Abstract
In this paper, a fully automatic approach to select the regions of interest (ROIs) of the liver images and an automatic hierarchical procedure to characterize normal, fatty and heterogeneous livers, using textural analysis of liver ultrasound images are described. The proposed algorithm contains two stages. The first stage, automatically assigns some ROIs in a liver ultrasound. In the second stage, discrimination between heterogeneous, fatty and normal livers is performed in a hierarchical method. This stage, first, classifies focal and diffused livers and then discriminates fatty and normal ones. The wavelet packet transform is used to analyze liver texture and obtaining a number of statistical features. A support vector machine classifier is employed to classify three classes. The fully automatic scheme to select the ROIs with low computational cost and the hierarchical classification scheme outperformed the non-hierarchical one-against-all schemes, achieving an overall accuracy of 97.9 %.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Liver is a vital organ of body and is necessary for survival and moreover, there is no way to compensate for the absence of liver function, therefore, liver diseases have attracted much attention [1].
Liver diseases can be classified into two main categories. The first category is heterogeneous diseases that abnormalities are concentrated within a small area in one or both of the liver lobes while the rest of the liver tissue remains normal such as solid lesion and fluid lesions. The second category is Diffused or homogenous diseases that the disease is distributed over the whole liver tissues such as fatty liver [1–3].
Fatty liver disease is caused by the accumulation of fat tissue in the liver. The most common causes of fatty liver are obesity, alcoholism, high blood triglycerides, diabetes and hepatitis [2, 5–7].
The best way to diagnose fatty liver disease is liver biopsy. In biopsy, a small sample of tissue is taken from the liver using a needle [10]. This method is highly invasive and costly. However, medical imaging techniques such as Ultrasound (US), Computed Tomography (CT) and Magnetic Resonance Imaging (MRI), are used for examination and since US is non-radiological, noninvasive, inexpensive, easy to operate and portable it is the most preferred diagnostic method for fatty liver. Extract information from the fatty liver ultrasound images are based on the changes of scanned image intensity. Fatty liver causes an increase of echogenicity or brightness in the liver tissue [2].
In order to find a gold standard method to diagnose and detect fatty liver disease, some authors recently proposed methods and tools based on computer-aided diagnosis (CAD) to help the physicians and experts to detect and classify fatty liver from normal liver or heterogeneous one using ultrasound images [1–4, 6–9].
Liver segmentation and classification are the main steps of CAD system. There are several methods to segment a proper region of liver. Most of them are manual and a few others are semi-automatic and automatic. Manual approaches require medical experts to determine the Region Of Interest (ROI) before leaving to the computer for processing. Whereas, automated segmentation methods segment with minimal user input or without the need of any medical expert assistance. In [18], the ROI for each image in the patient database is selected manually in the training phase. The threshold value to generate extremely stable edge pattern for the template image is then learned and stored back to the database. In the test phase, the ROI is detected from a query image based on a representative template using generalized Hough transform (GHT) to map the edge pattern of a database template to the edge pattern of image. Authors of [6] proposed a method to extract ROI semi-automatically. In their method, a medical expert has manually extracted ROIs from the training ultrasound images and the final ROIs are selected based on Continuous Wavelet Transform (CWT) and one-class support vector machine (SVM) classifier. The aim of the CAD systems is to minimize the user intervention, therefore automatic approaches are preferred. However, the ROI selection in [6, 18] is semi-automatic which results in high computational cost. Another semi-automatic method to select ROI is proposed in [19], this method is used for the liver surface detection, based on an image processing procedure that decomposes the US images of the liver parenchyma into two fields: the speckle image containing textural information and the de-speckled image containing intensity and anatomical information of the liver. Features extracted from the liver contour detected in the de-speckled field. The detected contour distinguishes the liver anatomy from its neighbors and the selected region is used as ROI, however, an efficient ROI should not include hepatic vessels, bile stores and other anomalies, therefore the segmentation stage of this scheme may not be efficient due to lack of anomalies removal.
Several approaches have been proposed for feature extraction and classification in liver tissue based on ultrasound images. The most common features used for diagnosis of fatty liver are the first order and the second order statistical features based on texture analysis of the ultrasound images [11, 12]. Texture analysis approaches can be divided in two groups: one based on the relation of neighbor pixels in spatial domain such as the Gray Level Concurrence Matrix (GLCM) [4, 14, 15], the Gray Level Difference Statistical (GLDS) [15], the Gray Level Run Length Matrix (GLRLM) [4] and fractal parameters [16]; and one based on the analysis of transform coefficients such as Fourier Power Spectrum (FPS) [16], Discrete Wavelet Transform (DWT) [13, 17] and Wavelet Packet Transform (WPT) [6, 17, 21].
As mentioned above, a fully automatic and efficient CAD system includes automatic selection of ROI, efficient feature extraction method and effective classification approach. In our proposed method, the ROIs are selected completely automatic without the need of radiologist assistance. In this method some ROIs have been selected by partitioning the ultrasound image, inspired by the clinical practice, and classification is performed by the use of these ROIs. This procedure presents a considerable low computational cost. Then the WPT is employed to extract some statistical features. Finally, in the classification part, we proposed a novel classification strategy based on a hierarchical method. The first stage of this method classifies heterogeneous liver from others and in the second stage, discrimination of fatty and normal is performed.
The rest of this paper is organized as follows. Section 2 explains the main methodology. Section 3 introduced the dataset used in this work. Segmentation part and the procedure of selecting the ROIs are described in Sect. 4. Sections 5 and 6 study texture analyzer and feature extraction, respectively. Section 7 provides detailed information of classifiers. In Sect. 8 the results are presented and Sect. 9 concludes the paper.
2 Methodology
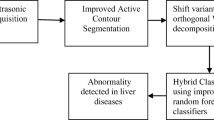
Block diagram of the proposed method is illustrated in Fig. 1. In this method after acquiring ultrasound images of liver, some optimum ROIs are selected in segmentation section. Then, WPT is applied to extract statistical features. Finally the hierarchical classification approach is employed.
3 Image Acquisition
The ultrasound images of 88 subjects contain 30 fatty, 39 normal and 19 heterogeneous liver images. The images are of size 560 × 450 and saved in bitmap format. All images were obtained using a Toshiba SSA 550 digital ultrasound imaging system at a 5 MHz frequency. This dataset is which has been used in [6] to enable us to have comparison results. Paper [6] is one of the most efficient and newest studies in classification of liver diseases and is the only study to discriminate fatty, normal and heterogeneous livers. Authors of [6] proposed an effective semi-automatic method to select a proper ROI in each ultrasound image.
4 Segmentation
The purpose of this section is to automatically select the proper regions of interest to perform accurate classification and diagnosis.
In the proposed method, as illustrated in Fig. 1, given ultrasound image of liver is first cropped to extract a wide region near the central lobe and the black region around the main part is removed. This is performed in order to reduce the computational cost of the process. Figure 2 shows a sample of the cropped image. Partitioning this wide region is performed in three levels. The wide region is partitioned to 9 equal size neighboring blocks without overlap in the first level. In order to utilize more information of image, overlapped blocks are also required. Therefore, in the second level, some other blocks formed at the intersection of each two blocks in each row. Thus, 6 blocks are generated which each one has 50 % overlap with each of two adjacent blocks. This process results in obtaining 15 overlapped blocks. The partitioning is continued at the intersection of each two blocks from 15 blocks in each column in the third level. Finally, 25 equal overlapped blocks are specified. Figure 3 shows this procedure. In order to find the proper ROIs, we select 64 × 64 regions in the center of each block, therefore, 25 ROIs of size 64 × 64 is obtained for each ultrasound image. However, using all the ROIs in classification is not efficient and increases the computational cost. Therefore, a \( \nu \) -linear support vector classifier (\( \nu - LSVC \)) [27] is employed as a preprocessing stage, and after classification process, 8 ROIs which have better results are selected. Finally, these selected ROIs are used in classification phase. The features which are used in this classification are explained in Sect. 6.
(a) The first level of partitioning (9 non-overlapped equal size blocks), (b) The second level of partitioning (the process of forming two overlapped blocks at the intersection of two previous blocks in each row), (c) The third level of partitioning (the process of forming two overlapped blocks at the intersection of two previous blocks in each column)
5 Wavelet Packet Transform (WPT)
Multi-resolution analysis feature extraction can be performed in the spatial domain which exists over small neighborhoods for example by using GLCM, GLDS and GLRLM, etc. Feature extraction in transform domain is performed over various scales by using multi-resolution schemes such as DWT and WPT. Using texture descriptors in transform domain is much more reasonable in the sense that human visual system processes images in a multi-scale way and scale is a dominant aspect for analysis of texture [20, 21].
In case of DWT, as only the low frequency subband is recursively decomposed, it may not be efficient for texture characterization as most significant texture information usually appears in the middle and high frequency bands [23]. The wavelet packet transform, in comparison, decomposes the detailed information of the image in the high frequency bands [22, 23]. The WPT tree up to second level of decomposition results in 16 subbands. Therefore, 4 and 16 subbands are obtained at the first and the second level of decomposition, respectively, that result totally in 20 subbands. The desired features are extracted from these 20 subbands as well as original ROIs.
6 Feature Extraction
The extracted features from WPT coefficients are Median, standard deviation and interquartile range. Median of the image shows the numerical value of intensity separating the higher half of pixel intensities in a window of the image, from the lower half. The median value of intensity in ultrasound fatty liver images is higher than the intensity of normal ones due to their increased echogenicity caused by fat accumulation [6]. This characteristic also exists in WPT subbands of ultrasound fatty liver images. Standard deviation shows the variation from the mean. The interquartile range measures the dispersion and is the difference between the upper and lower quartiles. The standard deviation and the interquartile range of the image represent the regularity or smoothness of the ultrasound texture. These two features may be good indicators to distinguish ultrasound images of focal and diffused diseases of liver.
As a spatial domain feature, the ratio of the maximum to the minimum value in the original image is computed the same as [6]. Therefore, by calculating the three explained features from each of 20 subbands and one feature from the original ROI image, the total number of 61 features is obtained.
7 Classification
In this section, it is aimed to discriminate normal, fatty and heterogeneous livers. To reach this aim, a hierarchical classification scheme is proposed. A block diagram of this scheme is illustrated in Fig. 4. The proposed hierarchical classification scheme is organized in two steps. The first step distinguishes heterogeneous liver from others and classification of fatty and normal livers is performed in the second step.
As already mentioned, in normal case, both lobes of liver are homogeneously clear and in fatty case, as it is a diffused disease, at least one lobe is accumulated homogeneously by fat, however, heterogeneous case is focal and a small region of liver is only affected. Therefore, the fatty and normal cases can be placed in the diffused category and the heterogeneous case placed in the focal category. In order to have a hierarchical scheme, in the first classification step, focal case is discriminated from diffused case, if a liver is classified as diffused in the first step, discrimination of fatty and normal is attempted in the second step.
For the classification in each step, the SVM classifier is implemented. The purpose of SVM is to find a decision plane that has a maximum distance (margin) from the nearest training pattern [24, 25]. In order to perform this aim, SVM maps the feature vector to a higher-dimensional space. In this space the SVM finds a hyperplane to separate the two classes with a decision boundary set by support vectors [24, 25]. An appropriate kernel function can reduced the high computational cost of mapping process. In this paper, a binary SVM classifier is adopted using the polynomial kernel.
8 Implementation Results
The first part of implementation is selecting ROIs which is performed as a fully automatic segmentation. The simplicity and reduction of computational cost of this method in comparison with the semi-automatic method of selecting ROI in [6], without degrading the classification performance, is noticeable. After selecting proper ROIs, classification is performed. At the first step of classification, the diffused and focal classes are denoted as class 1 and 2, respectively and at the second step, repeatedly, the fatty class is marked as class 1 and the normal one as class 2.
This experiment contains three tests over automatically selected ROIs and a comparison with the results of [6]. The first test is performed on only the 9 non-overlapped blocks. These blocks have the size of 76 × 84 pixels. 9 ROIs are formed by selecting 64 × 64 pixel regions at the center of each block. Each of these 9 ROIs are examined by SVM classifier, therefore, the total number of 9 class labels are obtained for each liver image. The performance of the SVM classifier is evaluated by means of leave one out cross validation (LOOCV) method, same as the method used in [6] to have a fair comparison with its results. Furthermore, this method is useful in cases with small amount of available data, as normally observed in medical problems. In LOOCV method, one case is left out as the testing set and the rest of the data is used as the training set. This process is repeated so that each case is given a chance as the testing case [26].
The performance of the algorithm was investigated by the following measures:
-
(1)
Sensitivity (Se):
$$ Se = \frac{TP}{TP + FN} $$ -
(2)
Positive Predictive Value (PPV):
$$ PPV = \frac{TP}{TP + FP} $$ -
(3)
Accuracy (Acc):
$$ Acc = \frac{TN + TP}{TN + TP + FN + FP} $$
TP (True Positives): TP is the number of instances of each class which have been correctly labeled to belong to that class [6, 28]. TN (True Negative): TN is the number of instances of other classes that have been labeled to belong to their class [6, 28]. FN (False Negatives): FN is the number of instances of each class which have been labeled to belong to any other class [6, 28]. FP (False Positives): FP is the number of instances of other classes that have been labeled to belong to a specified class [6, 28].
Tables 1, 2 and 3 illustrate the sensitivity and positive predictive value of each class in each step and the accuracy of each step using leave one out cross validation method of first, second and third tests, respectively. As these three Tables show, the sensitivity and the positive predictive value of each class and the accuracy of each step are increased by increasing the number of blocks. This fact shows the importance of selecting appropriate ROIs in diagnosing the diseases. The ROIs are placed at the center of blocks and cover 65 % of each one. Therefore, decreasing the number of blocks results in losing the regions around the edges. These regions may contain useful texture information which can be applied in the classification process by considering the overlapped blocks.
According to the results in Table 3, by considering 25 ROIs, the proposed method gives the sensitivity of 100 % for detecting both heterogeneous and fatty liver at the first and the second steps which shows the complete detection and quite correct diagnosis of these diseases. The high values of PPV given in this Table, also depicts the high precision of this method. Furthermore, we have achieved the overall accuracy of 97.9 % which is higher compare to the approach of [6] with overall accuracy of 95.4 % in the same condition. In addition, the method of selecting ROIs in the proposed scheme is completely automatic with lower computational cost than the method of [6]. In [6] the segmentation process is semi-automatic with high computational complexity and moreover, their classification method is not such a hierarchical scheme.
9 Conclusion
In this paper, an automatic segmentation and classification method to discriminate normal, fatty and heterogeneous liver images is proposed. The proposed algorithm is performed in two stages. The first stage, automatically selects some ROIs in a liver ultrasound. The WPT is applied to the selected ROIs as a multi-scale texture analyzer to extract some statistical features. In the second stage, a hierarchical binary classification method using SVM classifier is employed. The proposed hierarchical classification algorithm discriminates the heterogeneous case from the diffused case at the first step and classifies the fatty and the normal cases at the second step. The overall accuracy of 97.7 % indicates the efficiency of the hierarchical classification scheme. The implementation results illustrate the suitability of the proposed system to be used in a clinical environment to help radiologists in liver disease classification and improve diagnostic accuracy which can avoid biopsies in some cases. The completely automatic scheme to select the ROIs with the noticeable low computational cost is the other advantage of this system.
References
Suganya, R., Rajaram, S., Classification of liver diseases from ultrasound images using a hybrid kohonen SOM and LPND speckle reduction method. In: 2012 IEEE International Conference on Signal Processing, Computing and Control (ISPCC), pp. 1–6 (2012)
Icer, S., Coskun, A., Ikizceli, T.: Quantitative grading using grey relational analysis on ultrasonographic images of a fatty liver. J. Med. Syst. 36, 2521–2528 (2012)
Kadah, Y.M., Farag, A.A., Zurada, J.M., Badawi, A.M., Youssef, A.M.: Classification algorithms for quantitative tissue characterization of diffuse liver disease from ultrasound images. IEEE Trans. Med. Imag. 15(4), 466–478 (1996)
Andrade, A., Silva, J.S., Santos, J., Belo-Soares, P.: Classifier approaches for liver steatosis using ultrasound images. Procedia Technol. 5, 763–770 (2012)
Radu, C., Grigorescu, M., Lupsor, M., Vicas, C., Nedevschi, S., Badea, R., Grigorescu, M.D., Sparchez, Z., Crisan, D., Feier, D.: The diagnostic performance of attenuation coefficient computed on the ultrasound image compared to a biochemical marker - SteatoTest - for steatosis quantification in non-alcoholic fatty liver disease. In: 2010 IEEE International Conference on Automation Quality and Testing Robotics (AQTR), vol. 2, pp. 1–5 (2010)
Afsar Minhas, A., Sabih, D., Hussain, M.: Automated classification of liver disorders using ultrasound images. J. Med. Syst. 36, 3163–3172 (2012)
Mihailescu, D.M., Gui, V., Toma, C. I., Popescu, A., Sporea, I.: Automatic evaluation of steatosis by ultrasound image analysis. In: 2012 10th International Symposium on Electronics and Telecommunications (ISETC), pp. 311–314 (2012)
Yang, Y., He, Q., Hu, C., Liao, J., Meng, M.: Method of edge energy template for detection of ultrasonic based fatty liver. In: 2010 3rd International Congress on Image and Signal Processing (CISP), vol. 2, pp. 994–998 (2010)
Moldovanu, S., Moraru, L., Bibicu, D.: Computerized decision support in liver steatosis investigation. Int. J. Biol. Biomed. Eng. 6, 69–76 (2012)
Ceylan, R., Ceylan, M., Ozbay, Y., Kara, S.: Fuzzy clustering complex-valued neural network to diagnose cirrhosis disease. Expert Syst. Appl. 38, 9744–9751 (2011)
Wun, Y.T., Chung, R.: Ultrasound characterization by stable statistical patterns. Comput. Methods Prog. Biomed. 55(2), 117–126 (1998)
Badawi, A.M., Derbala, A.S., Youssef, A.B.M.: Fuzzy logic algorithm for quantitative tissue characterization of diffuse liver diseases from ultrasound images. Int. J. Med. Inf. 55(2), 135–147 (1999)
Yeh, W.C., Jeng, Y.M., Li, C.H., Lee, P.H., Li, P.C.: Liver fatty change classification using 25 MHz high frequency ultrasound. In: 2004 IEEE Ultrasonics Symposium, vol. 3, pp. 2169–2172 (2004)
Mukherjee, S., Chakravorty, A., Ghosh, K., Roy, M., Adhikari, A., Mazumdar, S.: Corroborating the subjective classification of ultrasound images of normal and fatty human livers by the radiologist through texture analysis and SOM. In: International Conference on Advanced Computing and Communications, 2007, ADCOM 2007, pp. 97–202. IEEE (2007)
Yali, H., Han, X., Tian, X., Zhao, Z., Zhao, J., Hao, D.: Texture analysis of ultrasonic liver images based on spatial domain methods. In: 2010 3rd International Congress on Image and Signal Processing (CISP), vol. 2, pp. 562–565 (2010)
Singh, M., Singh, S., Gupta, S.: A new measure of echogenicity of ultrasound images for liver classification. In: 2011 24th Canadian Conference on Electrical and Computer Engineering (CCECE), pp. 317–320 (2011)
Ribeiro, R., Marinho, R.T., Sanches, J.M.: Global and local detection of liver steatosis from ultrasound. In: Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. pp. 5547–5550 (2012)
Wu, Y.H., Lo, Y.C., Cheng, S.C., Lin, C.L.: Adaptive ultrasound image matching for automatic liver disease diagnosis using generalized hough transform. In: 2010 Sixth International Conference on Intelligent Information Hiding and Multimedia Signal Processing (IIH-MSP). IEEE (2010)
Ribeiro, R., Marinho, R., Velosa, J., Ramalho, F., Sanches, J.M.: Diffuse liver disease classification from ultrasound surface characterization, clinical and laboratorial data. In: Vitrià, J., Sanches, J.M., Hernández, M. (eds.) IbPRIA 2011. LNCS, vol. 6669, pp. 167–175. Springer, Heidelberg (2011)
Daugman, J.: An information-theoretic view of analog representations in the striate cortex. In: Schwartz, E.L. (ed.) Computational Neuroscience. MIT Press, Cambridge (1990)
Virmani, V., Kumar, V., Kalra, N., Khandelwal, N.: SVM-based characterization of liver ultrasound images using wavelet packet texture descriptors. J. Digit. Imag. pp. 1–14 (2013)
Gao, R.X., Yan, R.: Wavelet packet transform. In: Wavelets, pp. 69–81. Springer, US (2011)
Chang, T., Kuo, C.C.J.: Texture analysis and classification with tree structured wavelet transform. IEEE Trans. Image Process. 2(4), 429–441 (1993)
Sudha, S., Suresh, G.R., Sukanesh, R.: Speckle noise reduction in ultrasound images using context-based adaptive wavelet thresholding. IETE J. Res. 55(3), 135–143 (2009)
Duda, R.O., Hart, P.E., Stork, D.G.: Pattern Classification, 2nd edn. Wiley-Interscience, New York (2000)
Cawley, G., Talbot, N.: Efficient leave-one-out cross-validation of kernel fisher discriminant classifiers. Pattern Recogn. 36, 2585–2592 (2003)
Chen, P.H., Lin, C.J., Scholkopf, B.: A tutorial on ν-support vector machines. Appl. Stoch. Models Bus. Ind. 21(2), 111–136 (2004)
Zhu, W., Zeng, N., Wang, N.: Sensitivity, specificity, accuracy, associated confidence interval and ROC analysis with practical SAS® implementations, In: NESUG proceedings: health care and life sciences, Baltimore, Maryland, pp. 1–9 (2010)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this paper
Cite this paper
Owjimehr, M., Danyali, H., Helfroush, M.S. (2014). Diagnosing of Fatty and Heterogeneous Liver Diseases from Ultrasound Images Using Fully Automated Segmentation and Hierarchical Classification. In: Movaghar, A., Jamzad, M., Asadi, H. (eds) Artificial Intelligence and Signal Processing. AISP 2013. Communications in Computer and Information Science, vol 427. Springer, Cham. https://doi.org/10.1007/978-3-319-10849-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-10849-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10848-3
Online ISBN: 978-3-319-10849-0
eBook Packages: Computer ScienceComputer Science (R0)