Abstract
Stroke is a devastating disease that is a leading cause of death and disability in developed countries. However, therapeutic options are notably limited, so is mandatory to investigate repairing processes after stroke in order to develop therapeutic strategies able to promote brain repair processes. In this context, therapeutic angiogenesis and vasculogenesis hold promise to improve the prognosis of patients with stroke. In this regard, it is well established that circulating endothelial progenitor cells (EPCs) have been suggested to be a marker of vascular risk and endothelial function. Moreover, low EPC number has been found in patients with cerebrovascular diseases. Besides, EPC levels have been associated with good neurological and functional outcome as well as reduced infarct growth in patients with acute ischemic stroke. Finally, experimental and clinical studies indicate that EPC might mediate endothelial cell regeneration and neovascularization. Therefore, EPC-based therapy could be an excellent therapeutic option in stroke. Currently, clinical trials for evaluating EPC treatment in ischemic stroke are ongoing. In this chapter, we discuss the present status of knowledge about the possible therapeutic role of EPCs in stroke, molecular mechanisms, and the future perspectives and strategies for their use in clinical practice.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vascular Endothelial Growth Factor
- Endothelial Progenitor Cell
- Late EPCs
- Endothelial Cell Regeneration
- Cell Embryonic Stem Cell
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Stroke is the second to third most common cause of death in adults, and more than a third of people who survive a stroke will have severe disability (Lloyd-Jones et al. 2010). Most of the strokes, about 80 %, are ischemic strokes (IS). IS is caused by an occlusion of a cerebral artery, which prevents blood flow to reach brain parenchyma. The remaining 20 % of strokes are intracerebral hemorrhages (ICH) , caused by the rupture of a cerebral blood vessel. The ICH is usually more severe with greater rates of mortality and disability than IS. Therapeutic options currently are focusing on recanalization therapies for acute IS, mainly through intravenous or intra-arterial fibrinolyisis and thrombectomy (Mokin et al. 2014), but limitations restrict their use to a small proportion of patients. Moreover, no specific pharmacological treatments exist in order to improve the ICH prognosis, and treatment options for this disease are more limited than in IS, based primarily on surgical treatment.
Ischemic and hemorrhagic injury is a long and dynamic process involving a variety of mechanisms at different times. Stroke triggers many processes, including accumulation of excitatory amino acids, oxidative stress, alterations in gene expression, mitochondrial injury, brain edema, inflammation , and apoptosis, but also brain plasticity and endogenous repair mechanisms. Unfortunately, neurons at risk survive for only a few hours, and there is limited opportunity for effective therapeutic intervention. In this regard, although a wide range of neuroprotective substances has been effective in experimental models of stroke, they have repeatedly failed in clinical trials because of toxicity or loss of effectiveness (Tymianski 2013). Therefore, the development of strategies to increase plasticity and endogenous brain repair mechanisms in order to improve functional outcome in stroke are essential (Rodríguez-González et al. 2007). In this context, therapeutic angiogenesis and vasculogenesis hold promise to improve the prognosis of patients with stroke . In this regard, it is well established that circulating endothelial progenitor cells (EPCs) have been suggested to be a marker of vascular risk and endothelial function (Hill et al. 2003; Vasa et al. 2001; Werner et al. 2005). Moreover, low EPC number has been found in patients with cerebrovascular diseases (Ghani et al. 2005). Besides, EPC levels have been associated with good neurological and functional outcome as well as reduced infarct growth in patients with acute ischemic stroke (Sobrino et al. 2007; Chu et al. 2008; Yip et al. 2008; Navarro-Sobrino et al. 2010; Bogoslovsky et al. 2010; Paczkowska et al. 2013; Martí-Fàbregas et al. 2013). Finally, EPCs have been related to endothelial cell regeneration and neovascularization after tissue ischemia (Zhang et al. 2002; Mao et al. 2014). Therefore, EPC-based therapy could be an excellent therapeutic option in stroke. In this chapter, we discuss the present status of knowledge about the possible therapeutic role of EPCs in stroke, molecular mechanisms , and the future perspectives and strategies for their use in clinical practice.
2 Rationale for Therapeutic Use of EPCs to Treat Stroke
It is well established that circulating endothelial progenitor cells (EPCs) have been suggested to be a marker of vascular risk and endothelial function (Hill et al. 2003; Vasa et al. 2001; Werner et al. 2005). The number of circulating EPC has been reported to be decreased in patients with vascular risk factors such as smoking habit, hypercholesterolemia, diabetes and hypertension (Vasa et al. 2001; Sobrino et al. 2007; Zhao et al. 2013), many of which have been identified as prognostic markers of poor outcome following stroke. Moreover, low EPC number has been found in patients with cerebrovascular diseases (Ghani et al. 2005), and EPC levels have been associated with good neurological and functional outcome as well as reduced infarct growth in patients with acute IS and ICH (Sobrino et al. 2007, 2011a; Chu et al. 2008; Yip et al. 2008; Navarro-Sobrino et al. 2010; Bogoslovsky et al. 2010; Paczkowska et al. 2013; Martí-Fàbregas et al. 2013). However, existing evidence supports that EPCs not only work as biomarker but also might offer a new therapeutic strategy for stroke (Lapergue et al. 2007). In this regard, experimental and human studies indicate that EPC might mediate endothelial cell regeneration and neovascularization (Asahara et al. 1997; Werner et al. 2003; Kong et al. 2004; Werner and Nickenig 2006), and that EPC participate in the cerebral neovascularization present in adult brain after ischemia (Zhang et al. 2002; Mao et al. 2014). These protective vascular effects result from EPC proliferation. On the other hand, as stated above, EPCs have been suggested to maintain endothelial protection/repair and neovascularization and angiogenesis. Today it is known that angiogenesis is coupled with neurogenesis following ischemic injury (Thored et al. 2007). The underlying mechanisms include that the regenerated blood vessels provide nutritive blood flow and that EPCs, by secreting factors such as SDF-1 and VEGF, create a microenvironment for neural regeneration and survival (Imitola et al. 2004; Schänzer et al. 2004). Furthermore, neuroblasts migrate along these regenerated vessels to achieve neurogenesis in peri- infarct area. Consequently, suppression of angiogenesis substantially reduces migration of neuroblasts from the subventricular zone to the ischemic region (Zhang and Chopp 2009). Therefore, EPC-based therapy might be an excellent therapeutic option in stroke .
3 EPC-Based Cellular Therapy for Stroke
EPC levels have been associated with good neurological and functional outcome as well as reduced infarct growth in patients with acute IS (Sobrino et al. 2007; Chu et al. 2008; Yip et al. 2008; Navarro-Sobrino et al. 2010; Bogoslovsky et al. 2010; Paczkowska et al. 2013; Martí-Fàbregas et al. 2013) and ICH (Sobrino et al. 2011a; Paczkowska et al. 2013). Likewise, EPCs have been related to endothelial cell regeneration and neovascularization after tissue ischemia (Zhang et al. 2002; Mao et al. 2014). Therefore, EPC-based therapy could be an excellent therapeutic opportunity for stroke. The aim of cellular therapy is to restore brain function by replacing dead cells with new ones through transplantation or stimulation of endogenous stem or precursor cells (Hurtado et al. 2006). There is growing evidence that the adult stem cell system, including EPCs, is more flexible than previously thought and may be an excellent therapeutic option for stroke (Rodriguez-González et al. 2007). In this regard, it has been suggested that resident pools of adult stem cells, such as EPCs, can be used in two ways: (a) by isolating, harvesting and growing them in vitro and then administering them locally or systemically; (b) or by endogenous stimulating them (see factors associated to EPC increase in Table 15.1).
3.1 Exogenous Administration of EPCs
There are numerous concerns about the cell therapy with exogenous EPC transplantation . The optimal starting time point for administration of EPCs following stroke may be critical for their therapeutic efficacy. However, there are few studies on this important issue. Based on the capacity of EPCs to secrete several growth factors with protective effects on the brain, their transplantation in the early phase of stroke may have better efficacy. By contrast, it has also been suggested that oxidative stress and inflammation in the acute phase of stroke may limit the function on survival of transplanted EPCs (Locatelli et al. 2009). Therefore, preclinical and clinical studies are needed to evaluate the best time point after stroke onset for EPC transplantation.
Another important issue is about the autologous or allogeneic transplantation of EPCs , as well as the source of obtaining EPCs from stroke patients or healthy subjects. A recent proteomic study has analyzed differences in protein expression of early outgrowth colony forming unit-endothelial cell (CFU-EC) (Fig. 6.1) from IS patients and healthy subjects (see Brea et al. 2011 for review). Remarkably, the proteomic analysis revealed a greater expression of cell division control protein 42 homolog (CdC42) and endoplasmic reticulum protein 29 (ERp29) in EPCs from healthy subjects, and a greater expression of elongation factor 2 (eF2) and peroxiredoxin 1 (PRDX1) in EPCs obtained from IS patients. It has been reported that PRDX1 expression dramatically increases during processes such as spontaneous differentiation of human embryonic stem cells , targeted differentiation of neural progenitor cells and differentiation of human neural stem cell line respect to proliferating cells. eF2 is also up-regulated 4–7 days after differentiation of the human neuronal stem cell line, ReNcell VM. Therefore, these findings could be indicating that EPCs from IS patients are in a more advanced differentiation state than EPCs isolated from control subjects. On the other hand, CdC-42 and ERp29 were found to be up-regulated in EPCs from healthy subjects. Cdc42 regulates adhesion, migration, homing, and cell cycle progression of hematopoietic stem cells. ERp29 seems to be involved in cell proliferation . In view of the fact that Cdc42 and ERp29 are up-regulated in EPCs from healthy subjects, it is seems that EPCs isolated from healthy subjects show a more capacity of proliferation compared to EPCs from stroke patients. Moreover, it has been proposed the use of late EPCs as an optimal EPC-based therapy. However, another studies showed that infusion of early EPCs enhanced the long-term outcome in animal models of stroke (Zhao et al. 2013). In fact, currently coadministration of different types of progenitor/stem cells may constitute a novel therapeutic strategy for stroke (Foubert et al. 2008). Data from other studies show that EPCs obtained from stroke patients in the subacute phase have greater vasculogenic capacity than those from acute phase (Navarro-Sobrino et al. 2010). Finally, regarding to EPC transplantation in clinical practice, intravenous infusion should be the optimal route because intra-arterial infusion may be inconvenient and could provoke embolism. Likewise, intracerebral injection of EPCs is complex and might cause intracerebral hemorrhage and parenchymal damage. Although, it remains to be determined whether administration of autologous or allogeneic EPCs in the subacute period is more effective, it is tempting to postulate, based on the above data, that early and late EPCs obtained from stroke patients in the subacute phase could be the most suitable source of EPCs for cell therapy in stroke by using intravenous administration. However, larger clinical studies are needed to evaluate this hypothesis.
3.2 Endogenous Stimulation of EPCs
Restoring blood flow supply after ischemia and re-endothelization after hemorrhage may contribute to cell survival and tissue repair. Formation of new blood vessels in the adult brain after stroke is not only mediated by angiogenesis but also involves vasculogenesis mediated by EPCs, which are involved in processes of re-endothelization and repair of vascular endothelium in response to vascular trauma or tissue ischemia, promoted by biochemical factors that activate its proliferation . They have been described that EPCs migrate through the peripheral blood from bone marrow to sites of neovascularization where EPCs are able to differentiate into mature endothelial cells. Recruitment and incorporation of EPCs into ischemic or hemorrhagic tissues requires a coordinated multistep process including mobilization, chemoattraction, adhesion, migration , tissue invasion and in situ differentiation (Fig. 6.2) (Rodríguez-González et al. 2007). Many molecular and physiological-pathological factors, as well as drugs, are involved in these processes (For review: Arenillas et al. 2007; Brea et al. 2009; Sobrino et al. 2011a, b, 2012a, b; Zhao et al. 2013) (Table 6.1). For example, the activity of matrix metalloproteinase 9 (MMP-9), which causes a massive release of stem cell factor (SCF) and activation of membrane bound Kit ligand (mKitL) that favors the recruitment of progenitor cells, among which are EPC, from bone marrow. Likewise, active MMP-9 induces the release of cytokines that cause the mobilization of quiescent EPC (Rafii et al. 2002). Moreover, EPC release and mobilization are regulated by vascular endothelial growth factor (VEGF), stromal-cell-derived factor 1 (SDF-1), granulocyte colony-stimulating factor (G-CSF), erythropoietin (EPO), angiopoietin 1, endothelial NO synthase (eNOS), exercise, estrogens and several drugs such as statins, EPO or citicoline. In fact, clinical studies in IS and ICH patients demonstrated that serum levels of VEGF, SDF-1α and active MMP-9 increase in response to cerebral ischemia or ICH within the first 72 h from symptom onset, and that the magnitude of this increase is directly related to an EPC increment (Bogoslovsky et al. 2011a, b; Sobrino et al. 2011a, 2012b). On the other hand, the fact that serum levels of molecular markers at 24 h from stroke onset correlated with EPC increment during the 1st week, but not at admission, and that EPC increment during the 1st week, but not EPC counts at baseline, has been associated with better neurological outcome and reduced infarct growth supports the hypothesis that cerebral ischemia induces the activation of molecular pathways of EPC mobilization focused on promoting endogenous processes of vascular and neurorepair (Fig. 6.3). Furthermore, similar results were found in ICH patients (Sobrino et al. 2009, 2011a; Paczkowska et al. 2013). It has been reported in ICH patients a strong correlation between VEGF and SDF-1α serum levels and circulating concentrations of bone marrow-derived progenitor cells (BMPCs) at day 7 (Sobrino et al. 2011a). Given that the EPC is a subtype of BMPCs, it is tempting to hypothesize that similar molecular and cellular mechanisms are involved in the two major subtypes of stroke (ischemic and hemorrhagic stroke).
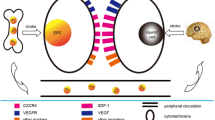
Mechanism of endothelial progenitor cells (EPC) mobilization in stroke. Stroke is a potent inducer of endogenous repair mechanisms, which are initially activated by a massive expression of growth factors such as vascular endothelial growth factor (VEGF), and other molecules such as matrix metalloproteinase 9 (MMP-9) and stromal cell-derived factor 1α (SDF-1α). These molecular factors, especially MMP-9 activity, induce the mobilization of bone marrow-derived stem cells, including EPCs, through membrane bound Kit ligand (mKitL) activation. These EPCs may migrate into the areas of brain injury, mediating repair mechanisms. Likewise, these mechanisms also enhance the endogenous neurogenesis
On the other hand, because EPCs express functional CXCR4 and VEGF receptors (Salcedo et al. 1999; Yamaguchi et al. 2003), an interaction between the SDF-1α/CXCR4 pathway and VEGF might form a positive-feedback loop which would increase the therapeutic effects of EPCs in cerebral neovascularization. Consequently, growth factors and SDF-1α might be an effective therapy in IS and ICH because of their role mediating the mobilization of circulating EPCs, as well as in restoring endothelium integrity and decreasing brain edema, inflammation , and perihematoma cell death (Krizanac-Bengez et al. 2004).
As a clinical implication, the number of circulating EPCs is inversely correlated with vascular risk factors such as diabetes, hypertension, hypercholesterolemia or smoking (Hill et al. 2003; Vasa et al. 2001; Werner et al. 2005), and also with molecular markers of endothelial dysfunction and inflammation such as homocysteine or C-reactive protein. Therefore, it can be rationally speculated that environment of circulation is essential for the living and functionality of EPCs, which would raise the perspective on the demand in managing risk factors of stroke (Zhao et al. 2013).
Finally, it has also been demonstrated that several drugs can modulate endogenous EPC behavior (Table 6.1). Statins treatment during acute phase leads to an increase in EPCs in IS patients (Sobrino et al. 2012a; Martí-Fàbregas et al. 2013). In view of the fact that statin treatment during the acute phase increases circulating EPC and statin withdrawal is associated with poor outcome in IS patients (Blanco et al. 2007), the positive effects of statin treatment during the acute phase on functional outcome in ischemic stroke could be mediated by EPC. Moreover, patients treated with statins showed also higher serum levels of VEGF, active MMP-9 and nitric oxide (NO)x at 24 h (Sobrino et al. 2012a). Statins induce the production of NO by eNOS, the expression of angiogenic factors such as VEGF, and the mobilization and proliferation of EPC (Endres 2005) , so these mechanisms may be interrelated. G-CSF is one of the early drugs discovered to be able to enhance EPC mobilization into the circulation after venous administration (Powell et al. 2005). Afterwards, other drugs such as Angiotensin II type 1 receptors blocker, Angiotensin-converting enzyme inhibitors, EPO, berberine, citicoline, recombinant tissue plasminogen activator (r-tPA) and PPAR-γ agonist have been shown to increase the number and functional activity of EPCs in vitro and in vivo (Arenillas et al. 2007; Rodríguez-González et al. 2007; Sobrino et al. 2011b, 2012a, b; Zhao et al. 2013). As these drugs are commonly used in clinical treatment of vascular diseases, all these clinical data may help to interpret the beneficial effects of these drugs on top of their known pharmacological actions. However, further studies are needed in order to facilitate the discovery of new drugs targeting EPCs.
4 Promising Strategies Related to EPCs
As a promising strategy for cellular-based therapies for stroke , induced pluripotent stem cells (iPSC) technology (Takahashi and Yamanaka 2006; Takahashi et al. 2007), which enables the reprogramming of a wide variety of cell types isolated from humans into embryonic stem cell-like pluripotent cells, offers a novel strategy for the patient-specific derivation of a lineage-specific cells from iPSC, such as EPCs (Choi et al. 2009; Park et al. 2010; Xu et al. 2012; Yoo et al. 2013). Moreover, the therapeutic potential of transplanted human iPSC-derived EPCs (hiPSC-EPCs) has been shown in animal disease models of hind-limb ischemia (Park et al. 2010; Rufaihah et al. 2011; Lai et al. 2013). Therefore, it is tempting to postulate that hiPSC-EPCs may represent a strategy for patient-specific EPC therapies in stroke.
Moreover, new strategies are necessary in order to increase the local number of EPCs in the ischemic or hemorrhagic areas. In this regard, nanomedicine may be useful to achieve this goal. A recent study has demonstrated the potential role of superparamagnetic iron oxide nanoparticles (SPION)-loaded EPCs by using a magnetic guidance to the ischemic tissue in animal models of cerebral ischemia . The authors demonstrate ex vivo cellular viability and maintained function following SPION load as well as successful guidance of the EPCs to the target site via magnetic resonance imaging (MRI) (Carenza et al. 2014). On the other hand, another recent study from our group (Agulla et al. 2014) has report a new theranostic nano-platform vectorized towards peri-infarct tissue, the key target for the treatment of cerebral ischemia . Anti-HSP72 (72 kDa heat shock protein) stealth immunoliposomes containing MRI probes were used to allocate the peri-infarct region in vivo and to achieve a superior therapeutic effect in comparison to other non-targeted drug delivery means (Fig. 6.4). Thus, despite the challenge of crossing the blood-brain barrier, this study demonstrates that theranostics inside the brain parenchyma is feasible and represents a good example of the potential that nanotechnology offers for the treatment of neurological disorders such as stroke . In this regard, these anti-HSP72 immunoliposomes, containing in their membrane one or more of the proteins involved in EPC recruitment shown in Table 6.1, could be used to locally increase the number of EPCs in the ischemic area after their exogenous transplantation or endogenous stimulation. Finally, another strategy in order to increase the local number of EPCs may be offered through interventional therapy for stroke, such as percutaneous transluminal angioplasty and stenting (PTAS) and thrombectomy. In addition, application of a bio-engineered EPC-capture stent, which accelerates re-endothelialization and reduces thrombogenicity, may reduce the rate of restenosis after PTAS (Larsen et al. 2012).
Schematic representation of theranostics feasibility inside the brain parenchyma despite the challenge of crossing the blood brain barrier (a). Representative MR images of ischemic brains from rats treated (i.v.) with 1 ml of saline (b), regular (non-vectorized) liposomes (c) or anti-HSP72 vectorized liposomes (d) showing the in vivo distribution of liposomes at 24 h after treatment. These anti-HSP72 immunoliposomes, containing in their membrane one or more of the proteins involved in EPC recruitment shown in Table 6.1, could be used to locally increase the number of EPCs in the ischemic area after their exogenous transplantation or endogenous stimulation
Abbreviations
- Ang-1:
-
Angiopoietin 1
- Cdc42:
-
Cell division control protein 42 homolog
- CFU-EC:
-
Early outgrowth colony forming unit-endothelial cell
- eF2:
-
Elongation factor 2
- Enos:
-
Endothelial nitric oxide synthase
- EPCs:
-
Endothelial progenitor cells
- EPO:
-
Erythropoietin
- G-CSF:
-
Granulocyte colony-stimulating factor
- ERp29:
-
Endoplasmic reticulum protein 29
- HIF-1:
-
Hypoxia-inducible factor 1
- HSP-72:
-
72 kilodalton heat shock protein
- ICH:
-
Intracerebral hemorrhage
- IGF-1:
-
Insulin-like growth factor 1
- IS:
-
Ischemic stroke
- mKitL:
-
membrane bound Kit ligand
- MMP-9:
-
Matrix metalloproteinase 9
- NO:
-
Nitric oxide
- PRDX1:
-
Peroxiredoxin 1
- r-tPA:
-
Recombinant tissue plasminogen activator
- SCF:
-
Stem cell factor
- SDF-1α:
-
stromal cell-derived factor 1α
- VEGF:
-
Vascular endothelial growth factor
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
References
Agulla J, Brea D, Campos F, Sobrino T, Argibay B, Al-Soufi W, Blanco M, Castillo J, Ramos-Cabrer P (2014) In vivo theranostics at the peri-infarct region in cerebral ischemia. Theranostics 4:90–105
Arenillas JF, Sobrino T, Castillo J, Dávalos A (2007) The role of angiogenesis in damage and recovery from ischemic stroke. Curr Treat Options Cardiovasc Med 9:205–212
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Blanco M, Nombela F, Castellanos M, Rodriguez-Yáñez M, García-Gil M, Leira R, Lizasoain I, Serena J, Vivancos J, Moro MA, Dávalos A, Castillo J (2007) Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology 69:904–910
Bogoslovsky T, Chaudhry A, Latour L, Maric D, Luby M, Spatz M, Frank J, Warach S (2010) Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology 75:2059–2062
Bogoslovsky T, Spatz M, Chaudhry A, Maric D, Luby M, Frank J, Warach S (2011a) Circulating CD133+ CD34+ progenitor cells inversely correlate with soluble ICAM-1 in early ischemic stroke patients. J Transl Med 9:145
Bogoslovsky T, Spatz M, Chaudhry A, Maric D, Luby M, Frank J, Warach S, NINDS Natural History of Stroke Investigators (2011b) Stromal-derived factor-1[alpha] correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 42:618–625
Brea D, Sobrino T, Ramos-Cabrer P, Castillo J (2009) Reorganisation of the cerebral vasculature following ischaemia. Rev Neurol 49:645–654
Brea D, Rodríguez-González R, Sobrino T, Rodríguez-Yañez M, Blanco M, Castillo J (2011) Proteomic analysis shows differential protein expression in endothelial progenitor cells between healthy subjects and ischemic stroke patients. Neurol Res 33:1057–1063
Carenza E, Barceló V, Morancho A, Levander L, Boada C, Laromaine A, Roig A, Montaner J, Rosell A (2014) In vitro angiogenic performance and in vivo brain targeting of magnetized endothelial progenitor cells for neurorepair therapies. Nanomedicine 10:225–234
Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, Slukvin I (2009) Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27:559–567
Chu K, Jung KH, Lee ST, Park HK, Sinn DI, Kim JM, Kim DH, Kim JH, Kim SJ, Song EC, Kim M, Lee SK, Roh JK (2008) Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 39:1441–1447
Endres M (2005) Statins and stroke. J Cereb Blood Flow Metab 25:1093–1110
Foubert P, Matrone G, Souttou B, Leré-Déan C, Barateau V, Plouët J, Le Ricousse-RoussanneS, Lévy BI, Silvestre JS, Tobelem G (2008) Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ Res 103:751–760
Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, Todd K (2005) Endothelial progenitor cells during cerebrovascular disease. Stroke 36:151–153
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T (2003) Circulating endotelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600
Hurtado O, Pradillo JM, Alonso-Escolano D, Lorenzo P, Sobrino T, Castillo J, Lizasoain I, Moro MA (2006) Neurorepair versus neuroprotection in stroke. Cerebrovasc Dis 21(Suppl 2):54–63
Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ (2004) Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 101:18117–18122
Kong D, Melo LG, Gnecchi M, Zhang L, Mostoslavsky G, Liew CC, Pratt RE, Dzau VJ (2004) Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation 110:2039–2046
Krizanac-Bengez L, Mayberg MR, Janigro D (2004) The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostatis and pathophysiology. Neurol Res 26:846–853
Lai WH, Ho JC, Chan YC, Ng JH, Au KW, Wong LY, Siu CW, Tse HF (2013) Attenuation of hind-limb ischemia in mice with endothelial-like cells derived from different sources of human stem cells. PLoS ONE 8:e57876
Lapergue B, Mohammad A, Shuaib A (2007) Endothelial progenitor cells and cerebrovascular diseases. Prog Neurobiol 83:349–362
Larsen K, Cheng C, Tempel D, Parker S, Yazdani S, den Dekker WK, Houtgraaf JH, de Jong R, Swager-ten Hoor S, Ligtenberg E, Hanson SR, Rowland S, Kolodgie F, Serruys PW, Virmani R, Duckers HJ (2012) Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model. Eur Heart J 33:120–128
Lloyd-Jones D et al (2010) Heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation 121:e46–e215
Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, Bresolin N, Comi GP, Corti S (2009) Stem cell therapy in stroke. Cell Mol Life Sci 66:757–772
Mao L, Huang M, Chen SC, Li YN, Xia YP, He QW, Wang MD, Huang Y, Zheng L, Hu B (2014) Endogenous endothelial progenitor cells participate in neovascularization via CXCR4/SDF-1 axis and improve outcome after stroke. CNS Neurosci Ther 20:460–468
Martí-Fàbregas J, Crespo J, Delgado-Mederos R, Martínez-Ramírez S, Peña EMarínR, Dinia L, Jiménez-Xarrié E, Fernández-Arcos A, Pérez-Pérez J, Querol L, Suárez-Calvet M, Badimon L (2013) Endothelial progenitor cells in acute ischemic stroke. Brain Behav 3:649–655
Mokin M, Khalessi AA, Mocco J, Lanzino G, Dumont TM, Hanel RA, Lopes DK, Fessler RD 2nd, Ringer AJ, Bendok BR, Veznedaroglu E, Siddiqui AH, Hopkins LN, Levy EI (2014) Endovascular treatment of acute ischemic stroke: the end or just the beginning? Neurosurg Focus 36:E5
Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Ribó M, Alvarez-Sabín J, Montaner J (2010) Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res 80:317–323
Paczkowska E, Gołąb-Janowska M, Bajer-Czajkowska A, Machalińska A, Ustianowski P, Rybicka M, Kłos P, Dziedziejko V, Safranow K, Nowacki P, Machaliński B (2013) Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: the role of endothelin-1. J Neurol Sci 325:90–99
Park SW, Jun Koh Y, Jeon J, Cho YH, Jang MJ, Kang Y, Kim MJ, Choi C, Sook Cho Y, Chung HM, Koh GY, Han YM (2010) Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood 116:5762–5772
Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, Finkel T, Cannon RO 3rd (2005) Granulocyte colony stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 25:296–301
Rafii S, Heissig B, Hattori K (2002) Efficient mobilization and recruitment of marrow-derived endothelial and hematopoietic stem cells by adenoviral vectors expressing angiogenic factors. Gene Ther 9:631–641
Rodríguez-González R, Hurtado O, Sobrino T, Castillo J (2007) Neuroplasticity and cellular therapy in cerebral infarction. Cerebrovasc Dis 24(Suppl 1):167–180
Rufaihah AJ, Huang NF, Jamé S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP (2011) Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol 31:e72–79
Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ (1999) Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol 154:1125–1135
Schänzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG (2004) Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol 14:237–248
Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Dávalos A, Lizasoain I, Castillo J (2007) The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke 38:2759–2764
Sobrino T, Arias S, Rodríguez-González R, Brea D, Silva Y, de la ONP, Agulla J, Blanco M, Pumar JM, Serena J, Dávalos A, Castillo J (2009) High serum levels of growth factors are associated with good outcome in intracerebral hemorrhage. J Cereb Blood Flow Metab 29:1968–1974
Sobrino T, Arias S, Pérez-Mato M, Agulla J, Brea D, Rodríguez-Yáñez M, Castillo J (2011a) Cd34+ progenitor cells likely are involved in the good functional recovery after intracerebral hemorrhage in humans. J Neurosci Res 89:979–985
Sobrino T, Rodríguez-González R, Blanco M, Brea D, Pérez-Mato M, Rodríguez-Yáñez M, Leira R, Castillo J (2011b) CDP-choline treatment increases circulating endothelial progenitor cells in acute ischemic stroke. Neurol Res 33:572–577
Sobrino T, Blanco M, Pérez-Mato M, Rodríguez-Yáñez M, Castillo J (2012a) Increased levels of circulating endothelial progenitor cells in patients with ischaemic stroke treated with statins during acute phase. Eur J Neurol 19:1539–1546
Sobrino T, Pérez-Mato M, Brea D, Rodríguez-Yáñez M, Blanco M, Castillo J (2012b) Temporal profile of molecular signatures associated with circulating endothelial progenitor cells in human ischemic stroke. J Neurosci Res 90:1788–1793
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O (2007) Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38:3032–3039
Tymianski M (2013) Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke 44:2942–2950
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:e1–e7
Werner N, Nickenig G (2006) Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol 26:257–266
Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G (2003) Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 93:e17–24
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Eng J Med 353:999–1007
Xu Y, Liu L, Zhang L, Fu S, Hu Y, Wang Y, Fu H, Wu K, Xiao H, Liu S, Yu X, Zheng W, Feng B, Huang H (2012) Efficient commitment to functional CD34+ progenitor cells from human bone marrow mesenchymal stem-cell-derived induced pluripotent stem cells. PLoS ONE 7:e34321
Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T (2003) Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107:1322–1328
Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW (2008) Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke 39:69–74
Yoo CH, Na HJ, Lee DS, Heo SC, An Y, Cha J, Choi C, Kim JH, Park JC, Cho YS (2013) Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases. Biomaterials 34:8149–8160
Zhang ZG, Chopp M (2009) Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 8:491–500
Zhang ZG, Zhang L, Jiang Q, Chopp M (2002) Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral Ischemia in the adult mouse. Circ Res 90:284–288
Zhao YH, Yuan B, Chen J, Feng DH, Zhao B, Qin C, Chen YF (2013) Endothelial progenitor cells: therapeutic perspective for ischemic stroke. CNS Neurosci Ther 19:67–75
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Conclusions
Conclusions
In brief, in response to stimuli such as stroke , EPCs are mobilized from bone marrow to peripheral blood and may participate in endothelial cell repair-regeneration and in tissue neovascularization processes. In this context, experimental and human studies have shown that neovascularization is present in the adult brain exposed to ischemia and that EPCs participate in cerebral neovascularization processes. Finally, we and others have observed that a higher increment in the number of circulating EPCs is associated with a better outcome in patients with stroke. Taken together, these findings suggest that EPCs may mediate neurorepair processes after stroke, and that exogenous supplementation or endogenous stimulation of EPCs have a great therapeutic potential for stroke. However, larger clinical trials are needed to evaluate the safety and efficacy of EPC transplantation for treating stroke. Furthermore, how to improve the strategies in order to maximize the endogenous stimulation of EPCs deserves also further studies.
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sobrino, T., Campos, F., Castillo, J. (2015). The Role of Endothelial Progenitor Cells in Stroke. In: Zhao, LR., Zhang, J. (eds) Cellular Therapy for Stroke and CNS Injuries. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-319-11481-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-11481-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11480-4
Online ISBN: 978-3-319-11481-1
eBook Packages: MedicineMedicine (R0)