies, is a federal health technology assessment process that considers clinical efficacy as well as cost-effectiveness but this process only makes recommendations about funding to the participating provincial, territorial and federal drug plans. Although private insurance pays for over one-third of all drug costs, it has very little role in either setting prices or containing costs. Overall Canada has among the highest drug costs among developed countries but a lack of drug insurance means that up to 35 % of low income people without insurance do not fill their prescriptions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
Health care in Canada is a complicated matter owing to the federal nature of the country and the way that the constitution divides power between the provinces and the federal government. Making the situation even more complex is the wording of the Canada Health Act that sets out which aspects of health care delivery are eligible for federal funding and which are solely reliant on provincial funding (Government of Canada 2012). Then there is the way that Canada has chosen to regulate the prices of patented and generic drugs and finally the role of health technology assessment and the role of the private insurance companies. This chapter explores all of these points and looks at how successful Canada has been at controlling drug costs and ends by examining whether or not drugs are affordable for all segments of the Canadian population.
2.2 Overview of the Canadian Health Care System
There actually is no Canadian national health care system. Under the Canadian constitution, written in 1867, “the provinces were responsible for establishing, maintaining and managing hospitals, asylums, charities and charitable institutions, and the federal government was given jurisdiction over marine hospitals and quarantine” (Canada’s Health Care System 2014). As a result, as health care became more important provinces individually developed their own mechanisms for funding and delivering health care. The beginning of publicly funded hospital care came in Saskatchewan in 1947 followed by federal legislation that provided for federal funding for half the cost of hospital care provided that the provinces agreed to offer universal coverage through a publicly administered system. Saskatchewan expanded its system to include universal public coverage for physician services in 1962 with the federal government once again following suit in 1966 with legislation to pay for 50 % of the cost to any province (Canada’s Health Care System 2014; Government of Canada 1966).
These two pieces of federal legislation were consolidated in 1984 in the Canada Health Act (CHA). The CHA lays out five conditions for the provision of federal funding to the provinces: portability (insurance continues when people move from province to province), accessibility (people cannot be charged extra for any service that is covered), universality (all Canadian citizens and permanent residents are automatically covered), comprehensiveness (all necessary medical services are covered), and public administration (the health care system is administered on a public not-for profit basis) (Canada’s Health Care System 2014). Although the CHA says nothing about the way that health care should be delivered almost all hospitals in Canada are run by private not-for profit corporations that are not investor owned and almost all of their funding comes from the provincial government (Sutherland et al. 2013). Doctors are not state employees but also receive nearly all of their income from the publicly run system (Blomqvist and Busby 2013).
While virtually all doctor and hospital services are covered without any form of patient copayment, it is important to note that aside from drugs administered while patients are in hospital, the CHA is silent about payment for prescriptions drugs. Canada is unique as being the only developed country with a national health care system that does not also cover drug costs. Therefore, as described below, each province has developed its own system for public payment, with the resultant provincial variation in what drugs are covered, what groups of the population are eligible for public insurance and what level of copayment, deductibles and user fees are levied. Further complicating the situation is that prices for patented drugs, i.e., brand-name drugs, are controlled differently from prices for generic drugs.
2.3 Pharmacy Services
As of 2011, there were a total of 8,869 pharmacies in Canada and of these 5,476 were part of a chain, with the remainder split almost equally between independently owned stores and pharmacy services located in food and other mass merchandising stores (IMS Brogan 2012). There are no publicly owned or private not-for profit pharmacies in Canada.
There were just over 30,600 pharmacists in 2012 with three-quarters working in community pharmacies and the remainder employed in hospitals and other health care facilities. “Most pharmacists reported their position as staff pharmacist, with approximately 30 % reporting themselves as pharmacy owners/managers” (Canadian Institute for Health Information 2013b). Pharmacy services are considered part of the delivery of health care and as such pharmacists and pharmacies are under the control of provincial governments.
2.4 Pharmaceutical Industry Ownership
The pharmaceutical industry in Canada is dominated by the subsidiaries of multinational companies. Out of the top ten companies in 2012, seven were multinational subsidiaries and three were generic companies of which two (Apotex and Pharmascience) were Canadian owned (see Table 2.1).
The brand-name industry is represented by Canada’s Research-Based Pharmaceutical Companies with 56 members. Although there is a scattering of small Canadian owned start up companies the vast majority are foreign owned (Canada’s Research-Based Pharmaceutical Companies 2014). As of 2006, there were 15 generic suppliers in Canada with 13 having manufacturing plants in the country; seven of the top ten companies were foreign based (Canadian Generic Drug Sector Study 2007). The Canadian Generic Pharmaceutical Association (CGPA) represents a subset of the generic industry and has nine members with a mix of Canadian and foreign owned companies (Canadian Generic Pharmaceutical Association 2014). Sandoz, one of the CGPA companies is actually owned by Novartis, a Swiss based brand-name company. There are no publicly owned generic or brand-name pharmaceutical companies.
Nearly all of the active ingredients that go into both brand and generic drugs are imported into Canada and manufacturing consists largely of combining the active ingredient with the excipients into the various dosage forms.
2.5 Canadian Drug Regulation
Health Canada is the federal department with the responsibility for approving new drugs for marketing, through two of its branches. The Therapeutic Products Directorate approves and monitors prescription and non-prescription drugs derived from chemical manufacturing whereas the Biologics and Genetic Therapies Directorate is responsible for biological and radiopharmaceutical drugs including blood and blood products, viral and bacterial vaccines, genetic therapeutic products, tissues, organs and xenografts. While dealing with different types of products, both Directorates function in an almost identical manner in terms of analyzing the laboratory, preclinical and clinical data that the drug companies submit when they fill a New Drug Submission. The basis for approving a new drug is efficacy, safety and manufacturing quality. Health Canada has no involvement in pricing issues (Lexchin 2008).
2.6 Patented Drug Pricing
2.6.1 Compulsory Licensing
Up until the early 1990s Canada allowed the import of active ingredients through a compulsory license in order to encourage competition and lower the price for brand-name drugs. During the 1960s, a series of three reports all pointed out that drug prices in Canada were among the highest in the world and all three reports identified patent protection as one of the major reasons for this situation (Restrictive Trade Practices Commission 1963; Royal Commission on Health Services 1964; Canada House of Commons 1967). The decision of the Liberal government of the day was to allow companies to receive a compulsory license to import a drug into Canada. Based on figures in the Report of the Commission of Inquiry into the Pharmaceutical Industry (the Eastman Report), in 1983 the multinationals had lost only 3.1 % of the Canadian market to generic competition but compulsory licensing was responsible for a reduction of $211 million in a total drug bill of $1.6 billion (Commission of Inquiry on the Pharmaceutical Industry 1985).
The compulsory licensing system significantly increased the availability of generic drugs and could lead to a price reduction on individual drugs of as much as 80 % depending on the number of generic competitors and the availability of low priced generics allowed for the development and expansion of provincial public drug plans (Lexchin 1993a). However, a combination of pressure from the United States (US) and free trade agreements—the Free Trade Agreement (Canada and the US), the North American Free Trade Agreement (Canada, Mexico and the US) and the Trade Related Aspects of Intellectual Property Rights Agreement, one of the agreements administered by the World Trade Organization, lead to first the weakening of compulsory licensing in 1987 and its ultimate abandonment in 1993 (Lexchin 1993b, 1997).
2.6.2 Patented Medicine Prices Review Board
Once the government made the decision to end the use of compulsory licensing it was faced with the question of how to ensure that drug prices were set at an affordable level. Having used the patent system for almost a quarter of a century, the government chose to continue to use the patent system to regulate prices for patented drugs and created the Patented Medicine Prices Review Board (PMPRB) in 1988. The PMPRB is a federal agency that operates at arms length from the Ministry of Health. The authority of the PMPRB extends over the $12.8 billion in sales of patented drugs or 59.3 % of total drug sales in Canada (Patented Medicine Prices Review Board 2013). “Under the Patent Act, patentees are required to file price and sales information about their patented drug products at introduction and twice a year thereafter for each strength of each dosage form of each patented drug product sold in Canada” until the patent expires (Patented Medicine Prices Review 2014b).
Once this information is received, the product is assessed by PMPRB’s Human Drug Advisory Panel (HDAP) to determine if it is a line extension, i.e., a new presentation of an existing product, or a new active substance (NAS), i.e., a molecule never marketed before in any form in Canada. If the product is a line extension it is priced in line with the already existing presentation of the medicine. If it is a NAS then the HDAP rates its therapeutic value and based on that rating the PMPRB applies a series of criteria in its guidelines including the price of existing products in the same therapeutic class and the median price in seven comparator countries (France, Germany, Italy, Sweden, Switzerland, United Kingdom and United States) to determine the maximum average potential price (MAPP) for the drug (Patented Medicine Prices Review 2014b). The MAPP is the maximum allowable introductory price for the drug. If the price that the company proposes is at or below the MAPP then no further action is taken but if the price exceeds the MAPP then the PMPRB first enters into negotiations with the company for a Voluntary Compliance Undertaking to reduce the price. If those negotiations are unsuccessful then the PMPRB can use its quasi-judicial powers to hold a public hearing. If the hearing finds that the price is excessive, the PMPRB may issue an order to reduce the price. PMPRB decisions are subject to judicial review in the Federal Court of Canada.
Finally, the PMPRB limits the rate of rise of prices of individual products to the rate of rise in the Consumer Price Index (CPI) over any 3 year period. Just examining the increase in prices for individual products, the PMPRB has been successful in controlling drug prices in Canada, with general price inflation, as measured by the CPI, exceeding the average increase in patented drug prices almost every year since 1988 (see Table 2.2).
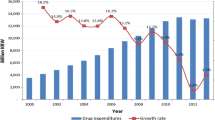
At the same time, Canadian prices are higher than those in four out of the seven comparator countries (Patented Medicine Prices Review Board 2013). At over $700 per person per year (US$ purchasing power parity), Canada spends more per capita on pharmaceuticals than any other country in the world except the United States (US) (OECD 2013). Similarly when measured against comparator countries in the Organisation for Economic Co-operation and Development (OECD), Canada’s growth in drug spending per capita (in real terms) between 2000 and 2009 was 4.3 % per year compared to the OECD average of 3.5 %. Although this rate fell to −0.3 % per year from 2009 to 2011, the OECD average fell to −0.9 % (OECD 2013). The high per capita expenditure, despite the control over introductory prices for patented drugs and the limitation on the rate of rise of their price, reflects the fact that the price of individual drugs is only one factor influencing expenditures. Far more important are “increased volume of use and changes in the mix of treatments being used…accounting for average annual growth of 6.2 % and 2.0 %, respectively. Both volume and mix effects were due in part to changes in treatment guidelines, increased disease prevalence and the uptake of new drugs” (Canadian Institute for Health Information 2012).
There are also inherent deficiencies in the PMPRB process that help to contribute to the high per capita expenditure level in Canada. When generic equivalents are marketed in Canada, the brand-name companies do not reduce their prices in an attempt to compete on price (Lexchin 2004). Since the PMPRB allows companies to set prices for new patented medicines up to the highest amount charged for other medicines in the same therapeutic market (Patented Medicine Prices Review 2014a) by not lowering brand-name prices, companies thereby enable new entrants into the same therapeutic market to charge higher prices. Lexchin has shown how this feature of the PMPRB guidelines concretely affects introductory prices. The mean introductory price of 33 new medications was 95.9 % of the price of existing brand-name products and 91.5 % of the price of the most expensive brand-name product in their class (Lexchin 2006).
Gagnon and Hébert criticize the comparator countries that the PMPRB uses noting that the list includes the four countries with the most expensive prices worldwide: Germany, Sweden, Switzerland and US. Since the Canadian price is the median of the seven comparator countries, they conclude that the PMPRB guidelines ensure that the price of patented medicines in Canada are normally the fourth most expensive price worldwide (Gagnon and Hébert 2010). Using other comparator countries could significantly affect the Canadian price. In 2006, the PMPRB conducted bilateral comparisons of the price of patented drugs in Canada and 11 other OECD countries including 6 of the 7 comparator countries that the PMPRB uses. (Sweden was excluded.) The mean of the average prices for the 6 comparator countries compared to the Canadian price was 1.04 whereas had all 11 countries been used the mean would have been 0.91 (Patented Medicine Prices Review 2006) (see Table 2.3).
2.7 Health Technology Assessment
In 2003, the Common Drug Review (CDR), operating as part of the Canadian Agency for Drugs and Technology in Health (CADTH), was established. The CDR provides advice to all of the provincial drug plans, except the one operated by the province of Quebec, the three territorial and six federal drug plans about the clinical efficacy and cost-effectiveness of a drug against other drug therapies so that public funds are optimally used. The CDR is funded by federal, provincial (except Quebec) and territorial governments and is governed by a 13-member jurisdictional Board of Directors appointed by the Federal/Provincial/Territorial Deputy Ministers of Health (Standing Committee on Health of the House of Commons 2007).
CDR reviews submissions from manufacturers for new drugs, new combination products, and drugs with new indications and in addition, the Formulary Working Group (composed of representatives from the federal, provincial, and territorial publicly funded drug plans and other related health organizations) or one or more of the participating drug plans “may request through a submission: (1) a review of the listing status of a particular drug that is already listed on one or more formularies, (2) a class review, or (3) a drug-related review other than that described in (1) and (2), and which may include a request for the review of a new drug or an old drug that is not currently listed on any participating Drug Plan formulary” (Common Drug Review 2013).
When CDR receives a submission it contracts a team that prepares a clinical review, including a systematic review of all relevant published and unpublished randomized controlled trials and in addition, examines and critiques the manufacturer’s pharmacoeconomic evaluation. The review team then prepares a report for the Canadian Expert Drug Advisory Committee (CEDAC), a body appointed by the Board of Directors for CADTH (see http://www.cadth.ca/index.php/en/cdr/committees/cedac). CEDAC uses the review to assess the clinical and economic value of the product and then makes a recommendation to the participating drug plans about listing taking into consideration the medication’s effectiveness, safety and cost-effectiveness compared to existing therapies (Tierney and Manns 2008). There are four different types of recommendations that the CDR can make: unrestricted listing, list in a manner similar to other drugs in the class, list with criteria and do not list. CDR recommendations are not binding on any of the participating drug plans, which are free to make their own funding decisions (Morgan et al. 2006).
Between May 2004 when the CDR made its first recommendation and May 2009, it considered 53 submissions and made a recommendation for listing in some form for 24 of these. Participating drug plans listed between 7 and 25 of these drugs and several drugs were listed on one or more formularies despite being given a “do not list” recommendation. (Quebec listed 12 of the 29 drugs given a “do not list” recommendation.) “The percent agreement between recommendations and decisions ranged from 60.4 to 96.2 %, irrespective of how agreement was defined” (Gamble et al. 2011).
A process similar to the one used by CDR is in place for providing advice to provincial and territorial plans about oncology drugs, the pan-Canadian Oncology Drug Review and as of April 1, 2014 responsibility for its administration was transferred to CADTH (Pan-Canadian Oncology Drug Review 2014).
2.8 Provincial Drug Formularies
Since coverage for medicines is not included under the CHA, each province and territory has developed its own public drug plan. Drug companies apply to get their drugs (patented, non-patented brand-name and generic) onto provincial formularies but there are differences in the number of new patented drugs that achieve listing. Out of 198 new patented drugs approved between May 1999 and May 2009, 152 (76.8 %) were listed on one or more formularies but the number listed on individual formularies varied from a low of 65 (32.8 %) to a high of 132 (66.7 %) (Gamble et al. 2011). When generic drugs are included, the variation in listing in 2006 ranged from 55 % of 796 drugs to 73 %. Rates of bilateral formulary agreement went from a low of 49 % to a high of 65 % depending on the provinces being compared. However, when national expenditure weights are applied to the drugs listed on the individual formularies then drugs listed on any of the nine provincial formularies (Prince Edward Island, the smallest of the ten provinces was excluded) accounted for an expenditure weighted share of 77 % of the market, suggesting that provincial drug coverage is more consistent than it would seem on the surface (Morgan et al. 2009).
2.8.1 Product Listing Agreements
Although the PMPRB sets a national price for patented drugs the provincial governments have started to play a role in how much they will pay for some of these drugs that they list on their formularies. Companies apply to have their drugs listed on provincial formularies and as part of that process produce pharmacoeconomic studies to show the value for money for their medicines. If the price of the product is felt to exceed its therapeutic value, i.e., it is not cost effective, then increasingly Canadian provinces are entering into product listing agreements (PLAs) with companies. These PLAs lead to lower, but confidential prices that may be achieved through “rebates that may or may not be tied to drug expenditures, utilization patterns or health outcomes” (Morgan et al. 2013b). According to a recent study into PLAs, they are being used by at least seven out of ten Canadian provinces although to varying degrees. Out of 12 drugs with a negative recommendation from the CDR, ten were funded with a PLA in at least one province (Morgan et al. 2013b). While PLAs reduce the price for drugs to the provinces, people who have to pay out-of-pocket or who are covered by private insurance do not benefit from these reduced prices.
2.8.2 Reference-Based Pricing
British Columbia is the only province to use reference-based pricing (RBP) to set and control drug prices in certain drug classes. The system was set up in 1995 and now covers five therapeutic classes of drugs. The assumption underlying RBP is that in certain drug classes the medications are essentially equally safe and effective and can be interchanged although they are not bioequivalent as generic are. A reference price is established for the class and RBP covers the cost of drugs priced at or below the reference price; if a physician prescribes a more expensive medication, the patient pays the difference. Overall, a Cochrane review found that RBP can reduce third party drug expenditures by inducing a shift in drug use towards less expensive drugs (Aaserud et al. 2006). The introduction of RBP for angiotensin converting enzyme inhibitors (drugs used to treat hypertension, congestive heart failure and coronary artery disease) lead to a saving of 6 % of all cardiovascular drug expenditures by the British Columbia drug plan (Schneeweiss et al. 2002). However, there have not been any recent evaluations of the effects of RBP to see if there are ongoing savings from the policy.
2.9 Generic Drug Pricing
Generic drugs account for just over 63 % of prescriptions filled in Canada but represent slightly less than 25 % of the expenditures on medications (Canadian Generic Pharmaceutical Association 2013). Previous reports have documented the high price of Canadian generics compared to those in other countries. In 2007, Canadian prices were higher than those in 11 other OECD countries (Patented Medicine Prices Review Board 2010) (see Table 2.4).
One reason for the higher Canadian prices is the level of generic competition in the market. Countries such as the US that have much larger populations tend to attract more generic companies into the market leading to lower prices. However, there still is substantial competition in Canada; work done by the PMPRB indicates that Canada ranks in the middle of 6 countries studied in terms of the average number of generic suppliers for each non-patented product (Canadian Generic Drug Sector Study 2007). The main reason for the higher Canadian prices is that competition among the generic companies takes place at the level of pharmacies and particularly pharmacy chains. Because of the dominance of pharmacy chains and franchises in the Canadian market they have been able to demand high rebates from generic manufacturers in return for stocking their products. Average rebates have been estimated to be 40 % and may be as high as 80 % for individual generic products (Competition Bureau Canada 2007).
The main mechanisms used by public drug plans to set the price that they will pay for generic products are capping the formulary price at a percentage of the brand name price and specifying a maximum reimbursable cost for a drug or group of interchangeable drugs. With a maximum reimbursable cost approach the plans obtain the cost of the generic from the manufacturer and use that cost to determine an appropriate formulary price (Bell et al. 2010).
In recent years provincial plans have significantly decreased what they will pay for generic drugs. Ontario has gone from 50 to 25 % of the originator price (with 20 % for the top ten drugs) and other provinces are at 25 and 35 %, although some are still paying 65 % of the originator price and when British Columbia reduced prices to 35 %, it granted over 600 exemptions for specific products (Law and Kratzer 2013). Despite these reductions Canadian generic prices still remain excessive. If Ontario paid prices available for generics in either New Zealand or the US Department of Veterans Affairs for the top 82 generics it covered, then it would have reduced it expenditures on these products from $190 to $61 million (Law 2013).
Most recently there is an interprovincial program to reduce generic prices, the Generic Pricing Initiative. Initially the program targeted six widely used drugs and set a price cap at 18 % of the originator price. When fully implemented this program could save up to $100 million annually Canada-wide (Council of the Federation 2013). As of April 1, 2014 four additional drugs were added (Ontario Public Drug Programs 2014).
2.10 Role of Private Insurance Companies
Twenty-three million Canadians (68 % of the population) have some form of private insurance coverage for medications (Canadian Life and Health Insurance Association 2012) and over one-third (35.4 %) of total prescription drug expenditures in Canada is covered by private insurance (Canadian Institute for Health Information 2013a), but these plans have little role in controlling drug prices or overall expenditures. In fact, over the period 2003–2012 private expenditures rose faster than public expenditures in 8 of the 10 years (Canadian Institute for Health Information 2013a). One of the key reasons that private insurers pay little attention to costs is because “the majority of private drug benefit plans are administered for companies by outside firms—mainly insurance companies—that are often paid a percentage of plan costs” leaving them no incentive to rein in prices (Silversides 2009).
Law and colleagues point out the weaknesses in how private insurers approach the issue of costs (Law et al. 2014). Private insurers are much more likely to list new drugs on their formularies compared to public plans. An analysis of new drugs approved by Health Canada between 2004 and 2011 found that 81 % of new drugs were insured by at least one private plan compared to 47 % by at least one public plan (CHPI 2013). Listing newer and typically more expensive drugs is only advantageous if these drugs offer significant therapeutic advantages over existing products but out of 336 NAS approved by Health Canada between January 1, 2000 and March 31, 2012 only 31 met that criterion (Lexchin 2014).
Private plans often pay prices that are in excess of the manufacturer’s list price. Although some provinces try to ensure that generic prices are equivalent in the public and private sector, a Competition Bureau report found that prices in private plans are 10 % higher than those in public plans for non-patented brand name drugs and 7 % higher for generic drugs (Competition Bureau Canada 2007). This difference in prices extends to patented brand-name products. In a “claims review of a large employer, drug prices submitted by pharmacies for certain brand drugs ranged from 9.2 to 37.2 % more than the…list price; and certain generic drugs were priced between 45 and 102.9 % more than the…list price. In other words, some pharmacies charged 102.9 % more for the same drug, in the same quantity, to the same drug plan” (Stevenson 2011) and the plan paid that price.
While all public drug plans require mandatory generic substitution only 67 % of employees belonged to private plans that had this requirement and only 19 % of employees were part of a private plan that used a multitiered formulary, i.e., a formulary that places drugs into different tiers with the first tier requiring the lowest copayment and typically including mostly generic drugs (Kratzer et al. 2013). According to Law and colleagues “[t]he very limited use of managed formularies—a list of the drugs covered by the Plan—in the past by private drug plans…has made it difficult, if not impossible, for insurers to negotiate…preferred discounts or rebates in exchange for preferential listing status. Industry estimates also suggest that the limited use of formularies resulted in private plans paying $3.9 billion more for drugs in 2012 where equally effective therapeutic alternatives were available” (Law et al. 2014).
2.11 Impact of Pricing on Accessibility
Provincial plans pay 38.5 % ($10,677.1 million) of total prescription drug costs in Canada, federal and social security funds pay an additional 6.0 % ($1,652.5 million), leaving private insurance to cover 35.4 % ($9,825.4 million) and out-of-pocket payment at 20.1 % ($5,578.5 million) (Canadian Institute for Health Information 2013a). While provincial drug plans are the largest payers they only cover about 25 % of the population (Demers et al. 2008). Estimates are that 13 % of the Canadian population is either uninsured or underinsured for prescription drug costs and people with no drug coverage and paying out of pocket are usually those with minimum wage jobs (Applied Management in Association with Fraser Group Tristat Resources 2000).
The individual prescription drug programs in each province vary considerably in their design in terms of who is eligible for coverage, what drugs are covered and how much people have to pay in the form of deductibles, copayments and user fees. Provincial plans are based on age (usually covering people 65 and older), income level (coverage on a sliding scale below a certain individual or family income) and employment (if employers offer health benefits to their workers then they must offer drug insurance and employees are obligated to purchase the insurance). In addition, all provinces cover social assistance recipients although sometimes these people are required to pay a co-payment (Daw and Morgan 2012; Morgan et al. 2013a).
Simulations were constructed to show the variation in costs to people in different provinces. One example was for a 65 year old woman whose family income was below the national average and who was taking medications for diabetes, hypertension and insomnia. Her annual out-of-pocket costs ranged from $8 to $504 depending on what province she lived in. A 40 year old social assistance recipient taking drugs for hypertension and high cholesterol would get his drugs for free in some provinces but would pay up to $200 in another. Overall, seniors, depending on their income, paid 35 % or less of their drug costs in two provinces but up to 100 % in others (Demers et al. 2008). These figures are based on the drug plans as of December 2006 and while plans have changed since then it is likely that this level of variation still exists. In recent years, “[u]niversal income-based catastrophic coverage appears to be emerging as an implicit national standard for provincial pharmacare. However, due to the variation and high level of patient cost-sharing required under these programs, convergence on this model does not equate to substantial progress towards expanding coverage or reducing interprovincial disparities” (Daw and Morgan 2012).
Cost related non-adherence to prescription medications across Canada is about 5.1 % (Kennedy and Morgan 2006) but this figure hides significant within-country differences. In Quebec where drug insurance is mandatory (either through employment or the provincial government) cost related non-adherence was 4.4 %. In Ontario where there was coverage for those 65 and over and social assistance recipients it was 8.8 % and in provinces with income-based coverage it was 12.1 % (Kennedy and Morgan 2009). Not surprisingly, cost-related non-adherence is significantly related to income and having insurance coverage. For those with a high income (annual household income >$80,000) and insurance it was 3.6 % while for those with a low income (annual household income <$20,000) and no insurance it was 35.6 % or ten times greater (Law et al. 2012).
2.12 Conclusion
Although superficially it would seem that Canada has been successful at controlling the price of patented medicines, a deeper examination and per capita expenditures shows that the mechanism that is used is deeply flawed and leads to Canadian prices being among the highest of all of the OECD countries. Similarly, despite recent moves by some of the provinces, generic prices remain much higher than those in places such as New Zealand or those from the US Veterans Affairs. Private insurance companies, despite playing a major role in paying for drugs have no incentive for trying to help contain costs because they often paid a percentage of how much the plan spends. The complicated federal nature of the country also means that while there is a health technology assessment process, participation is voluntary on the part of federal, provincial and territorial drug plans, and although all of them aside from Quebec have chosen to opt in, they are free to ignore the advice that comes out of the process. The lack of a federal role in ensuring coverage for drug costs means that each province and territory has its own unique plan with a divergence from province to province in terms of who is covered, what drugs are listed on formularies and how much people need to pay out of pocket in the form of copayments, user fees or deductibles. The ultimate result is that many lower income people who lack insurance forego filling the prescriptions that they receive.
References
Aaserud M, Austvoll-Dahlgren A, Kösters J, Oxman A, Ramsay C, Sturm H (2006) Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev (2): CD005979. doi:10.1002/14651858.CD005979
Applied Management in Association with Fraser Group Tristat Resources (2000) Canadian’s access to insurance for prescription medicines, vol 2, The uninsured and the under-insured. Health Canada, Ottawa
Bell C, Griller D, Lawson J, Lovren D (2010) Generic drug pricing and access in Canada: what are the implications? Health Council of Canada, Toronto
Blomqvist Å, Busby C (2013) Paying hospital-based doctors: fee for whose service? Commentary No. 392. C.D. Howe Institute, Toronto
Canada House of Commons (1967) Second (final) report of the special committee of the House of Commons on drug costs and prices. Canada House of Commons, Ottawa
Canada’s Research-Based Pharmaceutical Companies (2014) Member profiles. Rx&D. http://www.canadapharma.org/en/about-rxd/membership/rxd-members. Accessed 8 May 2014
Canada’s Health Care System (2014) Health Canada. http://www.hc-sc.gc.ca/hcs-sss/pubs/system-regime/2011-hcs-sss/index-eng.php. Accessed 7 May 2014
Canadian Generic Pharmaceutical Association (2013) The value of generic prescription medicines: are you taking full advantage. CGPA. http://www.canadiangenerics.ca/en/advocacy/docs/ValueofGenericPrescriptionMedicine2013.pdf. Accessed 8 May 2014
Canadian Generic Pharmaceutical Association (2014) About CGPA. CPGA. http://www.canadiangenerics.ca/en/about/members.asp. Accessed 8 May 2014
Canadian Institute for Health Information (2012) Drivers of prescription drug spending in Canada. CIHI, Ottawa
Canadian Institute for Health Information (2013a) Drug expenditure in Canada, 1985 to 2012. CIHI, Ottawa
Canadian Institute for Health Information (2013b) Pharmacist workforce, 2012—provincial/territorial highlights. CIHI, Ottawa
CHPI (2013) How good is your drug insurance? Comparing federal, provincial and private-sector plans, 2004–2011. Annual report—2013 edition. Toronto
Commission of Inquiry on the Pharmaceutical Industry (1985) Report. Ottawa
Common Drug Review (2013) Procedure for Common Drug Review. Canadian Agency for Drugs and Technology in Health, Ottawa
Competition Bureau Canada (2007) Canadian generic drug sector study. Gatineau
Council of the Federation (2013) Provinces and territories seek significant cost savings for Canadians on generic drugs. http://www.councilofthefederation.ca/phocadownload/newsroom-2013/nr-cof-generic_drugs_final-jan_18.pdf. Accessed 8 May 2014
Daw J, Morgan S (2012) Stitching the gaps in the Canadian public drug coverage patchwork? A review of provincial pharmacare policy changes from 2000 to 2010. Health Policy 104:19–26
Demers V, Melo M, Jackevicius C, Cox J, Kalavrouziotis D, Rinfret S, Humphries K, Johansen H, Tu J, Pilote L (2008) Comparison of provincial prescription drug plans and the impact on patients’ annual drug expenditures. CMAJ 178:405–409
Gagnon M-A, Hébert G (2010) The economic case for universal pharmacare: costs and benefits of publicly funded drug coverage for all Canadians. Canadian Centre for Policy Alternatives, Ottawa
Gamble J-M, Weir D, Johnson J, Eurich D (2011) Analysis of drug coverage before and after the implementation of Canada’s Common Drug Review. CMAJ 183:E1259–E1266
Government of Canada (1966) Medical Care Act. R.S.C. 1970, c. M-8. Queen’s Printer, Ottawa
Government of Canada (2012) Canada Health Act. R.S.C. 1985, c. C-6. Queen’s Printer, Ottawa
Canadian Life and Health Insurance Association (2012) Backgrounder—Canadian life and health insurance industry drug pooling agreement. CLHIA. http://www.clhia.ca/domino/html/clhia/CLHIA_LP4W_LND_Webstation.nsf/resources/Drug_Protection/$file/backgrounder.pdf. Accessed 8 May 2014
IMS Brogan (2012) Retail pharmacies by outlet type, Canada, 2005–2011
Industry Canada (2013) Pharmaceutical industry profile. Industry Canada. http://www.ic.gc.ca/eic/site/lsg-pdsv.nsf/eng/h_hn01703.html. Accessed 9 May 2014
Kennedy J, Morgan S (2006) A cross-national study of prescription nonadherence due to cost: data from the joint Canada–United States survey of health. Clin Ther 28:1217–1224
Kennedy J, Morgan S (2009) Cost-related prescription nonadherence in the United States and Canada: a system-level comparison using the 2007 international health policy survey in seven countries. Clin Ther 31:213–219
Kratzer J, McGrail K, Strumpf E, Law M (2013) Cost-control mechanisms in Canadian private drug plans. Healthcare Policy 9(1):35–43
Law M (2013) Money left on the table: generic drug prices in Canada. Healthcare Policy 8(3):17–25
Law M, Kratzer J (2013) The road to competitive generic drug prices in Canada. CMAJ 185:1141–1144
Law M, Cheng L, Dhalla I, Heard D, Morgan S (2012) The effect of cost on adherence to prescription medications in Canada. CMAJ 184:297–302
Law M, Kratzer J, Dhalla I (2014) The increasing inefficiency of private health insurance in Canada. CMAJ 186:E470–E474. doi:10.1503/cmaj.130913
Lexchin J (1993a) The effect of generic competition on the price of prescription drugs in the province of Ontario. CMAJ 148:35–38
Lexchin J (1993b) Pharmaceuticals, patents and politics: Canada and Bill C-22. Int J Health Serv 149:1401–1407
Lexchin J (1997) After compulsory licensing: coming issues in Canadian pharmaceutical policy and politics. Health Policy 40:69–80
Lexchin J (2004) The effect of generic competition on the price of brand-name drugs. Health Policy 68:47–54
Lexchin J (2006) Do manufacturers of brand-name drugs engage in price competition? An analysis of introductory prices. CMAJ 174:1120–1121
Lexchin J (2008) New directions in Canadian drug regulation: whose interests are being served? In: O’Donovan O, Glavanis-Grantham K (eds) Power, politics and pharmaceuticals. Cork University Press, Cork, pp 153–170
Lexchin J (2014) Postmarket safety in Canada: are significant therapeutic advances and biologics less safe than other drugs? A cohort study. BMJ Open 4:e004289
Morgan SG, McMahon M, Mitton C, Roughead E, Kirk R, Kanavos P, Menon D (2006) Centralized drug review processes in Australia, Canada, New Zealand, and the United Kingdom. Health Aff 25(2):337–347
Morgan S, Hanley G, Raymond C, Blais R (2009) Breadth, depth and agreement among provincial formularies in Canada. Healthcare Policy 4(4):e162–e184
Morgan S, Daw J, Law M (2013a) Rethinking pharmacare in Canada. C.D. Howe Institute, Toronto
Morgan S, Friesen M, Thomson P, Daw J (2013b) Use of product listing agreements by Canadian provincial drug benefit plans. Healthcare Policy 8(4):45–57
OECD (2013) Health at a glance 2013: OECD indicators. OECD Publishing
Ontario Public Drug Programs (2014) Executive Officer Notice: Pan-Canadian competitive value price initiative for generic drugs. Ministry of Health and Long-Term Care. http://www.health.gov.on.ca/en/pro/programs/drugs/opdp_eo/notices/exec_office_20140310.pdf. Accessed 8 May 2014
Pan-Canadian Oncology Drug Review (2014) About the Pan-Canadian Oncology Drug Review. pCODR. http://www.pcodr.ca/wcpc/portal/Home/AboutpCODR?_afrLoop=324795520801000&lang=en&_afrWindowMode=0&_adf.ctrl-state=n4wxkb1b8_458. Accessed 8 May 2014
Patented Medicine Prices Review Board (2006) Non-patented prescription drug prices reporting: Canadian and foreign price trends. PMPRB, Ottawa
Patented Medicine Prices Review Board (2010) Generic drugs in Canada: price trends and international price comparisons, 2007. PMPRB, Ottawa
Patented Medicine Prices Review Board (2013) Annual report 2012. PMPRB, Ottawa
Patented Medicine Prices Review Board (2014a) Compendium of policies, guidelines and procedures—reissued June 2013. PMPRB. http://www.pmprb-cepmb.gc.ca/english/View.asp?x=1733. Accessed 8 May 2014
Patented Medicine Prices Review Board (2014b) Regulating prices. PMRPB. http://www.pmprb-cepmb.gc.ca/english/View.asp?x=1440. Accessed 8 May 2014
Restrictive Trade Practices Commission (1963) Report concerning the manufacture, distribution and sale of drugs. Queen's Printer, Ottawa
Royal Commission on Health Services (1964) Report. Queen's Printer, Ottawa
Schneeweiss S, Soumerai S, Maclure M (2002) Reference drug pricing. CMAJ 167:126–127
Silversides A (2009) Ontario’s law curbing the cost of generic drugs sparks changes. CMAJ 181:E43–E45
Standing Committee on Health of the House of Commons (2007) Prescription drugs part 1—common drug review: an F/P/T process. Standing Committee on Health of the House of Commons, Ottawa
Statistics Canada (2014) Consumer price index, historical summary. Statistics Canada. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ46a-eng.htm. Accessed 8 May 2014
Stevenson H (2011) An end to blank cheques: getting more value out of employer drug plans. Reformulary Group, Toronto
Sutherland J, Crump R, Repin N, Hellsten E (2013) Paying for hospital services: a hard look at the options. Commentary No. 378. Toronto
Tierney M, Manns B (2008) Optimizing the use of prescription drugs in Canada through the Common Drug Review. CMAJ 178:432–435
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lexchin, J. (2015). Drug Pricing in Canada. In: Babar, ZUD. (eds) Pharmaceutical Prices in the 21st Century. Adis, Cham. https://doi.org/10.1007/978-3-319-12169-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-12169-7_2
Published:
Publisher Name: Adis, Cham
Print ISBN: 978-3-319-12168-0
Online ISBN: 978-3-319-12169-7
eBook Packages: Business and EconomicsEconomics and Finance (R0)