Abstract
Mitochondria are of an increasing interest in pharmaceutical and medical research since it has emerged as an intriguing target for treatment of many diseases with a great diversity of clinical appearance. The efficiency of drug action relies largely on how well it is able to reach its target or even its target inside the cell such as mitochondria. Subsequently, drug delivery to the specific intracellular organelle dramatically enhances drug action. Mitochondria play a major function in a range of cell processes and mitochondrial dysfunction contributes to several human diseases. Increasing interest in delivering large molecules such as nucleic acids, peptides, enzyme mimetics, drugs, and probes have led to the emergence of “Mitochondrial Medicine” as an entire pioneering field of biomedical exploration. Targeting of biologically active molecules to mitochondria in living cells open up ways for modifying mitochondrial functions, which may come with selective protection, repair or eradication of cells. Furthermore, nanoscience offers unique tools and materials to target therapeutic agents to mitochondria. This chapter deals with different aspects of mitochondrial drug delivery, current strategies of mitochondrial targeting and their possible therapeutic applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Targeting therapeutics to intracellular organelles of interest could be very effective in maximizing the drug effect and minimizing side effects. However, intracellular delivery and subsequent targeting to specific cellular compartments is challenging, especially for macromolecular drug delivery systems, due to a cell membrane that prevents their spontaneous entrance and that nanocarriers are taken up primarily by energy-dependent endocytosis process. Mitochondria are decisive cellular organelles known for their role in providing proficient energetic support through the chemiosmotic process of oxidative phosphorylation. The intrinsic pathway of apoptosis is controlled and governed by mitochondria by regulating the translocation of proapoptotic proteins from the mitochondrial intermembrane space to the cytosol and also participate in some forms of non-apoptotic cell death, such as necrosis.
In the context of drug delivery, effectiveness of any drug or gene therapy depends upon its delivery (site specific) within the cell (improve the efficacy) with the ability to reduce the toxicity. It is required to deliver drug to the specific cells as well as to inner cell compartments, which may contribute to a disease. Advancements in mitochondrial research and in medical technology have led to the fabrication of drugs that specifically target the mitochondria. Drugs that target mitochondria and exert their activity have become a focus of recent investigations due to their great clinical potential. The molecules, which selectively act on one or more mitochondrial sites for the palliative treatment and diagnosis of mitochondria associated diseases, are termed as “mitochondrial medicine” [1]. Nevertheless, targeting of mitochondria is particularly exigent as hurdles including biological barriers, additional barriers including intracellular diffusion/transport to the mitochondria and electrical potential across outer and inner mitochondrial membranes and toxicity affect the development of mitochondrial targeted therapeutics [2]. Moreover, processes such as the mobility of the system in the cytosol (because of the high concentrations of dissolved macromolecules), the rate of degradation in the cytosol and the rate of uptake into the targeted organelle limit intracellular targeting and thus affect targeting efficiency. Despite all these barriers and obstacles, drug delivery strategies are continuously being designed, and explored to target mitochondria specifically to make therapy more efficient and to minimize nonspecific side effects [3].
2 Importance of Mitochondrial Morphology
Mitochondria are typically elongated, thread-like organelles which differ from other organelles as they are double membranous structure with an unusual lipid composition. The mitochondrion is composed of four compartments each with different compositions, activities and functions: a porous outer membrane (lipid to protein ratio of 1:1), permeable to molecules smaller than about 6 kDa; an intermembrane space containing a number of specialized proteins; a convoluted and invaginated inner membrane containing the enzymes of oxidative phosphorylation and a series of metabolite carrier proteins; the mitochondrial matrix [4].
The mitochondrial matrix is the innermost space enclosed by the cristae membrane and contains enzymes of different breakdown pathways, including fatty acid oxidation, citric acid cycle, and the urea cycle, mitochondrial ribosomes, and specialized transfer RNAs as well as several copies of circular non-chromosomal mitochondrial DNA (mtDNA). mtDNA encodes 13 polypeptides, 22 tRNAs, and 2 rRNAs. All the 13 polypeptides are the components of mitochondrial enzymes. A number of transport proteins are present in the inner membrane—each of which is responsible for the transport of a specific ligand and compounds to the matrix space. For example, the ATP/ADP carrier (AAC) transferring ATP out of the matrix space, while simultaneously allowing ADP to cross the inner membrane. Mitochondria are found in all nucleated cells and are the principal generators of cellular ATP by oxidative phosphorylation. The number of mitochondria per cell is related to the energy requirements of the cell and varies according to the cell type, cell-cycle stage, proliferative state, and diseases related energy demands of the cell. Metabolically active organs such as liver, brain, cardiac and skeletal muscle tissues contain up to several thousands of mitochondria per cell while somatic tissues with low energy demands contain only a few dozen mitochondria [5]. Mitochondria have key implication in many pathways essential to both the life and death of cells. These organelles generate 80–90 % of ATP by oxidative phosphorylation needed for cell respiration and survival, regulate calcium flux, and have significant role in the integration of pro- and anti-apoptotic stimuli. Mitochondria cannot be formed de novo. They vary in shape, length, size and are dynamic organelles having complex, interconnected, and network like structures [6].
3 The Rationale for Mitochondria Drug Delivery
Mitochondria are the center of numerous fundamental metabolic pathways which are the prime target for pharmacological intervention. Cell’s energy metabolism, regulation of programmed cell death and intracellular calcium concentration are controlled by mitochondria. Furthermore, the mitochondrial respiratory chain is the major source of damaging reactive oxygen species. Consequently, a number of diseases including diabetes, cardiomyopathy, infertility, migraine, blindness, deafness, kidney, liver diseases, and stroke are the result of mitochondrial dysfunction. Somatic mutations in the mitochondrial genome contribute to aging, age-related neurodegenerative diseases as well as in cancer. Another concern is mitochondrial toxicity. A number of xenobiotics and therapeutics such as haloperidol and thiothixene exhibits mitochondrial toxicity by interfering with mitochondrial functions [7]. Membrane barriers as well as mitochondrial toxicity are significant limitations to be addressed for the effective mitochondrial therapeutics. In conclusion, the delivery of therapeutics into mitochondria may provide the basis for a large variety of future therapies. The natural aging process can be slowed down by delivery of antioxidants to the mitochondria. Mitochondrial DNA diseases may possibly be treated by the delivery of therapeutic DNA and RNA such as antisense oligonucleotides, ribozymes as well as plasmid DNA expressing mitochondrial encoded genes. The targeted delivery of drugs to mitochondrial-uncoupling proteins may be promising in obesity treatment. Designing therapeutic strategies specifically for killing cancer cells by exploiting their metabolic alterations may open up therapeutic possibilities for new anticancer therapies. Moreover, apoptosis-resistance of many cancer cells can be treated by delivering molecules which trigger apoptosis directly without involving mitochondria.
4 Mitochondrial Dysfunction and Related Disorders
Mitochondrial diseases include the disorders that are related to defects or absence of proteins that are utilized in mitochondria [8]. Dysfunctions caused by mutations mainly affect proteins of the respiratory chain and consequently normal energy production which can lead to severe diseases. Cancer, Neurological disorders such as Parkinson’s, Alzheimer’s, and Down syndrome are associated with mitochondrial dysfunction. Mitochondria also play a vital role in the pathology of many other diseases such as diabetes, amyotrophic lateral sclerosis, ischemic heart disease, hyperthyroidism, non-alcoholic fatty liver disease, and phenylketonuria. Mitochondrial dysfunction, which increases cellular glucose levels and consequently reduces insulin production [9] may cause diabetes. The maternally inherited type of mitochondria related diabetes is caused due to spot mutation/point mutation in one of the mitochondrial genes encoding tRNALeu (UUR). Amyotrophic lateral sclerosis is gradual and selective loss of motoneurons in cortex, brainstem, and spinal cord caused due to mutations in the enzyme superoxide dismutase 1 (SOD 1) in the mitochondria. Alzheimer’s (AD) and Parkinson’s disease (PD) are common age related diseases [10]. Patients with Alzheimer’s disease showed altered activities of enzymes in tricarboxylic acid (TCA) cycle, mutations of mitochondrial fusion proteins, and accumulation of mtDNA mutations. The importance of mitochondria in the pathology of cancer and diabetes is discussed below:
4.1 Cancer
Cancer cell mitochondria are structurally and functionally different from their normal counterparts signifying the role of mitochondrial dysfunctions in the entire process of cancer development and progression. Synthesis of respiratory chain proteins due to mutations of the mitochondrial DNA (mtDNA) leads to increased electron leakage and ROS over production. Increased ROS level favors cell proliferation, DNA damage, genetic instability, resistance to antitumor agent’s chromosomal instability, neoplasm metastasis, and carcinogenesis. Mitochondria regulate the intrinsic pathway of apoptosis by regulating the translocation of proapoptotic proteins from the mitochondrial inter-membrane space to the cytosol [11]. An intact outer membrane contains a variety of inactivated apoptotic proteins and once the integrity is lost; these apoptotic proteins are dumped into the cytoplasm and induce apoptosis. In cancer cells the antiapoptotic proteins are overexpressed with reduction in proapoptotic factors, enabling the cancer cells to be more resistant. In malignant lesions components of the permeability transition pore complex (PTPC) express and exhibit alterations, which control the exchange of metabolites and also mediate the permeability transition to trigger the release of cytochrome c. Thus, targeting of molecules or toxic substances which stimulate the permeability transition or alteration of mitochondria of tumor cells and devastate the mitochondrial DNA in mammalian cells to encourage apoptosis could be promising targeting strategy for cancer therapy [12].
5 Mitochondria-Targeted Agents Under Preclinical and Clinical Evaluation for Anticancer Therapy
Many of the compounds that target differences between mitochondria from normal cells and from cancer cells are currently under preclinical and clinical evaluation.
-
Agents targeting the transition of cell metabolism
Non-metabolically active glucose analog like 2-deoxyglucose inhibits glycolysis is currently under Phase I/II clinical trials. 3-bromopyruvate, an analog of lactic acid under preclinical testing, has shown beneficial property on tumor growth. Derivative of indazole-3-carboxylic acid, lonidamine (LND) inhibits glycolysis and improves the cytotoxicity of the doxorubicin and cisplatin is under multiple Phase III clinical trials. In addition, phloretin is known to sensitize cancer cells to daunorubicin for its anticancer activity and apoptosis to overcome drug resistance only under hypoxia. Another agent, dichloroacetate that inhibits the key enzyme pyruvate dehydrogenase kinase in cancer cells, is currently under Phase II clinical trials in brain tumor and some solid tumors.
-
Agents targeting cellular damage caused by abnormal ROS production
Several agents that increase ROS generation for cancer therapy are under clinical trials. Arsenic trioxide can cause an increase in electron leakage by interfering with the OXPHOS, therefore promoting ROS generation, leading to cancer cell apoptosis. Redox-inactive vitamin E analog alpha-tocopheryl succinate is under Phase II study in melanoma, prostate cancer, colorectal cancer, mesothelioma, and breast cancer for anticancer activity.
The Phase I/II studies have demonstrated that 2-methoxyestradiol; an estrogen derivative that selectively kills human leukemia cells is well tolerated and causes disease stabilization in patients with solid malignancies or with multiple myeloma. Additionally, buthionine sulfoximine and imexon are both in Phase I clinical trials.
-
Agents targeting the disabled apoptosis pathway
Bcl-2, an antiapoptotic protein is a promising target molecule in cancer therapy. Antisense oligonucleotides specific for Bcl-2 RNA sequences (such as G3139) suppress particularly the proliferation of cancer cells or augment their sensitivity to chemotherapeutic drugs. G3139 and Gossypol, a BH3 mimetic is under Phase III clinical trials in addition with chemotherapeutic agents in a number of tumors. Peripheral benzodiazepine receptor (PBR) ligands, such as PK11195, RO5-4864 and diazepam, have shown antitumor effects both in vitro and in vivo, either as alone or in combination with other chemotherapeutic agents and have entered clinical trials. The promising results have been achieved in patients with recurrent glioblastoma treated with diazepam plus lonidamine.
-
Agents targeting mutated mtDNA
Several compounds that target mtDNA or enzymes related to its replication illustrate potential clinical applications. Among them, cisplatin, a classic anticancer drug in clinical use, is found to bind preferentially to mtDNA more than to nuclear DNA and shows higher cisplatin–mtDNA adduct levels, resulting in the inhibition of NADH-ubiquinone reductase and the decrease of ATP generation. Ditercalinium, a bis-intercalating agent accumulates in the mitochondria causing specific elimination of mtDNA and inhibition of its replication. In vitro studies have shown that vitamin K3 exhibits anticancer activity in breast and pancreatic cancer cells by specifically inhibiting the affect on DNA polymerase ϒ, the mitochondrial enzyme responsible for mtDNA replication but its use as a potential anticancer agent remains to be evaluated.
6 Exploiting Mitochondrial Properties for Drug Targeting
Mitochondria exhibit some specific features that differ from other cellular compartments and also between normal and diseased mitochondria. These features can be exploited for developing a targeting strategy to transport biologically active molecules to and into the mitochondria within living mammalian cells. The distinct mitochondrial features, which govern the mitochondrial targeting strategies are:
-
The high membrane potential across the inner mitochondrial membrane,
-
The organelle’s protein import machinery
-
The mitochondrial fusion process
The first property that can be utilized for targeting is the mitochondrial membrane potential. Mitochondria are composed of a double membrane. Transmembrane electrochemical gradient is generated by the electron transport chain and ATP synthesis via oxidative phosphorylation that leads to a high membrane potential, negative inside and a pH difference, acidic outside [13]. Therefore, cationic molecules are attracted and preferentially taken up by mitochondria. The molecules get selectively accumulated in the mitochondrial matrix in response to their membrane potential. The inner mitochondrial membrane has to be crossed for the delivery of substances into the mitochondrial. Altogether, lipophilic as well as positively charged molecules can take the advantage of the membrane potential to accumulate in mitochondria [14]. Moreover, the outer membrane does not present a barrier to small molecules. These can basically diffuse through pores in the membrane formed by a membrane spanning protein.
Protein import pores the specific targeting sequences present in outer and inner membrane of mitochondria could potentially be utilized for transporting drug or DNA molecules to and/or into the matrix of mitochondria. In addition, any mitochondria-specific binding sites and unique protein receptor at the mitochondrial membranes could be exploited for drug targeting purposes. Mitochondrial targeting by mitochondrial fusion process so far has not been explored yet. Drug delivery using carriers might be a suitable approach to mitochondria targeting for drugs too large to pass the mitochondrial protein import pores.
7 Challenges of Targeting Mitochondria
Although mitochondria play an essential role in various significant pathologies, they have been an ignored target. It is due to the difficulty of selectively targeting molecules to this organelle in vivo. Various extracellular and intracellular barriers including cell membrane and the mitochondrial membrane impose formidable challenges to the drug delivery to mitochondria. The transportation through cell membrane is a prime requirement for drug delivery and therapy. For intracellular targeting a carrier system must cross the plasma membrane, a major barrier for large and charged molecules to enter the cytoplasmic space [15].
The challenge of targeting mitochondria by itself is to transfer the drug or drug carrier across two membranes in the case of mitochondrial matrix targeting. In targeting of the intermembrane space, it is sufficient to pass the outer mitochondrial membrane, but almost all potential targets are located in the mitochondrial matrix. Therefore, it is necessary to overcome both mitochondrial membranes which have only small pores and a highly lipophilic inner membrane. Theoretically, membrane impermeable probes can enter mitochondria through protein import pores (TOM and TIM complex), the pore protein porin, also referred to as the voltage dependent anion channel (VDAC), the mitochondrial permeability transition pore complexes (mPTPCs), through mitochondrial apoptosis-related channels and through apoptosis-related ceramide pores. But only the VDAC and the protein import pores are relevant in normal mitochondrial function. The others are related to apoptosis and dysfunction and are localized in the outer but not the inner mitochondrial membrane.
Size and hydrophobicity of the molecules are the factors affecting and determining the diffusion rate through mitochondrial membrane. Hydrophobic (log P > 5) and relatively small molecular weight (500 mw) substances are transported through the plasma membrane by passive diffusion whilst hydrophilic molecules are screened off. However, different endosomal mechanisms are also utilized by the cell for the transport of macromolecules and carriers. All eukaryotic cells exhibit one or more forms of endocytosis. Many endogenous and exogenous ligands enter a cell by receptor mediated endocytosis. Optimal drug therapy not only depends on the delivery of bioactive molecule/macromolecule to its target cell but also its appropriate localization within that cell. Receptor targeting for selective uptake and internalization of drugs has further expanded with the introduction of new macromolecular drugs including DNA, peptides and proteins. The accessibility of sophisticated nanotechnology approaches to encapsulate drugs, providing controlled release capacity as well as protection of macromolecules from degradation prior to reaching the site of action, has provided an additional level of advantages. Macromolecules are primarily entrapped in endosomes followed by maturation to form late endosome and ultimately fusion with lysosome in order to exert its therapeutic effects [16]. The particles that do enter cells via the endosomal pathway, must escape the endosome before its fusion with the lysosome in order to prevent drug and carrier degradation. Due to the stability problems at endosomal/lysosomal pH macromolecular drugs such as proteins, peptides, DNA and drugs should bypass the endocytic pathway for their proficient delivery in the cytosol or other cellular organelles [17].
Different molecules which can be targeted to the mitochondria and pathways for mitochondrial targeting are represented in Fig. 7.1. Peptides namely cell penetrating peptides (CPPs) constitute a novel class of molecules capable of transferring molecules directly to the cytosol and bypassing endocytic pathway [16]. CPPs represent short polycationic sequences of about 10–30 amino acids which can extraordinary facilitate cellular gene/drug delivery. There are some more peptides that are structurally similar in that they all contain a short sequence of less than 20 amino acids with a positively charged arginine and lysine residues. This sequence is called “protein transduction domain” (PTD) and are considered to be significant to establish carrier cellular contact [18].
(1) To design a successful drug delivery system, the therapeutic cargo must be encapsulated with therapeutic moiety depending on the physical characteristics, (2) intracellular trafficking of carrier including endosomal escape, (3) mitochondrial targeting, (4) showing the general route other than mitochondrial targeting
7.1 Cytosolic Barriers
The barriers that the molecules encounter before they reach mitochondrial organelle must be taken into account and special consideration should be given to diffusion of molecules from the plasma membrane through the cytosol. The cytosol has an active intracellular environment consisted of macromolecular species and specific components (water, ions and proteins) with minimal free space available [19]. The high concentration of macromolecules (up to 400 mg/mL) [20] in the cytosol is referred to as molecular crowding, which constitute a significant diffusion barrier for molecules. Moreover, collisional interactions and binding to intracellular components in the cytoplasm are additional obstacles encountered during drug targeting to the mitochondria [21].
7.2 Mitochondrial Membranes
Mitochondria offer two anatomical barriers, the outer and inner mitochondrial membrane to the therapeutic agents to be targeted to the mitochondrial matrix. The outer mitochondrial membrane is weak barrier compared to the inner membrane and contains voltage-dependent anion channels (VDAC, also referred to porin) [22]. VDACs nonspecifically transport molecules into the transmembrane space between the outer and inner mitochondrial membranes and also coordinate with the mitochondrial permeability transition pore complex [23]. VDAC also integrates with PBR which primarily translocates cholesterol and benzodiazepine derivatives into the mitochondria for metabolism.
The outer membrane also contains mitochondrial protein transporters that translocate proteins which are generally positively charged and contain alpha helical secondary structure into the mitochondria [24]. The drug has to cross the inner membrane to reach the mitochondrial matrix. The inner membrane creates a strong negative membrane potential of approximately −180 mV due to the presence of electron transport chain which accounts for the higher accumulation of cationic lipophilic drugs than other molecules including hydrophilic, neutral, and anionic molecules. This negative membrane potential can be exploited to target therapeutics to this organelle. Mitochondrial accumulation of therapeutics involves factors such as electric potential, ion-trapping, and complex formation with cardiolipin [14]. It is reported that lipophilicity, charge and polar surface area of the drug influence mitochondrial uptake. Transporter proteins assist the uptake of other drug/protein molecules with low affinity for mitochondria. Integrating mitochondrial translocation ligands and/or positively charged ligands into the therapeutic carrier design may help targeted mitochondrial delivery [25]. Figure 7.2 represents schematic representation of biological barriers to mitochondria delivery and the approaches that could surmount the drug delivery challenges.
Schematic representation of biological barriers for mitochondria delivery and the approaches to overcome the drug delivery challenges. Reproduced with permission from ref. [26]
8 Mitochondria Targeting Strategies
A range of strategies for targeting bioactives to mitochondria has been explored. These strategies utilize biophysical properties of mitochondria, unique mitochondrial enzymes and targeting based on the transporter-dependent delivery to mitochondria. Compounds exhibiting or possessing a positive charge are attracted to mitochondria (due to the negative potential) but are unable to enter the mitochondrial matrix because of the impermeability of inner mitochondrial membrane to polar molecules. Therefore, additional physicochemical properties are required for mitochondrial targeting. Although it has been widely accepted that molecules should be comprised of positive charge and lipophilicity/amphiphilicity for mitochondrial targeting, the idea is still in controversies. Moreover, correlation between physicochemical properties and extent of accumulation in mitochondria could not be demonstrated [14]. However, several strategies directed/designed for mitochondrial targeting are shown to selectively deliver the drugs or molecules into mitochondria are discussed in this chapter. Figure 7.3 represents various targeting strategies for mitochondrial targeting.
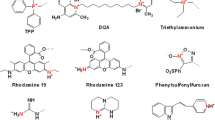
8.1 Lipophilic Cation-Based Non-peptide Targeting Strategies
Lipophilic cations can easily transverse across the plasma membrane and the mitochondrial membranes because of the cationic charge and hydrophobic surface area, which in effect lowers the activation energy for their movement across the membrane. The negative membrane potential enables the positively charged moiety to move through membranes and accumulate in the mitochondrial matrix. Furthermore, lipophilic cations do not require a specific import mechanism. The mitochondrial membrane potential is about 180–200 mV which favors a 200- to 400-fold higher accumulation of lipophilic cation in the mitochondrial matrix. The membrane potential of the plasma membrane is about 30–60 mV, negative inside thus accumulation of these compounds in mitochondria relative to the extracellular environment is several fold higher [27].
Triphenylphosphonium (TPP) cation is best characterized and most widely used delocalized lipophilic cation for delivery to mitochondria, which was originally used to assess the mitochondrial membrane potential [28]. Uptake of TPP into mitochondria is well recognized and it is comparatively straightforward to introduce it into a compound late in the chemical synthesis scheme, typically by displacing a leaving group with triphenylphosphine cation [27]. Moreover, TPP or a methyl derivative of TPP does not require any receptor to penetrate into mitochondria. Drugs can be attached through the functional groups to the phosphorous atom or modified phenyl rings and carried into the matrix space [29]. It has been applied in various studies for mitochondrial targeting of antioxidants with an aim of protecting them from oxidative damage [30]. A wide range of antioxidants have been targeted to mitochondria by conjugation to the TPP lipophilic cation, including ebselen, lipoic acid, vitamin E, nitrones, plastoquinone, and nitroxides. This principle has also been successfully exploited to transport peptide nucleic acid (PNA) of 3.4–4 kDa by conjugating them with TPP for mitochondrial targeting in order to inhibit the replication of mutated mtDNA. Other lipophilic cations such as rhodamine 123 or tetraguanidinium oligomers are also found to accumulate selectively within mitochondria [31]. A mitochondria targeted analog of α-tocopherol (MitoE2), reduces iron/ascorbate-induced mitochondrial damage and neuronal and astrocytic cell death in pyramidal neurons. MitoQ exhibits antioxidant properties upon mitochondrial uptake. In mitochondria, MitoQ is reduced to ubiquinol, which is the antioxidant species that detoxifies reactive oxygen species (ROS), and is thereby oxidized to ubiquinone; the ubiquinone thus formed is reduced to ubiquinol.
8.2 Mitochondria-Targeted Peptides
Studies describe a variety of peptide and amino acid based mitochondrial transporters which are designed to utilize charge-driven uptake into the mitochondria. These peptides appear to enter cells via direct mode of uptake, avoiding endosomal and/or lysosomal sequestration that would prohibit their ability to accumulate in mitochondria [32]. Peptides with antioxidant properties present a different mitochondria targeting strategy.
The peptide sequences are resistance to hydrolysis and, have considerable pharmacokinetic properties. The observed cell permeability of peptides is dependent on charge and lipophilicity. Szeto-Schiller (SS)-peptides (positively charged peptides) is an approach which could be used for mitochondrial targeting. The structural motif consisted of four alternating aromatic and basic amino acids and possesses three positive charges at physiological pH. Studies have revealed their rapid uptake through the plasma membrane and accumulation in mitochondria in isolated cells [33]. The uptake of Szeto-Schiller peptides into mitochondria does not depend upon the negative membrane potential and the mechanism behind their uptake is currently not clear [34]. The novel approach for targeted delivery of antioxidants to the inner mitochondrial membrane using Szeto-Schiller (SS) peptide antioxidants resulted in reduced ROS and cell death caused by t-butylhydroperoxide (tBHP) in neuronal N2A cells (EC50 in the nM range). These have been investigated in a variety of in vitro and in vivo disease models.
The peptides inhibit lipid peroxidation by scavenging hydrogen peroxide and peroxynitrite. Tyrosine and dimethyltyrosine residues may produce antioxidant action. SS peptide antioxidants inhibit the permeability transition (PT) and swelling, and reported prevented cytc release induced by Ca2+ in isolated mitochondria. Because ROS and PT have been implicated in myocardial stunning these peptide antioxidants can notably progress contractile force in an ex vivo heart model. Therefore, it can be speculated that these peptidal antioxidants can be effective antiaging treatment and diseases associated with oxidative stress [33].
Mitochondria-penetrating peptides (MPPs) are one more promising delivery vectors for specific and effective mitochondrial transport [35]. These consist of four or eight alternating positively charged, hydrophobic, and partly unnatural amino acids. Small molecules, biotin and trolox, a water soluble analog of vitamin E are delivered by MPPS into mitochondria [32]. Use of cell penetrating peptides (CPP) is another approach which is able to transport the cargoes of much higher molecular weight compared to their own [36]. CPP are larger peptides consisting of up to 30 positively charged or alternating positively charged and hydrophobic amino acids. Studies show that number of molecules such as proteins, peptides, nucleic acids, and even nanocarriers like liposomes can be delivered in this way into cells [37]. Natural mitochondrial leading sequences (MLSs), derived from mitochondrial proteins are competent to deliver molecules into mitochondria. They are synthesized in the cytosol, with 10–80 amino-terminal pre-protein to be translocated to their ultimate mitochondrial destination. These are positively charged, hydrophobic, and hydroxylated amino acids with an ability to form an amphipathic α-helix that presents one positively charged surface and one hydrophobic surface. These structural characteristics are imperative for the identification by the mitochondrial protein import pores and therefore when an MLS is attached to a non-mitochondrial protein, it can specifically direct the protein into mitochondria [38].
Most of these proteins are encoded in the nuclear genome and consequently delivered to mitochondria by mitochondrial targeting signal peptide (MTS) that is located at the N-terminus of the precursor protein [39]. These MTSs are typically consisting of 10–70 amino acids, which are removed in 1 or 2 proteolytic steps once delivered to the mitochondria. These MTS peptide can be used to deliver proteins to the matrix of the mitochondria. The MTS sequence leads the cargo protein to the mitochondria, and is then cleaved, allowing for the absolute localization and function of the fused protein [40].
Cyclosporin A (CsA) is a cyclic peptide that inhibit mPTPC formation and delays cell death caused by oxidative stress, and hence used on protecting the heart and the brain from ischemia–reperfusion injury. Although CsA is a potential drug for an anti-ischemic drug, it is difficult to predict the concentrations of CsA in mitochondria as there are additional targets of CsA in the cell. Therefore to achieve therapeutic benefit of CsA, mitochondria-specific drug carrier system should be developed [41].
8.3 Lipidic and Polymeric Nanocarriers for Mitochondrial Targeting
Nanocarriers offer numerous advantages in the design of therapeutics to overcome the challenges and limitations associated with mitochondrial targeting. Nanocarrier loaded with drug can be conjugated with organelle specific targeting moieties. Targeting moieties include peptide sequences and non-peptide molecules that can be recognized and interact with the mitochondrial membranes [42]. Mitochondrial delivery of drug requires escape of carrier from endosomes after endocystosis. The drug delivery system decorated with targeting moieties has to release the drug into the cytosol for the intracellular organelle specific targeting (Table 7.1). Liposomes, nanoparticles, micelles and multifunctional nanoparticles have been proposed for the delivery of hydrophobic drugs to various sub-cellular organelles including mitochondria. In a study fluorescently labeled micelles were found to be distributed through several cytoplasmic organelles including a majority of them being associated with the mitochondria. It was found that internalization of drug incorporated in micelles was better than the free drug [54].
It is demonstrated that the quantum dots that are enfolded in micelles and conjugated to a mitochondrial targeting peptide could preferentially accumulate in mitochondria when they are applied to the intact cells [55]. Another strategy that can be explored to target mitochondria is the utilization of water soluble fullerene derivatives that accumulate in mitochondria as well [56]. Moreover, drug conjugates of HPMA copolymer are also possible candidates which can be explored for mitochondrial targeting. Drug conjugates were synthesized using a photosensitizer mesochlorine 6 (Mce 6). Mitochondrial targeting of HPMA copolymer-bound Mce 6 enhanced cytotoxicity as compared to non-targeted HPMAcopolymer-Mce6conjugates [57].
8.4 Bolasomes
Dequalinium (DQA) is a cationic bolaamphiphile with delocalized charge centers. It forms liposome like aggregates in water called DQAsomes/bolasomes that act as a vector for the transportation of DNA to mitochondria in living cells. DQAsomes exhibited higher mitochondrial accumulation and retention. Positively charged DQAsomes are attracted towards the negatively charged mitochondrial transmembrane of the tumor cells. García-Pérez et al. [58] reported that DQA showed anticancer activities and induced a concentration-dependent oxidative stress by decreasing glutathione (GSH) level and increasing ROS in a cell type specific way. Inhibitors of the JNK and p38 stress regulated kinases potentiate DQA-induced NB4 cell death indicating a protective function for these enzymes.
DQA encapsulating paclitaxel exhibited enhanced apoptosis activity over unencapsulated paclitaxel in COLO25 cells [59]. They are capable of increasing the paclitaxel accumulation in the mitochondria. Apoptotic activity of paclitaxel was enhanced following the mitochondria-specific delivery at concentrations, at which the free drug does not have any significant cytotoxic effect. Paclitaxel loaded DQAsomes have led to an improved ability to inhibit the growth of human colon cancer tumors in nude mice. Decoration of DQAsomes containing paclitaxel with folic acid further improved the antitumor efficiency [60]. The folate receptor is over expressed in a large variety of human tumors. Conjugated nanocarriers are internalized in a tumor cell-specific manner through folate receptor-mediated endocytosis resulting in an increased toxicity of the encapsulated drug. Folic acid conjugated DQAsomes were studied for cell cytotoxicity using HeLa cells possessed improved antitumor activity as compared to plain paclitaxel loaded DQAsomes. They are found to be better delivery systems as they could deliver the drug not only to the cytosol but also to mitochondria whereas folic acid conjugated liposomes delivered the drug into the cytosol only [59]. Figure 7.4 shows untreated cells, cells incubated with empty DQAsomes and cells exposed to naked pDNA, respectively.
Fluorescence microscopic images of BT20 cells exposed to DQAsome/pDNA complex. The cells were incubated with MitoTracker Red CMXRos for staining mitochondria and with SYBR Green I for staining free DNA. Top row: Cells + DQAsome–pDNA; (a) MitoTracker, (b) SYBR. White arrows indicate plasmid DNA transported by DQAsomes to the site of mitochondria and released from the DQAsome–DNA complex upon contact with the mitochondrial membrane. Bottom row: Controls, stained with SYBR. (c) Untreated cells; (d) cells + empty DQAsomes; (e) cells + naked pDNA. (Reproduced with permission from D’souza et al. [60])
DQAsomes complexed with plasmid DNA can transport and release nucleic acid into mitochondria after interacting with mitochondrial membrane [60]. In a study DNA conjugated to mitochondrial targeting sequences (MLS) was entrapped into the DQAsomes and successfully used to deliver the DNA into the mitochondria [61]. Vaidya et al. [62] studied the antitumor activity of folic acid conjugated DQAsomes using HeLa cells. It was revealed that folic acid conjugated DQAsomes show improved antitumor activity as compared to un-conjugated DQAsomes, folic acid conjugated liposomes and paclitaxel solution.
8.5 Lipidic Nanocarrier-Liposomes
Liposomes are the most potent and investigated nanocarrier system for mitochondrial targeted therapeutics because of their biocompatibility and safety. Proteoliposomes prepared by incorporating a crude mitochondrial membrane fraction into liposomes was the first research that signifies the utility of liposomes for targeting mitochondria [63]. Liposomes can be fused with mitochondrial membranes releasing their drug load into mitochondria. This approach takes benefit of the fact that mitochondria are able to fuse with one another [64].
Cationic liposomes made up of DOPE (1,2-dioleoyl-sn-glycero-3-phosphoe-thanolamine) and DOTAP (dioleoyl-1,2-diacyl-3-trimethylammoniumpropane) were used to deliver pro-apoptotic peptide D-(KLAKLAK)2 together with an antisense oligonucleotide into mitochondria of the cell in order to treat cancer [65]. Conjugation of a lipophilic cationic ligand to the liposomes could target drugs to the mitochondria. In a study ceramide (anticancer agent that targets ROS production) loaded liposomes functionalized with stearyl triphenyl phosphonium (STPP) were developed and assessed for antitumor efficiency [54]. STPP shows both cationic and lipophilic properties. STPP functionalized liposomes were localized within the mitochondria of 4T1 breast cancer cells and found to induce more apoptosis in 4T1 breast cancer cells, compared to unconjugated liposomes. Survival rates of tumor induced BALB/c mice models were amplified as compared to non-targeted liposomes or no treatment control since the drug was targeted to the mitochondria [54]. Further, STPP functionalized liposomes enhanced the efficacy of sclareol against colon cancer and leukemia. These liposomes were found to enhance the apoptosis, caspase-8 activity, and caspase-9 activity in COLO205 cells compared to non-targeted liposomes. The amount of drug required for an effective therapeutic response by functionalized liposomes was less compared to non-functionalized liposomes. It is also reported that STPP-liposomes directed successful accumulation of rhodamine labeled phosphatidylethanolamine into mitochondria of live cells [51].
MITO-Porter is a liposomal carrier system with octaarginine modified at the surface. It promoted fusion with mitochondrial membranes and was shown to specifically deliver contents into mitochondria. Macropinocytosis rather than clathrin-dependent endocytosis is the main mechanism of internalization of MITO-Porter in the cells [3]. MITO-porter liposomes have also been used to deliver green fluorescent protein [66] as well as propidium iodide [67] to mitochondria suggesting their efficacy in delivery of small and large molecules to mitochondria. Moreover, MITO-Porter was modified with R8 peptide, which mimics the trans-activating transcriptional activator (TAT) and was found to function as a useful moiety for cellular uptake as well as mitochondrial targeting [68].
The multifunctional envelope-type nano-device (MEND) permits efficient and simple packaging of plasmid DNA, proteins or other macromolecules for gene delivery. It consists of lipid envelope equipped with various functional devices to mimic envelope-type viruses [53]. MEND is based on the new packaging concept ‘programmed packaging’, is similar to envelope-type viruses, comprises a condensed core, such as plasmid DNA, and a lipid envelope equipped with various functional devices, which keep their unique basic properties and exist as separate structures in the compound. The merits of MEND include enzymes protection, improved packaging efficiency, controlled size and release over a short period of time. To locate these nanoparticles selectively in the mitochondria of cancer cells, different strategies like peptides for endosomal escape, ligands for specific receptors and mitochondrial targeting drugs could be easily incorporated into the core and onto the surface of particles. These unique characteristics make this design a capable module for drug delivery [69].
Lipoplexes are small partially stable particles formed by mixing of nucleic acids with cationic liposomes which protect nucleic acid from nuclease degradation and also enhance cellular transfection. They also facilitate nucleic acids release from the intracellular vesicles before they reach the destructive lysosomal compartments [70]. Lipofectin is a cationic liposome that is commercially available as a transfection reagent used for intracellular delivery of DNA [71]. It is composed of the cationic lipid 2,3-bis-(oleoyl)oxipropyl-trimethyl ammonium chloride (DOTMA) and di-oleoylphosphatidylethanolamine (DOPE). A spontaneous electrostatic interaction between the negatively charged DNA and the positively charged liposomes results in condensation of the nucleic acids. Moreover, the resulting cationic liposome/DNA complexes display a net positive charge that is important for their association with the negatively charged cell surface. Intracellular release of complexed DNA is facilitated due to the fusogenic properties of the cationic liposomes based formulation that can induce fusion and/or destabilization of the plasma membrane. Intact DNA can be delivered into the cytoplasm by the virtue of cationic liposomes. The DNA released into the cytosol consequently move to the mitochondrial matrix through protein import machinery if DNA is conjugated with MLS. DNA is first conjugated with the MLS and this complex is entrapped within the cationic liposomes. The entrapped DNA is released from the liposomes in to the cytosol; subsequently DNA is carried by the MLS peptides to the mitochondrial matrix (Fig. 7.5).
Dose-dependent cytotoxicity of dendrimers against NIH-3T3 cells at 24 and 48 h. Data are expressed as mean SD of three experiments carried out in triplicate. Difference between G(5)-D and G(5)-D-Ac/G (acylated dendrimer) (5)-D-Ac-TPP (TPP-anchored dendrimer) were analysed. (Reproduced with permission from Biswas et al. [26, 47])
8.6 Polymer Nanocarriers
Polymeric nanoparticles are the system of choice because of their stability, biodegradability, and biocompatibility. Moreover, polymeric nanoparticles can be chemically conjugated and modified to targeting ligands and/or drugs. Poly-lactide-co-glycolide (PLGA) is nontoxic and biocompatible polymer most commonly explored for drug delivery applications. PLGA nanoparticles loaded with super-oxide dismutase (SOD), was found to be more efficacious in preventing of H2O2 induced neuronal cell death compared to SOD alone and SOD conjugated to polyethylene glycol [72]. These nanoparticles were fabricated using a w/o/w double emulsion method and it was speculated that the H2O2 produced by SOD as a result of ROS reduction, depletes intracellular antioxidants such as catalase, which neutralize H2O2. The study demonstrates the potential of antioxidant loaded polymeric nanoparticles in protecting cells from oxidative stress induced cell death. Marrache et al. [73] successfully induced immune response through mitochondria-targeted biodegradable polymeric nanoparticle containing zinc phthalocyanine (ZnPc) (photosensitizer).
Multifunctional nanoparticles are investigated to provide a promising method for mitochondria-targeted cancer treatment. Several multifunctional mitochondrial nanoparticles have been effectively used for cancer therapy. For example, mitochondrial photo damage upon photo-irradiation by dendrimer phthalocyanine-encapsulated polymeric micelles and αvβ3 integrin-targeting by ligand conjugated doxorubicin (DOX)-micelles can increase DOX mitochondrial accumulation [74]. Biswas et al. [26, 47] conjugated mitochondriotropic ligand triphenylphosphonium (TPP) on the surface of the poly(amidoamine) (PAMAM) dendrimer. The newly developed TPP-anchored dendrimer (G(5)-D-Ac-TPP) was efficiently taken up by the cells and demonstrated good mitochondrial targeting. In vitro cytotoxicity experiments carried out on normal mousefibroblast cells (NIH-3T3) showed greater cell viability in the presence of the G(5)-D-Ac-TPP compared to the parent unmodified G(5)-D. To assess the effect of surface modification on the cytotoxicity, a dose dependent cell viability experiment with all three dendrimers was performed in normal mousefibroblast cell line (NIH-3T3) at 24 and 48 h incubation periods. The results demonstrated that the modified dendrimers were significantly less toxic than the starting material G(5)-D (Fig. 7.5).
8.7 Metal Nanoparticles
Mitochondrial targeting can be successfully executed by nanoparticles prepared from metal elements including gold, platinum and titanium dioxide. They have distinctive properties including smaller size (<10 nm), antioxidant capabilities, and ease of attachment of targeting ligands. Bimetallic nanoparticles may act as the delivery vehicle and therapeutic agent and are also capable of acting as antioxidant species. Conjugation of targeting ligands (peptides, proteins or nucleic acids) to these bimetallic nanoparticles may be a useful approach to target the mitochondria. However, the cellular toxicity of these nanoparticles is yet to be tested. Different types of metal nanoparticles are discussed below. Gold nanoparticles have been prepared with various sizes from <10 nm to approximately 100 nm [75]. Surface of the gold nanoparticles generate a magnetic field upon laser irradiation due to plasmon resonance. Altering this surface of the nanoparticle with proteins or DNA alters their surface plasmon resonance properties. This property has been used in development of novel functional drug delivery systems, diagnostic systems [76], sensitive biosensors for determination of protein–ligand binding reactions, biosensing assays [77]) and micro-electromechanical systems. Only very few studies allude to the potential application of these devices as novel mitochondrial targeting systems.
Gold nanoparticles are known to have detrimental effects on the mitochondrial as they are found to disrupt the integrity of the outer mitochondrial membrane and release of cytochrome c from the mitochondrial electron transport chain, which is known to cause cell death [22]. It is reported that gold nanoparticles of 3 nm could permeate the outer mitochondrial membrane of heart mitochondria whereas nanoparticles of 6 nm were not able to permeate. Therefore, the delivery of gold nanoparticles is size dependent whereby particles smaller than or equal to 3 nm are capable of crossing the outer mitochondria membrane. It was found that nanoparticles entry into the mitochondrial is dependent on VDAC as the entry is inhibited by VDAC inhibitors. This pathway is of therapeutic value as it is involved in cellular apoptosis as well. Surface charge and nanoparticle concentration are important determinants other than size as they govern cellular toxicity [78]. Further, toxicity was characterized by lysis of anionic 1-stearoyl-2-oleoylphosphatidylcholine (SOPC)/stearoyl-oleoyl-phosphatidylserine (SOPS) liposomes and neutral SOPC liposomes. Cationic gold nanoparticles lysed tenfold more anionic liposomes (about 20 %) than anionic gold nanoparticles (about 2 %) in 5 min. Lysis of neutral liposomes (about 15 %) was nearly the same as for anionic and cationic gold nanoparticles at 5 min; however, anionic gold nanoparticles lysed neutral liposomes more (about 5 %) compared to anionic liposomes (about 2 %) at 5 min. In addition to the diameter of the gold nanoparticles, the charge of the nanoparticle and concentration also contribute to cellular toxicity. Although gold nanoparticles exhibit toxicity, they also exhibit therapeutic effects.
Surface modified chitosan functionalized gold nanoparticles were found to be more proficient at eliminating ROS in an H2O2/FeSO4 system than ascorbic acid [79], and the antioxidant activity was further augmented with an increase in chitosan concentration. However, the activity was independent of size of gold nanoparticles. Although the results suggest that chitosan may be responsible for the antioxidant activity when complexed with gold, chitosan has no known antioxidant effects and may exhibit cytotoxicity [80].
Gold nanoparticles functionalized with polyamidoamine (PAMAM) dendrimers are capable of acting as antioxidants by reducing ROS to water and oxygen [81]. PAMAM dendrimer functionalized gold nanoparticles were fabricated by reducing the HAuCl4–dendrimer mixture with NaBH4 under stirring for 30 min. The gold nanoparticle may contribute to the antioxidant effects of dendrimer functionalized gold nanoparticles as PAMAM dendrimer alone was not capable of eliminating free hydroxyls. PAMAM functionalized gold nanoparticles with terminal carboxyl groups had a rate constant 85 times faster than ascorbic acid. The dendrimer functionalized gold nanoparticles showed smaller size than chitosan-gold nanoparticles hence may be more capable than the chitosan-gold nanoparticles. They also have slightly higher efficiency at eliminating ROS. The gold nanoparticles can be used to functionalize and provide for mitochondrial targeting of the dendrimer along with the drug content.
A study demonstrated preferentially induced cell death in cancer cells, but not in normal cells by gold nanorods functionalized with CTAB (cetyltrimethylammonium bromide, a cationic lipophilic molecule). They accumulated in the mitochondria of cancer cells, but not in the mitochondria of normal cells [49, 50]. CTAB is known to induce toxicity and may be partially responsible for cell death induced by CTAB functionalized gold nanorods [82]. Preferential uptake of cationic CTAB functionalized gold nanorods may be attributed to the extremely negative membrane potential across the inner mitochondrial membrane. Mitochondrial membrane potential disruption induced cancer cell death as determined by formation of ROS by flow cytometry and a JC-1 dye assay. Cancer cells had significantly higher levels of ROS than non-cancerous cells with gold nanorods. Gold nanoparticles conjugated to TPP are under investigation for their possible benefits as targeted delivery option. Further, these studies demonstrate that surface modification of gold nanoparticles enhances mitochondrial delivery. Gold nanoparticles elicit anticancer therapeutic effect in the absence of drug. Gold nanoparticles have the ability to act as drug carrier as well as an antioxidant, which make them an attractive nanoparticle for mitochondrial delivery.
Titanium dioxide particles have been also investigated as targeted nano-therapeutics to enter the mitochondria for gene regulation control [25]. Surface of the nanoparticle modified with mitochondrial-specific oligonucleotides exhibit their mitochondrial specificity. It is reported that the dopamine complex with TiO2 nanoparticles is much more stable than the glycidyl isopropyl ether coating, which is also stable in the presence of sunlight. Intense red color was generated due to the charge transfer between dopamine and TiO2 that confirms the conjugation of the dopamine labeled oligonucleotide to the nanoparticle. Oligonucleotide functionalized TiO2 nanoparticles were applied to MCF-7/WS8 breast cancer cells and were subjected to electroporation as a transfection tool. TEM images revealed the accumulation of nanoparticles within the mitochondria of the cell. The authors also confirmed mitochondrial targeting in a rat pheochromocytoma cell (PC12) line. These results demonstrated that mitochondrial intracellular targeting is possible with nanoparticles coated with oligonucleotides specific to the mitochondrial DNA. Interestingly, targeted mitochondria delivery is achieved by non-functionalized titanium dioxide particles of 5–23 nm with minimal nuclear delivery [83]. However, the mechanism by which the oligonucleotide conjugated nanoparticle is endocytosed and reached the mitochondria is not clear. Since larger titanium dioxide particles (5,000 nm) were unable to enter mitochondria, mitochondrial delivery appears to be affected by nanoparticle size and probably also by nanoparticle materials since titanium dioxide can enter mitochondria even without functionalization. An important consideration in drug delivery using metallic nanoparticle is toxicity. Tests should be included in early stages of nanoparticle development in appropriate conditions (buffer, pH, temperature) to ensure higher rates of success in in vivo models with no toxicity. Similarly, platinum nanoparticles also exhibit unique antioxidant properties [84] but safety of the particles has not been confirmed [85] in cell free and cell based assays. Platinum nanoparticles are of approximately 20–30 nm and of varying shapes (nanoflowers, spheres, and multipods). The studies confirm that platinum nanoparticles of various shapes do not exhibit cytotoxicity as they do not produce ROS in a cell free system or inflammatory responses (interleukin-6, and tumor necrosis factor-α) in a human umbilical vein endothelial cell (HUVEC) system up to 50 μg of platinum nanoparticles. They have been known for their antioxidant properties and potential mitochondria therapeutic effects. Hikosaka et al. demonstrated that platinum particles functionalized with pectin were capable of oxidizing NADH to NAD+ [86]. This property may be used to normalize the redox potential by regenerating NAD+ species for glycolysis and other cellular pathways to function properly. It is also demonstrated that platinum nanoparticles (with a size of 5 nm) are capable of quenching superoxide anion radical (O2−) and hydrogen peroxide (H2O2) [87]. They may mimic the functions of complex I since pectin functionalized platinum nanoparticles oxidize NADH to NAD+ and reduce ubiquinone (CoQ) to ubiquinol (CoQH2). These nanoparticles have unique therapeutic benefits in diseases with complex I deficiencies such as Alzheimer's disease. Moreover, polyacrylic acid (PAA) protected platinum nanoparticles of approximately 2 nm are also capable of acting as antioxidants in vitro.
The polyacrylic acid (PAA) coated platinum nanoparticles scavenge the superoxide anion radicals in a dose dependent manner. Since PAA alone was not capable of scavenging the radicals, the platinum could be responsible for the antioxidant activity. In a pulmonary inflammation model, antioxidant properties were demonstrated by platinum nanoparticles in vitro as well as in vivo. PAA coated platinum nanoparticles can effectively scavenge ROS and may be more effective than available current antioxidant treatments. It has been reported that zinc oxide (ZnO) nanoparticles lead to the alteration of the electron transport chain, triggering the apoptotic pathways of the cell and induce mitochondrial cytotoxicity in human colon carcinoma LoVo cells [88].
9 Conclusion
Mitochondria play a crucial role in various metabolic processes of the cell and mitochondrial dysfunction is related to several diseases. The mitochondrion is a novel intracellular target involved in the pathology of many degenerative and metabolic diseases. Although mitochondria appeared to be a potential target for many diseases, the knowledge about functional characteristics in relation to diseases and drug delivery into the mitochondria is still in its infancy. This is certainly due to the barriers that have to be circumvented to achieve a selective targeting and accumulation in mitochondria. Even though several approaches to mitochondrial drug delivery have already been accomplished, there is still a demand for more selective targeting strategies with improved drug efficiency and the therapeutic outcome in various diseases. Currently, the relationships between mitochondrial dysfunction and related disease are being investigated and established. Pharmaceutical nanocarriers offer great promise for site specific mitochondrion delivery. However, the development of these multifunctional nanoparticles for clinical applications has proven to be challenging and they are still at an early stage.
Abbreviations
- CPPs:
-
Cell penetrating peptides
- CsA:
-
Cyclosporin A
- CTAB:
-
Cetyltrimethylammonium bromide
- DOPE:
-
Di-oleoylphosphatidylethanolamine
- DOPE:
-
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP:
-
Dioleoyl-1,2-diacyl-3-trimethylammoniumpropane
- DOTMA:
-
2,3-Bis-(oleoyl)oxipropyl-trimethyl ammonium chloride
- GSH:
-
Glutathione
- Mce 6:
-
Photosensitizer mesochlorine 6
- MEND:
-
Multifunctional envelope-type nano-device
- MLS:
-
Mitochondrial targeting sequences
- MPPs:
-
Mitochondria-penetrating peptides
- mPTPCs:
-
Mitochondrial permeability transition pore complexes
- MTS:
-
Mitochondrial targeting signal peptide
- PAA:
-
Polyacrylic acid
- PAMAM:
-
Poly(amidoamine) dendrimer
- PLGA:
-
Poly-lactide-co-glycolide
- PT:
-
Permeability transition
- PTD:
-
Protein transduction domain
- PTPC:
-
Permeability transition pore complex
- SOD:
-
Super-oxide dismutase
- SOPC:
-
1-Stearoyl-2-oleoylphosphatidylcholine
- SOPS:
-
Stearoyl-oleoyl-phosphatidylserine
- STPP:
-
Stearyl triphenyl phosphonium
- TAT:
-
Trans-activating transcriptional activator
- tBHP:
-
t-Butylhydroperoxide
- TPP:
-
Triphenylphosphonium
- TPP:
-
Triphenylphosphonium
- VDAC:
-
Voltage dependent anion channel
- ZnO:
-
Zinc oxide
- ZnPc:
-
Zinc phthalocyanine
References
Ferreira L, Karp JM, Nobre L, Langer R (2008) New opportunities: the use of nanotechnologies to manipulate and track stem cells. Cell Stem Cell 3:136–146
Durazo SA, Kompella UB (2012) Functionalized nanosystems for targeted mitochondrial delivery. Mitochondrion 12:190–201
D’Souza GGM, Weissig V (2009) Subcellular targeting: a new frontier for drug-loaded pharmaceutical nanocarriers and the concept of the magic bullet. Expert Opin Drug Deliv 6:1135–1148
Murphy MP, Smith RAJ (2000) Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev 41:235–250
Szewczyk A, Wojtczak L (2002) Mitochondria as a pharmacological target. Pharmacol Rev 54:101–127
Detmer SA, Chan DC (2007) Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8:870–879
Wallace KB, Starkov AA (2000) Mitochondrial targets of drug toxicity. Annu Rev Pharmacol Toxicol 40:353–388
DiMauro S (2004) Mitochondrial diseases. Biochim Biophys Acta 1658:80–88
Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387
Gibson GE, Karuppagounder SS, Shi Q (2008) Oxidant-induced changes in mitochondria and calcium dynamics in the pathophysiology of Alzheimer’s disease. Mitochon Oxid Stress Neurodegen Disord. Ann N Y Acad Sci 1147:221–232
Pathania D, Millard M, Neamati N (2009) Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev 61:1250–1275
Yamada Y, Shinohara Y, Kakudo T, Chaki S, Futaki S, Kamiya H et al (2005) Mitochondrial delivery of mastoparan with transferring liposomes equipped with a pH-sensitive fusogenic peptide for selective cancer therapy. Int J Pharm 303:1–7
Muratovska A, Lightowlers RN, Taylor RW, Wilce JA, Murphy MP (2001) Targeting large molecules to mitochondria. Adv Drug Deliv Rev 49:189–198
Horobin RW, Trapp S, Weissig V (2007) Mitochondriotropics: a review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J Control Release 121:125–136
Langner M (2000) The intracellular fate of non-viral DNA carriers. Cell Mol Biol Lett 5:295–313
Bulmus V (2005) Biomembrane-active molecular switches as tools for intracellular drug delivery. Aust J Chem 58:411–422
Gupta B, Levchenko TS, Torchilin VP (2005) Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev 57:637–651
Torchilin VP (2006) Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng 8:343–375
Luby-Phelps K (2000) Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int Rev Cytol 192:189–221
Ellis RJ, Minton AP (2003) Cell biology: join the crowd. Nature 425:27–28
Seksek O, Biwersi J, Verkman AS (1997) Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J Cell Biol 138:131–142
Salnikov V, Lukyanenko YO, Frederick CA, Lederer WJ, Lukyanenko V (2007) Probing the outer mitochondrial membrane in cardiac mitochondria with nano-particles. Biophys J 92:1058–1071
Crompton M, Virji S, Ward JM (1998) Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem 258:729–735
Jensen RE, Dunn CD (2002) Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons. Biochim Biophys Acta 1592:25–34
Paunesku T, Vogt S, Lai B, Maser J, Stojicevic N, Thurn KT et al (2007) Intracellular distribution of TiO2-DNA oligonucleotide nanoconjugates directed to nucleolus and mitochondria indicates sequence specificity. Nano Lett 7:596–601
Biswas S, Dodwadkar NS, Piroyan A, Torchilin VP (2012) Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials 33:4773–4782
Smith RAJ, Murphy MP (2011) Mitochondria-targeted antioxidants as therapies. Discov Med 11:106–114
Liberman E, Skulachev V (1970) Conversion of biomembrane-produced energy into electric form. IV. Biochim Biophys Acta 216:30–42
Jauslin ML, Meier T, Smith RA, Murphy MP (2003) Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J 17:1972–1974
Sheu SS, Nauduri D, Anders MW (2006) Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta 1762:256–265
Fernandez-Carneado J, VanGool M, Martos V, Castel S, Prados P, de Mendoza J, Giralt E (2005) Highly efficient, nonpeptidic oligoguanidinium vectors that selectively internalize into mitochondria. J Am Chem Soc 127:869–874
Yousif LF, Stewart KM, Horton KL, Kelley SO (2009) Mitochondria-penetrating peptides: sequence effects and model cargo transport. Chembiochem 10:2081–2088
Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW et al (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279:34682–34690
Szeto HH (2006) Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J 8:E277–E283
Horton KL, Stewart KM, Fonseca SB, Guo Q, Kelley SO (2008) Mitochondria-penetrating peptides. Chem Biol 15:375–382
Sebbage V (2009) Cell-penetrating peptides and their therapeutic applications. Bioscience Horizons 2:64–72
Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ et al (2002) Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem 278:585–590
Pfanner N, Geissler A (2001) Versatility of the mitochondrial protein import machinery. Nat Rev Mol Cell Biol 2:339–349
Schatz G (1996) The protein import system of mitochondria. J Biol Chem 271:31763–31766
Zhang C, Sriratana A, Minamikawa T, Nagley P (1998) Photosensitisation properties of mitochondrially localised green fluorescent protein. Biochem Biophys Res Commun 242:390–395
Weissig V (2003) Mitochondrial-targeted drug and DNA delivery. Crit Rev Ther Drug Carrier Syst 20:1–62
Sneh-Edri H, Likhtenshtein D, Stepensky D (2011) Intracellular targeting of PLGA nanoparticles encapsulating antigenic peptide to the endoplasmic reticulum of dendritic cells and its effect on antigen cross-presentation in vitro. Mol Pharm 8:1266–1275
Zhou J, Zhao W-Y, Ma X, Ju R-J, Li X-Y, Li N, Sun M-G, Shi J-F, Zhang C-X, Lu W-L (2013) The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials 34:3626–3638
Li N, Zhang CX, Wang XX, Zhang L, Ma X, Zhou J, Ju RJ, Li XY, Zhao WY, Lu WL (2013) Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signaling pathways. Biomaterials 34:3366–3380
Yu Y, Wang Z-H, Zhang L, Yao H-J, Zhang Y, Li R-J, Ju R-J, Wang X-X, Zhou J, Li N, Lu W-L (2012) Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials 33:1808–1820
Malhi SS, Budhiraja A, Arora S, Chaudhari KR, Nepali K, Kumar R, Sohi H, Murthy RS (2012) Intracellular delivery of redox cycler-doxorubicin to the mitochondria of cancer cell by folate receptor targeted mitocancerotropic liposomes. Int J Pharm 432:63–74
Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP (2012) Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J Control Release 159:393–402
Solomon MA, Shah AA, D’Souza GG (2013) In Vitro assessment of the utility of stearyl triphenyl phosphonium modified liposomes in overcoming the resistance of ovarian carcinoma Ovcar-3 cells to paclitaxel. Mitochondrion 134:64–72
Wang L, Liu Y, Li W, Jiang X, Ji Y, Wu X et al (2011) Selective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapy. Nano Lett 11:772–780
Wang X-X, Li Y-B, Yao H-J, Ju R-J, Zhang Y, Li R-J, Yu Y, Zhang L, Lu W-L (2011) The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials 32:5673–5687
Patel NR, Hatziantoniou S, Georgopoulos A, Demetzos C, Torchilin VP, Weissig V et al (2010) Mitochondria-targeted liposomes improve the apoptotic and cytotoxic action of sclareol. J Liposome Res 20:244–249
Liguori L, Marques B, Villegas-Mendez A, Rothe R, Lenormand J-L (2008) Liposomes-mediated delivery of pro-apoptotic therapeutic membrane proteins. J Control Release 126:217–227
Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, Yamashita K, Kobayashi H, Kikuchi H, Harashima H (2008) MITO-Porter: a liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim Biophys Acta 1778:423–432
Boddapati SV, D’Souza GG, Erdogan S, Torchilin VP, Weissig V (2008) Organelle-targeted nanocarriers: specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett 8:2559–2563
Hoshino K, Fujioka T, Oku S, Nakamura M, Suga Y, Yamaguchi K et al (2004) Quantum dots targeted to the assigned organelle in living cells. Microbiol Immunol 48:985–994
Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P et al (2002) Cellular localization of a water-soluble fullerene derivative. Biochem Biophys Res Commun 294:116–119
Cuchelkar V, Kopeckova P, Kopecek J (2008) Novel HPMA copolymer-bound constructs for combined tumor and mitochondrial targeting. Mol Pharm 5:776–786
García-Péreza AI, Galeanob E, Nietoa E, Sancho P (2011) Dequalinium induces human leukemia cell death by affecting the redox balance. Leuk Res 35:1395–1401
D’Souza GG, Cheng SM, Boddapati SV, Horobin RW, Weissig V (2008) Nanocarrier-assisted sub-cellular targeting to the site of mitochondria improves the pro-apoptotic activity of paclitaxel. J Drug Target 16:578–585
D’Souza GG, Rammohan R, Cheng SM, Torchilin VP, Weissig V (2003) DQAsome-mediated delivery of plasmid DNA toward mitochondria in living cells. J Control Release 92:1–2
Gao X, Kim KS, Liu D (2007) Nonviral gene delivery: what we know and what is next. AAPS J 9:E92–E104
Vaidya B, Paliwal R, Rai S, Khatri K, Goyal AK, Mishra N, Vyas SP (2009) Cell-selective mitochondrial targeting: a new approach for cancer therapy. Cancer Ther 7:141–148
Inoki Y (2000) Proteoliposomes colocalized with endogenous mitochondria in mouse fertilized egg. Biochem Biophys Res Commun 278:183–191
Miller BR, Cumsky MG (1991) An unusual mitochondrial import pathway for the precursor to yeast cytochromec oxidase subunit Va. J Cell Biol 112:833–841
Herrmann JM, Koll H, Cook RA, Neupert W, Stuart RA (1995) Topogenesis of cytochrome oxidase subunit II. Mechanisms of protein export from themitochondrial matrix. J Biol Chem 270:27079–27086
Rajendran L, Knölker HJ, Simons K (2010) Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov 9:29–42
Stover TC, Sharma A, Robertson GP, Kester M (2005) Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res 11:3465–3474
Yamada Y, Furukawa R, Yasuzaki Y, Harashima H (2011) Dual function MITO-Porter, a nano carrier integrating both efficient cytoplasmic delivery and mitochondrial macromolecule delivery. Mol Ther 19:1449–1456
Yamada Y (2007) Mitochondrial drug delivery and mitochondrial disease therapy – an approach to liposome-based delivery targeted to mitochondria. Mitochondrion 7:63–71
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312
Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M et al (1987) Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci 84:7413–7417
Reddy MK, Wu L, Kou W, Ghorpade A, Labhasetwar V (2008) Superoxide dismutase-loaded PLGA nanoparticles protect cultured human neurons under oxidative stress. Appl Biochem Biotechnol 151:565–577
Marrache S, Tundup S, Harn DA, Dhar S (2013) Ex vivo programming of dendritic cells by mitochondria-targeted nanoparticles to produce interferon-gamma for cancer immunotherapy. ACS Nano 7(8):7392–7402
Nishiyama N (2009) Enhanced photodynamic cancer treatment by supramolecular nanocarriers charged with dendrimer phthalocyanine. J Control Release 133:245–251
Link S, El-Sayed MA (1999) Size and temperature dependence of the plasmon absorp-tion of colloidal gold nanoparticles. J Phys Chem B 103:4212–4217
El-Sayed IH, Huang X, El-Sayed MA (2005) Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett 5:829–834
Flanagan MT, Pantell RH (1984) Surface plasmon resonance and immunosensors. Electron Lett 20:968–970
Goodman CM, McCusker CD, Yilmaz T, Rotello VM (2004) Toxicity of gold nano-particles functionalized with cationic and anionic side chains. Bioconjug Chem 15:897–900
Esumi K, Takei N, Yoshimura T (2003) Antioxidant-potentiality of gold-chitosan nanocomposites. Colloids Surf B Biointerfaces 32:117–123
Qi LF, Xu ZR, Li Y, Jiang X, Han XY (2005) In vitro effects of chitosan nanoparticles on proliferation of human gastric carcinoma cell line MGC803 cells. World J Gastroenterol 11:5136–5141
Esumi K, Houdatsu H, Yoshimura T (2004) Antioxidant action by gold-PAMAM den-drimer nanocomposites. Langmuir 20:2536–2538
Isomaa B, Reuter J, Djupsund BM (1976) The subacute and chronic toxicity of cetyl-trimethylammonium bromide (CTAB), a cationic surfactant, in the rat. Arch Toxicol 35:91–96
Suzuki H, Toyooka T, Ibuki Y (2007) Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ Sci Technol 41:3018–3024
Aiuchi T, Nakajo S, Nakaya K (2004) Reducing activity of colloidal platinum nanopar-ticles for hydrogen peroxide, 2,2-diphenyl-1-picrylhydrazyl radical and 2,6-dichlorophenol indophenol. Biol Pharm Bull 27:736–738
Elder A, Yang H, Gwiazda R, Teng X, Thurston S, He H et al (2007) Testing nanomaterials of unknown toxicity: an example based on platinum nanoparticles of different shapes. Adv Mater 19:3124–3129
Hikosaka K, Kim J, Kajita M, Kanayama A, Miyamoto Y (2008) Platinum nanoparticles have an activity similar to mitochondrial NADH: ubiquinone oxidoreductase. Colloids Surf B Biointerfaces 66:195–200
Kajita M, Hikosaka K, Iitsuka M, Kanayama A, Toshima N, Miyamoto Y (2007) Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic Res 41:615–626
De Berardis B, Civitelli G, Condello M, Lista P, Pozzi R, Arancia G et al (2010) Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol Appl Pharmacol 246:116–127
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Controlled Release Society
About this chapter
Cite this chapter
Agrawal, U., Sharma, R., Vyas, S.P. (2015). Targeted Drug Delivery to the Mitochondria. In: Devarajan, P., Jain, S. (eds) Targeted Drug Delivery : Concepts and Design. Advances in Delivery Science and Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-11355-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-11355-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11354-8
Online ISBN: 978-3-319-11355-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)