Abstract
The median suboccipital approach is the most commonly used approach to the posterior fossa in pediatric neurosurgery. In contrast to the retrosigmoid and far lateral approaches, it provides access to midline tumors in the cerebellum, particularly those arising from the fourth ventricle and the cerebellar vermis. Hemispheric cerebellar tumors may also be removed through this route, with a slight modification of the approach towards the side of the lesion. The most common pediatric posterior fossa tumors specifically pilocytic astrocytomas, medulloblastomas, or ependymomas can be removed with this approach. It provides access to the pineal region and permits the removal of those brain stem tumors with an exophytic component in the fourth ventricle.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Posterior Fossa
- Fourth Ventricle
- External Ventricular Drain
- Posterior Inferior Cerebellar Artery
- Bone Flap
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The median suboccipital approach is the most commonly used approach to the posterior fossa in pediatric neurosurgery. In contrast to the retrosigmoid and far lateral approaches, it provides access to midline tumors in the cerebellum, particularly those arising from the fourth ventricle and the cerebellar vermis. Hemispheric cerebellar tumors may also be removed through this route, with a slight modification of the approach towards the side of the lesion. The most common pediatric posterior fossa tumors specifically pilocytic astrocytomas, medulloblastomas, or ependymomas can be removed with this approach. It provides access to the pineal region and permits the removal of those brain stem tumors with an exophytic component in the fourth ventricle.
Historically, surgery to the posterior fossa has not been without significant morbidity. Allen Starr, in 1893, published a series of 16 patients with cerebellar tumors, who had undergone attempted surgical resection. The tumor was not found in 11 patients, and of the 5 who had had tumor resection, 3 died [1]. In 1903, with improvement of surgical technique, the same author described 58 surgical cases of cerebellar tumors with 16 being successfully removed and 8 deaths [2]. In 1909, the successful excision of a posterior fossa tumor in one patient was described. Interestingly it was noted that the major problem following tumor removal was control of bleeding, with the condition of the patient becoming “grave. The blood pressure fell… and a rapid closure was made with catgut.” After a stormy immediate postoperative course, this patient survived [3]. Over the last century, neurosurgical techniques improved substantially thus removing a posterior fossa tumor via a median suboccipital approach became standard practice. In 1996, Yasargil [4] modified the technique by dividing the median suboccipital approach into superior and inferior corridors. In the present day, the superior median approach provides good exposure of the inferior tentorial incisura, the dorsal mesencephalon, and the superior cerebellar hemisphere, while the inferior median approach provides access to the fourth ventricle and the posterior aspect of the pontobulbar region.
This chapter will highlight the general surgical principles necessary to enter the posterior fossa in the pediatric population. The relevant anatomy of the posterior fossa will be described with respect to the median suboccipital approach, with the different parts of the cerebellum accessed through this approach highlighted. Aspects of patient preparation relevant to this approach will be emphasized. The surgical techniques used in our department will be described incorporating the considerations of postoperative management.
2 Brief Overview of the Anatomy of the Posterior Fossa
The surgical anatomy of the posterior fossa has been extensively illustrated by many authors. This section aims to provide a relevant overview only and readers are referred to the works of Yasargil and Rhoton for more detail [4, 5]. Briefly, the bony posterior fossa is surrounded anteriorly by the dorsum sellae and clivus and posteriorly and laterally by the occipital bone and the petrous and mastoid parts of the temporalis bone. On either side of the posterior fossa, the superior part of the petrous portion of the temporal bone provides attachment to the tentorium and contains the superior petrosal sinus. Posteriorly, both transverse sinuses delimit the superior aspect of the posterior fossa. They represent an important landmark to delineate the upper limit of the suboccipital craniotomy. The foramen magnum is positioned in the center of the floor of the posterior fossa and is delimited laterally by the occipital condyles, above which is situated the hypoglossal canal for the hypoglossal nerve. The jugular foramen is located between the lateral part of the occipital bone and the petrous portion of the temporal bone. Superior to the jugular foramen lies the internal acoustic meatus for the facial and acoustic nerves and internal auditory artery [6]. Posterior and superior to the foramen magnum are located the bilateral inferior occipital fossae which support the hemispheres of the cerebellum and are useful landmarks for burr hole placement. They are separated from each other by the internal occipital crest, which provides attachment to the falx cerebelli. It houses the occipital sinus, which can be a source of significant blood loss if not carefully closed during dural opening. The circular sinus, which traverses the posterior aspect of the foramen magnum, can also be a site of significant bleeding if not recognized during surgery [7].
The median suboccipital approach provides direct exposure of the cerebellum. The cerebellum presents three distinct surfaces: (1) the tentorial surface which faces the tentorium cerebelli and may be exposed in a superior median suboccipital approach, (2) the petrosal surface, retracted to access the cerebellopontine angle (usually via a retrosigmoid and far lateral approach), and (3) the suboccipital surface exposed in a median suboccipital craniotomy and through which access for most cerebellar tumors including access to the fourth ventricle is provided. This surface presents the cerebellar vermis and the two cerebellar hemispheres. The vermis is subdivided into the tuber superiorly and the pyramid and uvula inferiorly, separated by the horizontal suboccipital fissure which also divides the cerebellar hemispheres into superior and inferior parts. The suboccipital fissure delimits a superior and inferior surgical triangle providing access to either the superior part of the vermis including the culmen (superior triangle) or to the fourth ventricle, the inferior vermis, cerebellar hemispheres, and the foramen magnum (inferior triangle) (Fig. 8.1). The cerebellomedullary fissure is a natural cleft between the cerebellum and the medulla oblongata. It extends between the cerebellum and the medulla and forms part of the roof of the fourth ventricle [5]. It provides access to the fourth ventricle via the well-described telovelar approach through the tela choroidea [8].
The fourth ventricle, tentlike in appearance, is located between the cerebellum and brain stem. Dorsally, the roof is divided into superior and inferior parts. The ventricular surface of the superior part of the roof of the fourth ventricle is further divided into a median part formed by the superior medullary velum and two lateral parts formed by the inner parts of the cerebellar peduncles. Access to the fourth ventricle is therefore either by the cerebellomedullary fissure described above or through the vermis.
Understanding posterior fossa vasculature, in particular the supply to the cerebellum and the brain stem, is crucial to the safety of median suboccipital approaches. Three neurovascular complexes in the posterior fossa have been described by Rhoton [9]: an upper complex related to the superior cerebellar artery (SCA) which contains the midbrain; cranial nerves III, IV, and V; the cerebellomesencephalic fissure; the superior cerebellar peduncle; and the tentorial surface of the cerebellum. The middle complex, supplied by the anteroinferior cerebellar artery (AICA), contains the pons; the cranial nerves VI, VII, and VIII; the middle cerebellar peduncle; the cerebellopontine fissure; and the petrosal surface of the cerebellum. The lower complex, related to the posterior inferior cerebellar artery (PICA), contains the medulla and lower cranial nerves IX, X, XI, and XII. The PICA has the most tortuous course of the posterior circulation arteries and is exposed through a median suboccipital craniotomy. It is closely related to the cerebellomedullary fissure; coursing around the cerebellar tonsils, it enters the cerebellomedullary fissure and traverses the lower half of the roof of the fourth ventricle to finally exit the cerebellomedullary fissure. It provides branches that are distributed to the vermis and hemispheres of the suboccipital surface.
Veins of the posterior fossa may become an issue when enlarged due to tumor growth. They are divided into four groups: superficial, deep, brain stem, and bridging veins [7]. Of relevance to the median suboccipital approach are the large inferior vermian veins, which give rise to inferior hemispheric veins. These need to be carefully divided in midline approaches through the vermis.
This drawing represents an enlarged view of the suboccipital surface of the cerebellum. The suboccipital fissure divides the cerebellum into a superior and inferior part. Three different parts of the vermis are represented here: the tuber lies above the suboccipital fissure, the pyramid and the uvula below. The foramen of Magendie is located below the uvula, in the midline. The cerebellar tonsils lie lateral to the foramen of Magendie.
3 Goals of the Surgery
The aims of surgery for posterior fossa tumors, associated with brain stem compression and hydrocephalus, are (1) to decompress the brain stem and cerebellum, (2) to restore normal cerebrospinal fluid pathways through the fourth ventricle and the foramina of Luschka or Magendie, and (3) to attempt complete resection, without new neurological deficit, in order to improve postoperative survival.
4 Surgical Planning and Types of Approaches
The median suboccipital approach provides access to most pediatric tumors in the posterior fossa. Careful assessment of preoperative imaging to determine tumor location within the fourth ventricle, the vermis, or cerebellar hemisphere is essential to determine the most appropriate surgical route and strategy. Depending on the tumor localization, approaches through a median suboccipital craniotomy can be classified into five major categories: (1) transcortical, (2) transvermian, (3) telovelar, (4) infratentorial supracerebellar, and (5) combined, involving a telovelar approach and a small inferior vermian incision or involving a telovelar approach and a supracerebellar approach as described recently [10]. Brain stem tumors can be resected either by a transvermian or telovelar approach (Figs. 8.2 and 8.3).
Median suboccipital approaches to the posterior fossa. The main classical approaches to the posterior fossa via a median suboccipital approach are represented. (a) Sagittal view of the posterior fossa. Three surgical corridors are represented. In yellow, the infratentorial supracerebellar approach which gives access to pineal region tumors or to the superior part of voluminous posterior fossa tumors; in green, the transvermian approach, a classical route to access tumors from the vermis or within the fourth ventricle; in red the telovelar approach, which gives access to the fourth ventricle via the cerebellomedullary fissure after retraction of the cerebellar tonsils and dissection of the tela choroidea. (b) Axial view of the posterior fossa and the brain stem. Two surgical corridors are represented: in gray is the combination of the three approaches described above which are seen as one on this view. In blue is the corridor to access hemispheric tumors which have a median component
(a, b) Tumors of the fourth ventricle with compression of the vermis but no extensive vermian infiltration. These cases are good candidates to telovelar approach. (c) Tumor of the fourth ventricle with limited dimensions (<3 cm) but with extensive infiltration of the vermis. In this case telovelar approach with limited incision of the lower vermis was sufficient to achieve complete removal. (d) Tumor of the fourth ventricle with largest diameter >4 cm and extensive compression/infiltration of the lower vermis. In similar cases incision of the lower vermis for traditional transvermian approach is usually necessary. (e) Large medulloblastoma of the upper vermis without involvement of the fourth ventricle. Transvermian approach with incision of the upper vermis is here the only option. (f, g) Medulloblastoma of the upper vermis with extensive lateral adhesion to tentorium. The tumor was completely removed through a supracerebellar approach
Initially, standard neurosurgical practice involved splitting the vermis to access “midline” posterior fossa tumors [11]. This approach was often associated with the known “cerebellar mutism syndrome” due to surgical incision of the vermis and lateral retraction to the dentate gyrus and its outflow tract [12–14].
The telovelar approach is now a well-accepted surgical route and should be performed alone or in combination with incision of the inferior vermis dependent on the tumor size and its superior extension [15]. The microsurgical anatomy of this approach has been well described [8]. It involves gentle retraction of the tonsils laterally, which exposes both the tela choroidea and the inferior medullary velum. The tela choroidea is a thin arachnoid membrane and forms the lower portion of the inferior half of the roof of the caudal wall of each lateral recess. The foramen of Magendie delimits the lowest part of the membrane. In its superior parts and laterally to the uvula, it prolongs into the telovelar junction followed by the inferior medullary velum (Fig. 8.4). Opening of the tela gives access to the floor of the fourth ventricle, from the obex to the aqueduct. Further rostral opening of the velum gives additional access to the superior half of the roof of the fourth ventricle, the fastigium and the superolateral recess, a technique well described by Mussi [8].
Although this surgical corridor appears restricted, it has been shown to be sufficient to remove large fourth ventricle tumors without splitting the inferior vermis [15]. In cadaveric dissection it has been quantified, using triangles from defined anatomical points, that the telovelar approach with the removal of C1 posterior arch provided a larger working area than the transvermian approach except for a limited angle to the rostrum of the fourth ventricle [16]. These findings have been confirmed by other studies [17]. For ependymomas with lateral extension near the brain stem or cranial nerves, this surgical corridor offers attractive possibilities, preventing potentially devastating complications associated with the transvermian approach [18]. For giant posterior fossa midline tumors, some surgeons have proposed a combined transventricular and infratentorial supracerebellar approach, highlighting that this was a safe technique, avoiding the incision of the vermis [10]. In contrast however, others have raised concerns regarding utilizing the telovelar approach for large fourth ventricular tumors, suggesting that a staged dissection of the tela and the uvulotonsillar cleft to be superior [19].
It is therefore imperative to consider on the basis of preoperative imaging the different approaches that are available and to define surgical strategy accordingly.
This drawing represents the surgical anatomy of the cerebellomedullary fissure. The tonsils have been retracted on both sides, and exposure of the tela choroidea on the left side of the drawing is seen. The inferior limit of the tela corresponds to the teania and the superior part to the inferior medullary vellum. On the right side of the drawing, the tela has been removed, showing exposure of the fourth ventricle with the foramen of Magendie on its lowest aspect and the lateral limits represented by the inferior cerebellar peduncle.
5 Preoperative Consideration
5.1 Surgical Consent
Often, the diagnosis of a posterior fossa tumor in children is unexpected. Special care therefore needs to be taken in relaying the diagnosis and potential surgical risks to the parents [20]. Although surgical morbidity is low in most cases, significant neurological deficits are possible [21]. While the radiological appearances of the tumor may point to distinct pathologies, explicit management strategy discussions, particularly with respect to adjuvant chemotherapy and radiotherapy, should be delayed until the formal pathology is known. Signed consent is obtained from the parents. Age-appropriate information should be provided to the child regarding the proposed surgical procedure.
5.2 Anesthetic Considerations
Ideally, a specialized pediatric neuroanesthetic team should be involved with children, undergoing posterior fossa craniotomy for tumor excision. A full description of neuroanesthetic principles is however beyond the scope of this chapter. Of critical importance during surgery is monitoring for air embolism and hemodynamic fluctuations due to brain stem irritation or blood loss. The latter is of particular relevance in young children where rapid hypotensive shock can ensue. Routine anesthetic practice in our institution would include central venous and invasive arterial pressure monitoring and an indwelling urinary catheter. Mannitol is not given routinely. Perioperative antibiotic prophylaxis (cephazolin) and dexamethasone is used routinely.
5.3 Management of Hydrocephalus
The presence of hydrocephalus is assessed on preoperative imaging, and the necessity for its rapid treatment is dependent on the clinical state of the patient. If hydrocephalus does not need to be treated urgently, surgery to remove posterior fossa tumors should be performed within 48 h of presentation and potentially on the day of admission if the child is critically unwell.
Different strategies are available to manage hydrocephalus in patients with posterior fossa tumors, including the insertion of an external ventricular drain (EVD), a ventriculoperitoneal shunt (VP) shunt, or performing a third ventriculostomy or the anticipated restoration of normal CSF flow secondary to tumor removal. There is no consensus yet as to which is the best option, often being dependent on surgical expertise and preference. The reader is referred to Chap. 7 for a full discussion regarding hydrocephalus management in posterior fossa tumors. Recognition should be given, however, that unless critical the author’s preference is to not treat the hydrocephalus separately to tumor removal.
Where significant hydrocephalus is present on preoperative imaging, the author’s preference is to place an occipital EVD, prior to commencing the posterior fossa craniotomy. Reducing intracranial pressure in this way allows for a safer dural opening by reducing venous hypertension and reducing venous congestion of the cerebellum during the early phase of tumor removal. Where hydrocephalus is not severe, similar drainage of CSF may be enabled by entering into the cisterna magna initially prior to fully opening the dura. We would not recommend inserting a permanent VP shunt prior to surgery.
6 Operative Techniques
Different surgical techniques are available to remove posterior fossa tumors. General surgical principles, however, differ little between centers and thus we describe the surgical techniques used in our department.
6.1 Patient Positioning
Patient positioning depends on the surgeon’s preference, and removing a pediatric posterior fossa tumor can be done in a prone “concorde,” sitting or lateral position. The sitting position has the advantage that hemorrhage and CSF drain easily providing a clear operative field. It also provides superior exposure of the superior vermis and pineal region. It has the disadvantage of increased risk of air embolism, systemic hypotension, and intracranial air accumulation in the subdural and/or subarachnoid spaces. The surgeon’s position during this approach with extended arms can be difficult to maintain.
In our neurosurgical unit all patients are operated upon in the prone position. The head is fixed in Mayfield pins if tolerated by the age of the patient; otherwise a soft horseshoe headrest is used. For small children extra padding is added to the horseshoe and to the dependent side of the head of the child to prevent pressure sores. Special attention is made to avoid pressure on the eyes. The eyes are filled with lubricant and covered to avoid inadvertent contamination by preparation solutions and blood. The patient is placed on a gel mat supported by gel rolls placed under the upper chest and pelvic regions. This allows the abdomen to be noncompressed improving venous return and aiding ventilation. A soft pad is placed under the patella tendon to allow the patella to have no contact with the mat, and the feet are elevated on a soft pillow. The rest of the patient is checked to avoid pressure areas.
The head is moderately flexed on an extended neck which opens the space between the foramen magnum and the posterior arch of C1. It is important not to compromise venous return thereby increasing cerebellar swelling. The bed is slightly tilted to place the occiput and the neck uppermost. View to the infratentorial space may be limited, due to the restricted head flexion during positioning, particularly in patients with a low-lying torcular.
A navigation system is utilized for all cases with posterior fossa tumors, even if positioning in pins is not possible. However, its utility once CSF and tumor cysts are drained is questionable.
After the patient’s positioning has been checked, shaving is performed with the clipper which has been associated with a lower postoperative infection rate compared to the use of the razor [22]. At this stage of the preparation, it is important to have a visual concept of the anatomy of the posterior fossa and its landmarks including the torcular, the transverse and sigmoid sinuses, and foramen magnum. It can be useful to draw the projection on the skin. An incision line is marked from the occipital protuberance to the level of C2 and the site of the potential insertion of the EVD.
7 Surgical Steps
7.1 Soft Tissue Preparation
The skin is prepped in three steps with chlorhexidine soap, alcoholic chlorhexidine solution, and alcohol. Care is taken not to allow the preparation solution to run down loosening taping of tracheal tubes and penetrating into the eyes. After draping, long-lasting local anesthetic is injected into the wound to improve the management of postoperative pain. A midline incision is carried out from the external occipital protuberance to the level of C2. In our experience, a midline incision is sufficient for most midline and hemispheric cerebellar tumors. Using monopolar cauterization, division of the fascia is performed in the avascular midline plane of the ligamentum nuchae. Either a linear midline incision can be made down to the bone with lateral exposure or a T-shaped incision of the fascia is made leaving a horizontal rim of tissue along the superior nuchal line (Fig. 8.5) used for later closure. The occipital bone is exposed to the foramen magnum. The posterior arch of C1 is exposed using a subperiostal dissection technique with a combination of the monopolar cautery on low settings and sharp periosteal elevation. Bleeding from emissary veins is controlled with bone wax. In small children the posterior arch of C1 may be incompletely fused, and special attention is made not to cauterize the arch in the midline. The posterior arch of C1 is removed laterally up to the beginning of the groove of the vertebral artery. The atlantooccipital membrane is exposed. Careful dissection needs to be performed laterally to avoid injury to the vertebral arteries. A large circular sinus may cause significant bleeding but can often be controlled with the use of Surgicel® and pressure or bipolar electrocoagulation. Strong curved retractors are used to maintain adequate retraction of the muscles.
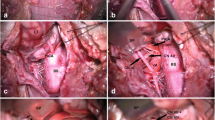
Surgical steps to perform the suboccipital craniotomy flap. Representation of some of the different steps used to achieve a suboccipital craniotomy. (a) The incision line is drawn on the skin of the patient from the posterior occipital protuberance down to a level of C2. (b) Image representing the exposure of the occipital bone with the posterior arch of C1 and the atlantooccipital membrane. The T-shaped incision of the muscle fascia along the superior nuchal line can be appreciated. (c) The edge of the craniotomy flap has been drilled with the M8 Midas Rex drill®. (d) Picture representing the line of the craniotomy edge which has been drilled (curved dashed line). The posterior aspect of the foramen magnum is exposed (white line)
7.2 Suboccipital Craniotomy
It is now well recognized that a suboccipital craniotomy with replacement of the bone flap is to be preferred to a craniectomy. It limits postoperative pseudomeningocele formation and headache [23]. The limits of the craniotomy can be drawn on the occipital bone. To elevate the bone flap, two options are possible: one is the placement of two burr holes on each side of the occipital plate just below the transverse sinus. The opening is then completed with the high-speed craniotome cutting a curved line down to the foramen magnum. The cut joining the two bur holes just below the torcular can be initiated with the craniotome and completed with the drill or rongeurs to remove the triangular occipital crest. The second option is to elevate the entire bone flap with the high – speed M8 Midas Rex drill® (Fig. 8.5), which is our preferred option especially in small children. It allows preservation of the maximum size of the bone flap and provides also a safe option for bone removal in the midline below the torcular, and note should be made that posterior fossa tumors, particularly pilocytic astrocytomas, may cause remodelling and marked thinning of the occipital bone. On elevation of the bone flap, the dura around the foramen magnum may be quite adherent. This can be separated by detaching the membranes with a scalpel or sharp periosteal elevator, always maintaining contact with the bone to avoid cerebellar/brain stem injury, and occasionally a fine point diathermy on low settings.
7.3 Opening of the Dura
Usually, the dura is opened in a Y-shaped or extended U-shaped fashion, starting above the cerebellar hemispheres and working down towards the midline at the level of the circular sulcus. Opening the dura in a tight posterior fossa, however, may be a difficult task due to the significant bleeding which may occur during division of the midline occipital sinus and due to tumor/cerebellar herniation. In this instance an EVD inserted to drain associated hydrocephalus or the tumor cyst may be drained. Alternatively the dura may be opened over the cisterna magna first allowing CSF egress. There are different ways of approaching the occipital sinus, we tend to use metal clips (Weck clips™) to progressively occlude and cut the sinus from each side and avoid major sudden bleeding. Alternatively the sinus may be oversewn. Once the dura is opened, a suture is inserted to the apex of the dura flap, which is reflected upward to limit bleeding from epidural spaces. Sutures are used around the Y-shaped dura opening to increase surgical exposure to the cerebellum and cisterna magna and limit extradural bleeding.
7.4 Tumor Excision
Midline cerebellar and fourth ventricular tumors can be often seen by gentle retraction of the cerebellar tonsils, division of the tela choroidea, and occasionally inferior vermian incision. Bilateral retractors are placed laterally to maintain exposure. Particular attention needs to be made to the inferior vermian and retromedullary portion of the PICA in this approach. Hemispheric tumors can be appreciated due to discoloration and swelling of the cerebellar surface. Cystic hemispheric tumors may require cyst drainage with a Cushing needle prior to tumor dissection if swelling is significant otherwise initial preservation of the cyst is advantageous for the gradual dissection of the tumor wall from the cerebellar parenchyma.
As a general concept for tumor excision, the use of the Cavitron ultrasonic aspirator (CUSA) enables definition of the plane between the tumor and the adjacent cerebellar parenchyma. Dissection in this plane is performed, in general, prior to tumor debulking particularly in vascular tumors. This limits blood loss and improves the chance of complete excision particularly in low-grade astrocytomas.
Intraventricular tumors may be detached from the floor of the fourth ventricle via the CUSA except when direct tumor infiltration has occurred. In this case, resection should be stopped to avoid permanent neurological damage. The upper limit of the dissection of intraventricular tumors requires visualization of the aqueduct of Sylvius in general in order to confirm CSF flow reconstitution.
Once the tumor has been removed, the margins must be carefully inspected for residua.
7.5 Hemostasis
A critical part of the surgery is to achieve perfect hemostasis, which is done by coagulation of focal bleeding within the cavity of resection and local tamponade with cotton balls. If hemostasis cannot be achieved this way due to diffuse bleeding, hemostatic material such as fibrillary Surgicel® may be used. Hemorrhage into the ventricular system is to be avoided to minimize postoperative hydrocephalus, thus may be achieved by temporarily plugging the aqueduct of Sylvius with a small cottonoid. This should be removed when closing. Similarly the spinal subarachnoid space should be protected with temporary cotton pledgets.
7.6 Closure
A critical part of the surgery is dural closure. Opinions on whether the dura can be left open or not vary. As a principle we advise for watertight closure to prevent the formation of pseudomeningocele, to reconstitute normal CSF pathways, and to restire normal anatomy should reoperation be necessary. In our practice we use a 5.0 Prolen® continuous suture and a pericranial duroplasty when necessary. Once the dura is closed, we place a square of Surgicel® over the suture line.
The bone flap is repositioned and attached to the edge of the craniotomy with 3.0 vicryl sutures or plates, but in children we recommend the use of sutures to secure the bone flap.
Muscle layers are closed with 3.0 vicryl sutures in the deeper layers. It is important that the “dead space” left between the dura and the inferior part of the craniotomy near the foramen magnum is minimized during this closure. The fascia layer is closed with 3.0 vicryl sutures in interrupted sutures. Perfect closure of this layer is critical to prevent CSF leak through the wound. The subcutaneous layers are closed with 3.0 vicryl sutures and the skin with running 5.0 vicryl rapide. The wound is then cleaned; the hair of the patient washed and a trimmed primapore dressing placed over the wound. A crepe bandage is applied.
8 Postoperative Management
In our practice patients are awoken and extubated directly after surgery. During this transition period it is important to minimize patient coughing to minimize the risk of venous bleeding. Once extubated patients are observed in recovery for 1–2 h. Neurological observations and vital parameters are obtained frequently and regularly. Once patients have regained an adequate conscious state, they are transferred to the neurosurgical ward in the high dependency unit, for continuous monitoring and neurological observation. Hemoglobin, blood cell count, electrolytes, and the C-reactive protein are monitored in the immediate postoperative period and for the following days until normalization. In our experience monitoring in an intensive care unit is not routinely necessary. Antibiotics are maintained for 24 h. Pain is relieved if a morphine infusion via a pump or patient controlled anesthesia (PCA) is administered. The wound is checked regularly for signs of CSF leakage or infection. Patients are kept in the hospital until they have regained independent safe mobility and normal fluid and dietary intake, typically a period of 5–7 days.
9 Morbidities
Although safe in experienced hands, removal of a posterior fossa tumor in a child may still be associated with a range of minor to serious complications which can occur during the operative and perioperative periods.
Intraoperative morbidity can be due to a number of events including (1) significant bleeding in a highly vascularized tumor making it difficult to differentiate tumor, from adjacent important structures; (2) neurological impairment due to direct surgical trauma to the floor of the fourth ventricle, the medulla, or the lower cranial nerves particularly in tumors invading the fourth ventricular floor or extending into the cerebellomedullary and cerebellopontine cisterns, to the dentate nucleus leading to cerebellar mutism, or to the superior vermis leading to cerebellar ataxia; and (3) vascular injury to arterial brain stem perforators and PICA with resultant brain stem or cerebellar infarction.
Mild new postoperative neurological deficits, as temporary ataxia, mild diplopia, or mild facial hemiparesis, occur in about a third of the patient population and are reversible [21].
Postoperative complications may occur including the formation of a pseudomeningocele, CSF leakage through the wound, infection, meningitis, and persistent hydrocephalus.
10 Conclusions
The median suboccipital approach provided access to most posterior fossa tumors in the pediatric population. Different techniques can be used to perform the craniotomy and to remove the tumor. We have highlighted our goals and methods for this surgery. We feel that modern tools such as the high-speed drill, the ultrasonic aspirator, and a neuronavigation system should be used routinely to operate on these children in order to achieve maximum tumor removal and avoid neurological deficits to the patient.
References
Starr A (1893) Brain surgery. William Wood & Company, New York
Starr MA (1903) Organic nervous disease. Lea Brothers & Co, New York
Diller T (1909) The writings of Benjamin Franklin pertaining to medicine and the medical profession. Aesculapian 1(2):65–84
Yasargil (1996) Surgical approaches. In: Yasargil (ed) Microneurosurgery of CNS tumours. Thieme, Stuttgart, New York
Rhoton AL Jr (2000) Cerebellum and fourth ventricle. Neurosurgery 47(3 Suppl):S7–S27
Rhoton AL Jr (2000) The foramen magnum. Neurosurgery 47(3 Suppl):S155–S193
Rhoton AL Jr (2000) The posterior fossa veins. Neurosurgery 47(3 Suppl):S69–S92
Mussi AC, Rhoton AL Jr (2000) Telovelar approach to the fourth ventricle: microsurgical anatomy. J Neurosurg 92(5):812–823
Rhoton AL Jr (2000) The cerebellar arteries. Neurosurgery 47(3 Suppl):S29–S68
Hermann EJ, Rittierodt M, Krauss JK (2008) Combined transventricular and supracerebellar infratentorial approach preserving the vermis in giant pediatric posterior fossa midline tumors. Neurosurgery 63(1 Suppl 1):ONS30–ONS35; discussion ONS35–ONS37
Epstein FJ, Goh KC (2000) Ependymomas of the posterior fossa. In: Kaye AH, Black P (eds) Operative neurosurgery. Churchill Livingstone, London, pp 429–436
Kotil K et al (2008) Cerebellar mutism following posterior fossa tumor resection in children. Turk Neurosurg 18(1):89–94
Dailey AT, McKhann GM 2nd, Berger MS (1995) The pathophysiology of oral pharyngeal apraxia and mutism following posterior fossa tumor resection in children. J Neurosurg 83(3):467–475
Kellogg JX, Piatt JH Jr (1997) Resection of fourth ventricle tumors without splitting the vermis: the cerebellomedullary fissure approach. Pediatr Neurosurg 27(1):28–33
El-Bahy K (2005) Telovelar approach to the fourth ventricle: operative findings and results in 16 cases. Acta Neurochir 147(2):137–142; discussion 142
Deshmukh VR et al (2006) Quantification and comparison of telovelar and transvermian approaches to the fourth ventricle. Neurosurgery 58(4 Suppl 2):ONS-202–ONS-206; discussion ONS-206–ONS-207
Tanriover N et al (2004) Comparison of the transvermian and telovelar approaches to the fourth ventricle. J Neurosurg 101(3):484–498
Shimoji K et al (2009) Surgical considerations in fourth ventricular ependymoma with the transcerebellomedullary fissure approach in focus. Child Nerv Syst 25(10):1221–1228
Rajesh BJ et al (2007) Telovelar approach: technical issues for large fourth ventricle tumors. Child Nerv Syst 23(5):555–558
Greenberg ML, Hargrave D, Bond J (2004) Information needs for children and families. In: Walker DA (ed) Brain and spinal tumors of childhood. Arnold, London
Di Rocco C (1999) Cerebellar astrocytomas. In: Choux M (ed) Pediatric neurosurgery. Churchill Livingstone, London
Alexander JW et al (1983) The influence of hair-removal methods on wound infections. Arch Surg 118(3):347–352
Kurpad SN, Cohen AR (1999) Posterior fossa craniotomy: an alternative to craniectomy. Pediatr Neurosurg 31(1):54–57
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jenny, B., Maixner, W.J. (2015). Median Suboccipital Approach. In: Özek, M., Cinalli, G., Maixner, W., Sainte-Rose, C. (eds) Posterior Fossa Tumors in Children. Springer, Cham. https://doi.org/10.1007/978-3-319-11274-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-11274-9_8
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11273-2
Online ISBN: 978-3-319-11274-9
eBook Packages: MedicineMedicine (R0)