Abstract
Boron (B) is a vital nutrient for plant growth and metabolism. Lack of B in plant tissues causes reductions in crop yields, whilst an excess supply of B may also seriously damage plant tissues and sometimes leads to plant death. Appropriate amounts of B in plants are crucial for normal growth and it significantly increases seed germination and seedling growth. Moreover B has positive effects on the uptake and utilization of other nutrients at the whole plant level and it may improve nutrient use efficiency (NUE) and nutrient demand and supply (NDS). NUE mainly reflects efficiency of extraction of mineral nutrients from soil along with their integration and recycling, whereas NDS nutrient shows how efficiently plant can fulfil the demand and supply rate of required nutrient at different stages and conditions of plant life cycle. Despite a substantial existing literature, the understanding of B interactions with other nutrients remains unclear.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Boron
- NUE (nitrogen use efficiency)
- Soil interactions
- Deficiency
- Reproductive growth
- Photosynthesis
- Nutrient interactions
Introduction: Boron in the Soil and in the Plant
Boron belongs to the metalloid elements having properties of both metals and non-metals (Marschner 1995). There is very low abundance of B in nature (Kot 2009) but it is broadly distributed in all the layers of the soil. B abundance range in rocks averages about 10–20 mg B kg−1. In sea water it can range from 1 to 10 mg B kg−1 and as far as river is concern the B concentration is about 1/350 that of sea water (Power and Woods 1997).
Warington (1923) established the requirement of B for plant growth and functioning, and many recent reports suggest an essentiality of B for all vascular plants. B deficiency or toxicity affects various metabolic and physiological processes (Blevins and Lukaszewski 1998; Bolaños et al. 2004). Less than 10 mg kg−1 B in the soil is considered to be a B deficient soil (Woods 1994). Moreover, most of this B is in a bound form in rocks and is not readily available to plants. Boric acid is the most common form of B liberated during weathering of rocks (Nable et al. 1997) and is easily absorbed by plant roots, but this represents only 10 % of the total B in the soil (Power and Woods 1997).
Soil pH, texture, temperature, and organic matter affect soil B availability; among these the soil pH is one of the most significant characteristics for B availability (Goldberg 1997). Boric acid is a very weak acid and when the pH is below 7, it appears in its undissociated form; at alkaline pH, boric acid dissociates to form the borate anion:

Therefore, at common soil pH values (5.5–7.5), B exists mainly as soluble uncharged boric acid (B(OH)3), and in this form B is absorbed by plant roots (Hu and Brown 1997; Power and Woods 1997; Sathya et al. 2013). In flooded conditions, B is easily solubilised and leached resulting in a deficiency of B for growing plants.
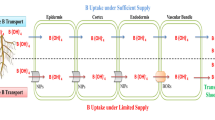
A plant requirement for B varies between species and is dependent on the conditions in which they grow. The B requirement for one plant species may be toxic or deficient for other species (Blevins and Lukaszewski 1998). There are three main groups of plants based on B requirements, one consists of graminaceous species, a second are monocotyledons and a third are dicotyledons and lactifers, having minimum, moderate and high B demands, respectively (Blevins and Lukaszewski 1998; Goldbach et al. 2001). It is necessary to study mechanisms of B uptake and transport, as well as translocation in plant systems, to optimise agricultural production. For example, B deficiency results in major disorders, which may decrease plant growth in soil with excess water. Molecular mechanisms involved in B transport from the soil to root cells and xylem have two different routes. Transport of B is dependent mainly on its availability and it is mostly transported into the plant from the soil by passive diffusion, which is possible when adequate B is available. When B availability is low or under deficiency conditions, uptake is facilitated by active transport, which requires energy.
B absorbed by roots must be delivered to the xylem for further transport around the plant. When there is sufficient B availability, absorbed B moves by passive diffusion involving MIPs channels (Dannel et al. 2002; Miwa and Fujiwara 2010). In limited or deficient B conditions, transport of B towards the xylem is facilitated via a specific B transporter (BOR), which is an energy dependent transport process (Fig. 6.1). Takano et al. (2002) had identified such a transporter of B under limiting conditions, known as BOR1 in Arabidopsis. Subsequently, BOR1-like genes have been reported in Eucalyptus (Domingues et al. 2005) and in rice (Nakagawa et al. 2007). Expression of two genes, NIP5;1 and BOR1, are decreased by transcriptional and post-translational regulation respectively, under sufficient B supply (Miwa and Fujiwara 2010; Yang et al. 2013).
After loading in the xylem, B is transported to the shoot through the transpiration stream (Wimmer and Eichert 2013; Bogiani et al. 2014). Transport of B through phloem was also reported, and such transport differs between species (Brown and Hu 1996; Brown and Shelp 1997; Ganie et al. 2013). In certain plants, B is transported and translocated to reproductive and vegetative tissue via the phloem (Matoh and Ochiai 2005). It is also suggested that there is a formation of a boron-diol complex involving a sugar alcohol, which acts as the transport molecule (Brown and Hu 1996; Hu et al. 1997). Transgenic tobacco and rice with enhanced sorbitol production had a higher ability to transport B through the phloem towards the plant shoot (Brown et al. 1999; Bellaloui et al. 2003). Plants that produce sugar alcohols like sorbitol and trehalose, have the ability of B transport via the phloem, whereas plants without any production of sugar alcohols have no such transport system (Stangoulis et al. 2001; Takano et al. 2001; Matoh and Ochiai 2005).
Role of Boron in Plant Functioning
Since the beginning of twentieth century, B has been considered to be an important micronutrient for plant growth, but there are very few records regarding its actual biochemical role. Deficiency of B is common all over the world, which has an important agronomic impact (Gupta 1979). In soils with high percolatory water, B is easily leached downward in the soil and hence it is not readily available for plants (Blevins and Lukaszewski 1998). Adequate B nutrition is required for high production as well as for quality of crops. B deficiency results in biochemical, metabolic and physiological abnormalities, and causes diverse disease symptoms in plants and hence adequate supply is a critical challenge for plant nutrition.
In the last 10 years, several roles have been demonstrated for B in plant function, including as a cell wall component, an involvement in membrane structure and integrity, and involvement in metabolic process (Bolaños et al. 2004). To date, one of the most accepted roles of B in plant physiological function is the formation of an ester with one apiose residue of rhamnogalacturonan ІІ (RG II) in the cell wall (Kobayashi et al. 1996), which is essential for maintaining cell wall permeability and also for rigidity (Fleischer et al. 1999; Ryden et al. 2003). Moreover, B deficiency led to a decrease of gene transcription of various hydrolytic enzymes such as xyloglucan endotransglucosylase/hydrolases (XTHs), expansins, pectin methylesterases, and pectin lyases in Arabidopsis roots (Camacho-Cristóbal et al. 2002). These enzymes play key roles in cell wall loosening, necessary for cell elongation (Cosgrove 1999). Camacho-Cristobal et al. (2011) suggested an influence of B in transcriptional level regulation of genes, which are responsible for cell wall synthesis and its modification.
Many research reports suggested roles of B in plasma membrane transport processes, and in membrane integrity by cross-linking the membrane molecules containing hydroxlated ligands such as glycoproteins and glycolipids (Goldbach et al. 2001; Wimmer et al. 2009). The membrane potential in Daucus carota is changed under limited B (Blaser-Grill et al. 1989) and activity of the proton-pumping ATPase was reduced in Helianthus annuus roots (Ferrol and Donaire 1992). Similarly, it has been reported that B deficiency alters plasma membrane permeability for ions and other solutes (Wang et al. 1999; Carmen Rodriguez Hernandez et al. 2013). The impact of B on ion fluxes can be mediated by direct or indirect effects of B on plasma membrane-bound proton-pumping ATPase (Cara et al. 2002). The activity of the K+ stimulated ATPase in B-deficient maize roots was considerably lower than in control plants (Pollard et al. 1977). These results indicated that the action of B could be associated with membrane components. It is still unclear whether B directly interacts with membrane proteins or indirectly modifies membrane properties with subsequent changes in enzymatic activities.
The literature indicates possible roles of B in several other metabolic functions. For instance, it has been shown that B deficiency causes qualitative and quantitative changes in phenol metabolism (Pandey and Archana 2012; Hajiboland et al. 2013). Additionally B deficiency affects nitrogen metabolism (Bolaños et al. 1994). B-deficient plants showed lower nitrate reductase activity and enhanced amounts of nitrate; these observations strongly suggest a role of B in the de novo synthesis of the nitrate reductase protein or facilitation of nitrate absorption (Ruiz et al. 1998).
Effect of B on Photosynthesis
Photosynthesis is a complex series of reactions that culminate in the reduction of carbon dioxide. The effect of B nutrition on photosynthetic processes has been rarely studied. Decreases in B supply reduce soluble proteins and chlorophyll in leaves, which are important constituents for Hill reactions and photosynthesis (Mukhopdhyaya et al. 2013).
There was no direct involvement of B on rate of photosynthesis, but from recent research it is evident that B has a positive effect on photosynthesis under normal (optimum) level. Moreover, there are reports of increases in chlorophyll pigment and carotenoids by foliar spraying of B leading to increases in photosynthetic rate (Thurzo et al. 2010). Ganie et al. (2013) also showed that net photosynthetic rate was increased due to increases in plant light harvesting pigments such as chlorophyll and carotene in the leaves.
B deficiency negatively affects photosynthesis by decreasing photosynthetic oxygen evolution rates and hence the efficiency of photosystem II (Kastori et al. 1995; El-Shintinawy 1999). Photosynthetic rate (P n ) drastically decreased in cotton plants when grown in B deficient soil (Zhao and Oosterhuis 2002). It was reported that inhibition of photosynthesis was a result of reduced Hill reactions and low intercellular CO2 concentrations (Sharma and Ramchandra 1990). Some experimental evidence indicated close relationships between gas exchange parameters and B deficiency, implying that these parameters were possibly affected by external B supply and in turn prejudiced growth. Previous studies, under B deficiency, supported a change in photosynthetic enzyme activities that were undoubtedly involved in a decrease in P n (Sharma and Ramchandra 1990). B deficiency was followed by reduction in the leaf stomatal conductance (g s ) and rate of photosynthesis (Huang et al. 2005; Han et al. 2008). Related declines in plant g s at B deficiency were initiated by high oxidative damage in leaves (Huang et al. 2005). Moreover, low g s also decreases E (Han et al. 2008). It was advocated that the presence of free hexoses can elicit regulation of the Calvin cycle and, hence, can obstruct P n (Han et al. 2008). A diminution in pigments under B deficiency was shown for citrus (Han et al. 2008). The synthesis of unnecessary starch possibly disrupts chloroplast structure, leading to poorer CO2 assimilation and decreased chlorophyll content (Han et al. 2008). It is likely that B deficiency caused changes in chloroplast structure, which eventually affected pigment content (Pandey and Pandey 2008). B deficiency may also affect photosynthetic responses by the modifications in the structure and function of chloroplast thylakoids.
Effect on Reproductive Growth
According to Loomis and Durst (1992) B is essential for generative growth. Boron is involved in metabolism of carbohydrates and phenolic acids, which are crucial for growth of pollen tubes. Decreases in crop propagative yield or seed/fruit quality in low B soils can be due to diminished reproductive development early or late in the flowering/fruiting cycle. It has often been seen that reproductive growth, mainly flowering, fruit and seed set and seed yield, is particularly sensitive to B deficiency compared to asexual growth (e.g. Woodbridge et al. 1971; Dear and Lipsett 1987; Noppakoonwong et al. 1997). Likewise, substantial yield decreases can arise without expression of indications of deficiency during prior somatic growth. B also plays an important role in synthesis and metabolism of nucleic acids (Hundt et al. 1970).
As described above, B is responsible for enhancing chlorophyll content and rate of photosynthesis (P n ), as well as inducing dry matter production in plants, and therefore may result both in enhancing flowering and also the transport of photosynthetic products to reproductive stages, ultimately leading to yield improvement (Du Ying Qiong et al. 1999).
Factors influencing the impact of a low external B supply on sexual reproduction in flowering plants are likely to include: the capacity of roots to obtain B from soil (Hu and Brown 1997); the mobility of B in the phloem (Brown and Shelp 1997); the relative sink size in floral parts for photosynthate; the capacity to redistribute B from vegetative tissues to reproductive organs; the rate of transpiration by floral organs; the functional necessity for B in reproductive tissues; and the distribution and richness of B-binding compounds in the apoplastic pathway between the vein endings and the most distal floral tissue. The B requirement for flowering is indicated by the sensitivity of pollen development to low B and the generally high concentrations of B that occur in reproductive parts of the flower. Under conditions of low external B supply, levels in the anthers and pistils do not decline to the low levels measured in leaves. B concentrations are higher in the stamen than in the pistil. The physiological roles for B in sexual reproduction have yet to be fully defined and there is a need for experimentation in this area.
Many of the studies that have been undertaken do not give definitive information as to whether plants were critically deficient in B at the time of flowering, or the B status of floral tissues was not determined at the time of impairment in cellular development/function, or cell structure and metabolism were examined long after the primary effect of B took place. An example is the observation that B deficiency results in male sterility, a condition that can be induced by deficiencies in other nutrients (e.g. Mn, Cu) or unfavourable environmental conditions (e.g. water deficit, high/low temperature). These observations reveal nothing about the processes that are being affected by low B supply and do not enable us to conclude whether the effect of low B supply on male sterility is a direct or indirect event. As root function is greatly impaired in severely B-deficient plants in containers and this can impact on whole plant physiology, the requirement of pollen development for B should be studied under conditions of controlled external supply.
Flowering Response
There is no evidence that B deficiency prevents initiation of or delays floral development. When plants are grown without B or are transferred into B-free nutrient solutions in pot experiments, the apical meristems may abort and therefore flowers do not develop, as shown for peach (Prunus persica; Kamali and Childers 1970). However, under field conditions, where B deficiency stresses occur more gradually, plants may have time to adapt to deficiency. For example, in peanut (Arachis hypogaea), the flowering period was extended in B-deficient plants (Harris and Brolman 1966), resulting in low-B plants producing as many flowers as B-adequate plants. In species where the flowers occur in compact inflorescences, and these are terminal on the stem (e.g. sunflower, wheat), low B has a greater impact on reproduction because the plant has less ability to modulate reproductive growth than species with axillary inflorescences and indeterminate growth. The first group of plants is more prone to pollen sterility under low B than the latter group of plants. Experiments on wheat by Li et al. (1978), suggested that absorbed B was transported from soil to floret and spikelet organs, which resulted in accumulation of B in seeds. Finally, low B can result in plants being functionally male sterile (e.g. wheat, Li et al. 1978; rice, Garg et al. 1979; barley, Simojoki 1972), although cases of female sterility have been reported (e.g. maize, Vaughan 1977; avocado, Coetzer and Robbertse 1987). The external B supply does not appear to alter the frequency of unisexual flowers, although some workers have attempted unsuccessfully to do this by applying B sprays (Singh 1994).
Effect of B on Nutrient Use Efficiency, Demand and Supply
Nutrient use efficiency (NUE) may be expressed as productivity of the plant per content of applied nutrients. Enhancement of NUE is vital for improvement of crop production in marginal lands with poor nutrient availability. NUE for plants is reliant on the ability to efficiently take up nutrients from the soil, but also on translocation, storage and usage within the plant, and on the environment (North et al. 2009). NUE is largely dependent on nutrient availability in the soil or applied medium. Nutrient demand and supply (NDS) is how efficiently the plant can fulfil the demand and supply rate for required nutrients at different stages and conditions of plant growth and development. Under limiting conditions of nutrients, plants showed decreases in nutrient uptake compared to sufficient nutrient conditions (preserving the nutrition for future demand).
Large variations in defining nutrient efficient plants and methods used in calculating nutrient use efficiency, makes it difficult to compare results of different studies. The effort to measure yield response to an applied nutrient is further confounded by other factors, such as variable soil fertility levels, climatic conditions, crop rotations, and changes in production practices that affect nutrient use efficiency (Stewart et al. 2005). In simple terms, efficiency is the ratio of output (economic yield) to input (fertilisers) for a process or complex system (Crop Science Society of America 1992).
Plant nutrients rarely work in isolation. Interactions among nutrients are important because a deficiency of one restricts the uptake and use of another. Figure 6.2 shows interactions of major plant nutrients with each other (Khan Towhid Osman 2013). Numerous studies have demonstrated that interactions between B and other nutrients, primarily N, P and K, impact crop yields and nutrient efficiency.
Mulder’s chart shows some of the interactions between plant nutrients. Interaction: A decrease in availability to the plant of a nutrient by the action of another nutrient (see direction of arrow). Stimulation: An increase in the need for a nutrient by the plant because of the increase in the level of another nutrient (dotted line)
For example, for experiments carried out in our research group on groundnut it was evident that uptake of almost all macro and micronutrients by straw and seeds showed a linear relationship ( p < 0.05) among the different nutrients, which were absorbed by roots. B at a level of 1 kg ha−1 resulted in significant uptake of macro and micronutrients from soil to seed and straw. Data strongly support improvement of NUE as well as NDS in groundnut at 1.0 kg ha−1 B supply. Other levels of B treatment did not significantly increase the uptake of nutrients in groundnut. The same trend of nutrient uptake by plants at different levels of applied B has also been reported by Nadia et al. (2006) and 200 ppm B sprayed on groundnut plant showed an increase in uptake of N, P, K and Fe, Mn and Zn. The highest value of yield and yield components were received from the plant treated with 200 ppm B (Nadia et al. 2006). Leaf venation, xylem stream, and transpiration are the primarily factors involved in the accumulation of B in leaves (Oertli and Richardson 1970; Shelp and Shattuck 1987).
These results are similar to the observations of McIlrath et al. (1960). It has been suggested that selected nutrients in the soil have antagonist and/or synergistic effect on the uptake of other nutrients by roots of developing plants (Malvi 2011). Moreover, B interactions, either synergistic and/or antagonistic, may affect plant nutrition under both deficiencies as well as in toxic conditions (Tariq and Mott 2007).
Deficiency, sufficiency and toxicity of B may exert an effect on mineral nutrient content of plants but such an interaction has not been well studied or reported. The results of many reports in this direction are conflicting, which may be due to different experimental systems with different crop plants and varieties (Lombin and Bates 1982; Mozafar 1989). B is directly or indirectly involved in many physiological and biochemical processes and may affect other plant nutrients (Bolaños et al. 2004). Therefore, one might expect relationships between B and other nutrient utilization to be very complex. Examples of effects of B on availability and uptake of plant nutrients other than B are described below.
Parks et al. (1944) were the first researchers who reported that with graded B levels, the concentrations of NH4-N, NO3-N, Org-N, P, K, Ca, Mg, Na, Zn, Cu, Fe, Mn, Mo and B were altered in the tomato leaflets as much as several-fold. In addition, they stated that B supply had specific effects, and the trends found were completely dissimilar with respect to different elements. In the absence of B, the concentrations of N, K, Ca, Mg, Na, Cu and Mn in tobacco leaves were increased and the concentrations of P, Fe and Al were decreased as compared to plants fed with a B adequate nutrient solution (Steinburg et al. 1955).
Baker and Cook (1959) reported that P, K and Mg were higher and Ca was lower in severely B deficient alfalfa plants compared to healthy plants, perhaps due to the dilution effect which occurred in the healthy plants. In increasing B conditions, the concentration of Cu, Fe, Mn, Mo, and B were increasing in perennial fodder grass, but the reverse trend occurred in the case of uptake and ash content for micronutrients accept B (Mcllarth et al. 1960). Cu and K content in grass showed highly significant positive correlations, while Ca and Mg contents showed negative correlations with B contents for 98 grasses at the flowering stage when grown in increasing B nutrition (Tolgyesi and Kozma 1974). Touchton and Boswell (1975) observed that P, K, Ca, Mg, Na, Zn, Cu, Fe, Mn, Mo and Al concentrations varied slightly with location, but were not affected by the method or rate of B application. Only the B concentration in tissues was significantly increased with regard to method rate and location. Increasing B nutrition enhanced phytotoxicity and some interactions among nutrients due to increased concentrations of Zn, Cu, Fe and Mn in the leaves, stem and roots of bush bean plants (Wallace et al. 1977). But contradictory results were reported by Leece (1978), who observed that with high levels of applied B, the concentrations of N, P, K, Ca, Zn, Cu, Fe and Mn (not Mg) in maize crop were depressed. The reverse results were obtained when no B was applied. Increasing B supply in soil resulted in the decrease of leaf N and P in tomato, suggesting B antagonism. The contrary was the case with a B effect on leaf K, Ca, Mg and Na (Aduayi 1978). Yadav and Manchanda (1979) noted that with an increase in the B content of soil, Ca and Mg concentration in wheat and Gramineae crops significantly decreased, whereas N, P and K contents were significantly increased.
Moreover, with differential supply of B in nutrient solution, the concentration of Fe, Mn, P and Ca in the shoots and roots of tomato increased, and B reduced the translocation of Mn, P and Fe whilst Ca remained unchanged (Alvarez-Tinaut et al. 1980). Addition of B in the nutrient solution decreased the absorption of N, P Ca, Mg and B, induced K accumulation, while Na remained unaffected in lamina stem and roots of Cabernet sauvignon wine plants (Downton and Hawker 1980). Gomez-Rodriguez et al. (1981) found a highly significant inverse correlation between B and Mn concentrations in leaves of sunflower, while Cu, Fe and Zn concentrations were not changed by different B levels in the nutrient solution. Marked reductions in Fe and Mn adsorption, but an increase in Zn uptake were recorded in bean plants grown in B deficient medium. The transport of Fe, Mn and Zn was increased in the trifoliate leaves, while that in shoots was reduced. It appears that, B is involved in the physiological processes controlling uptake and transport of nutrients like Mn, Fe and Zn (Dave and Kannan 1981).
Lombin and Bates (1982) found that with increasing B levels, the uptake of K, Mn, Zn, Cu, Mo and B was increased in alfalfa, peanut and soybean crops, but had no apparent effect on that uptake of Ca ad Mg in all crops. Similar detrimental effects of B on the uptake of Ca and Mg were reported by Singh and Singh (1983), who observed varying B level significantly increased the concentration of N, P, K, Na and B and decreased Ca and Mg concentrations in lentil plants. Applied B increased the N, P, K, Na and B content but decrease Ca and Mg contents of barley crops, whilst uptake of N, P, Na and B in grain and straw significantly increased, and K uptake remained unaffected (Singh and Singh 1983). Francois (1986) reported that with increasing B in the soil solution the concentration of B, P, K and Mg tended to increase in tomato leaves, whilst Ca and Na showed inconsistence trends. Studies on the chemical composition of radish, using sand culture techniques, indicated that Ca and P concentrations decreased significantly and K, Mg and Na remained unchanged with the increasing B levels (Francois 1986). Morsey and Taha (1986) reported that applied soil B and foliar application increased the concentration and uptake of N, P, K, Mn and B in both shoots and roots of sugar plants. Patel and Golakia (1986) demonstrated the effect of soil B on the uptake of N, P, K, Ca, Zn, Cu, Fe and Mn by a groundnut crop. Interestingly they outlined the mechanisms of action for some nutrients in relation to the B effect: for example, B increased an uptake and could be responsible for a favourable effect on nodulation. A positive effect of B and P uptake, which altered the permeability of plasma lemma at the root surface, resulted in increased P absorption. Uptake of K increased because of their mutual synergistic relationship, but Ca decreased due to antagonistic effect. Uptake of Fe and Cu were positively correlated, while Mn and Zn negatively correlated with applied B. The deficient state of B resulted in decreased the leaf N, P, Ca, Mg, Fe, Cu, Zn and B in tomato. On the other hand, excess B increased the concentration of nutrients with greater significance for K, Mg and Fe followed by Ca and Mn and in smaller quantity Cu and Zn (Carpena-Artes and Carpena-Ruiz 1987).
B toxicity had no consistent effects on the tissue concentration of P, K, Ca, Mg, Zn, Cu, Fe and Mn for five barley and six wheat cultivars grown in nutrient solution and no interactions were found among B nutrients and cultivars (Nable 1989). Higher levels of applied B significantly depressed the N and enhanced P and K contents in three cuttings of Trifolium alexandrinum (berseem; Pal et al. 1989). Singh et al. (1990) reported that the concentration of P, Mg and Zn in wheat increased and Ca, K, Cu, Fe and Mn decreased with increasing B in soil. On the other hand, an increasing supply of B significantly decreases the uptake of P, K, Ca, Mg and Mn, while that of Zn, Cu and Fe increased. They concluded that high levels of applied B had an antagonistic effect on the uptake of nutrients and this could be due to the toxic effect of B on root cells, resulting in impaired nutrient absorption processes. Alvarez-Tinaut (1990) found positive correlations between B and Fe and Cu contents of sunflower, suggesting that B could indirectly affect catalase activity via Fe and Cu. Positive correlations between Zn and B also indicated that B could indirectly affect the enzyme through modification of the Zn content. Concentrations, total uptake and ratios of certain nutrients in radish top and root change with differential B supply to nutrient solution (Tariq 1997). However, this study also suggested that changes occurring were mainly due to the B effect and partially due to antagonism between Ca and B. It is clear from the reported literature, that B interactions, either synergism or/and antagonism, can affect plant nutrition under both deficiency and toxicity conditions.
With increasing B supply in nutrient medium, leaf content of P became high (usually younger leaves shows higher P concentration) and there was a minor decrease in K, Ca and Mg compared to the average concentration for leaves (Furlani et al. 2003).
Yang and Gu (2004) demonstrated the effect of B on Al toxicity for soybean seedlings. They showed that high supply of B was found to induce Al toxicity by significantly increasing growth parameters including root length at 2 mM, and fresh weight at 5 mM Al for two different cultivars. Similar results have been described by Hossain and Hossain (2004), who confirmed the relationship of B with Al. The ratio between Ca and B in the plant is sometimes used to identify B deficiency. In one study, the supply of Ca and B to four maize cultivars considerably improved shoot dry matter production (Kanwal et al. 2008).
B is responsible for changes in other nutrients in soil-plant interactions (Tariq and Mott 2006). They also showed optimum productivity of radish plants at 0.5 mg l−1 B supply. Toxic effects confirmed by significant productivity decreases were found at increased levels of B supply. The amount of B, Zn and Cu in plants was increased and amount of Fe, Mn and Mo were reduced. Except B, the net uptake of all microelements decreased with increasing levels of B in the nutrient supply, and exerted close connection to the growth response of radish plants. Moreover, Zn/Cu ratio increased and ratio of Mn/Fe and Mn/Zn decreased, while Fe/Cu exerted unpredictable trend with increasing B levels. Inoculation with biofertilisers (Rhizobium strains) alone or combined with different levels of B increased significantly the uptake of N, P, K, Fe, Mn, Zn and B by shoot and seeds of peanut in both seasons as compared with the corresponding treatments without biofertilisers. The highest values of N, P, K, Fe and Mn uptake by straw and seeds of peanut plants were obtained by using (200 ppm of B + Rhizobium spp.) in two successive seasons, while the highest values of Zn and B uptake by straw and seeds in both seasons were obtained by using (300 ppm of B + inoculation with Rhizobium; Nadia et al. 2006). B interactions (synergism and/or antagonism) can affect plant nutrition under both deficient and toxic levels (Tariq and Mott 2007).
There is no significant effect on the residual Fe in the soil when using B fertiliser. Results suggest that Zn and B fertilisers had no role in the changes of residual Fe and Mn in the soil relative to the normal levels, and other factors are operative on the accumulation of residual Fe and Mn in the soil relative to its normal levels. The effect of Zn and B interaction on the residual Fe and Mn in the soil was insignificant (Aref 2010).
Our findings showed that different level of B applied to the groundnut plant affect uptake of nutrients in an irregular fashion. Interactions between nutrients and applied B indicted uptake of N, P, K, Mg, Mn, Zn and Fe are indirectly dependent on increasing supply of B resulting in increasing NUE and NDS, but at a certain level. Our recent observations suggested that at 1 kg ha−1 B level, NUE of groundnut plant was higher in terms of absorption of mineral nutrients compared to the 0.5 kg ha−1 B level. At the 2.0 kg ha−1 B level, groundnut varieties showed decreases in nutrients uptake capacity, resulting in decreases in NUE. Increases in supply of nutrients may affect nutrient uptake capacity of plants, which was confirmed when levels of absorbed macro- and micronutrients in groundnut plants at 2.0 kg ha−1 B supply were determined. At 0.5 and 1.0 kg ha−1 B, NDS in groundnut plants was higher. NDS was improved in both varieties of groundnut at 1.0 kg ha−1 B supply. Higher levels of B might be exerting antagonist effects on uptake of nutrients, which might be affecting NUE and NDS to groundnut plants. Observations of nutrient uptake capacity of groundnut plants in all three conditions (limiting, sufficient and toxic) strongly suggested that 1.0 kg ha−1 B improved NUE and NDS in groundnut plants. Despite substantial literature, the mechanism of B interaction with other nutrients still remains unclear and needs more investigation in terms of improvement in NUE and NDS. NUE is usually studied for only one nutrient and improving NUE for any one nutrient may affect the NUE of other nutrients; this is still a question of interest.
Concluding Remarks
Boron is a crucial element for normal development and growth of plants. This chapter highlights the positive effects of boron on crops and also describes the interaction of B with the uptake of other nutrients. It further highlights the importance of understanding the mechanisms of B action in plants and determining the molecular mechanisms of plant responses to toxicity and deficiency of B, to allow improvement of crops for tolerance to both conditions. Deficient or toxic amounts of B may both have adverse effect on plants and alter the uptake of other nutrients by direct or indirect interactions. Increasing supply of B may result in increasing nutrient use efficiency (NUE) and nutrient demand and supply (NDS). Alternatively higher levels of B may exert antagonist effects on uptake of nutrients, negatively affecting NUE and NDS. The mechanism underlying the B interaction with other nutrients still remains unclear and requires further investigation. NUE is studied typically for only one nutrient at a time and improving NUE for any one nutrient almost certainly affects the NUE of other nutrients. However, the interactions of B with other plant elements are complex and may exert antagonistic or synergistic effects, which may be specific for species, growth medium and different environments.
References
Aduayi EA (1978) Role of boron on growth components and elemental composition of Ife plum tomato. Commun Soil Sci Plant Anal 9:1–11
Alvarez-Tinaut MC, Leal A, Recalde-Martinez L (1980) Iron-manganese interaction and its relation to boron levels in tomato plants. Plant Soil 55:377–388
Alvarez-Tonaut MC (1990) Correlation between boron and other micronutrients. In: Behaviour, function and significance of boron in agriculture. Report on an International Workshop at St. John’s College, Oxford, England. 23–25 July, 1990. Borax Consolidated Limited, London, SW 1P 1HT
Aref F (2010) Influence of zinc and boron interaction on residual available iron and manganese in the soil after corn harvest. Am Eur J Agric Environ Sci 8:767–772
Baker AS, Cook RL (1959) Green house studies on alfalfa with soil type, soil reaction and borax fertilization as variables. Agron J 51:1–4
Bellaloui N, Yadavc RC, Chern MS, Hu H, Gillen AM, Greve C, Dandekar AM, Ronald PC, Brown PH (2003) Transgenically enhanced sorbitol synthesis facilitates phloemboron mobility in rice. Physiol Plant 117:79–84
Blaser-Grill J, Knoppik D, Amberger A, Goldbach H (1989) Influence of boron on the membrane potential in Elodea densa and Helianthus annuus roots and H+ extrusion of suspension cultured Daucus carota cells. Plant Physiol 90:280–284
Blevins DG, Lukaszewski KM (1998) Boron in plant structure and function. Annu Rev Plant Physiol Plant Mol Biol 49:481–500
Bogiani JC, Sampaio TF, Abreu-Junior C, Rosolen CA (2014) Boron uptake and translocation in some cotton cultivars. Plant Soil 375:241–253
Bolaños L, Esteban E, de Lorenzo C, Fernández-Pascual M, de Felipe MR, Garate A, Bonilla I (1994) Essentiality of boron for symbiotic dinitrogen fixation in pea (Pisum sativum) rhizobium nodules. Plant Physiol 104:85–90
Bolaños L, Lukaszewski K, Bonilla I, Blevins D (2004) Why boron? Plant Physiol Biochem 42:907–912
Brown PH, Hu H (1996) Phloem mobility of boron is species dependent: evidence of phloem mobility in sorbitol-rich species. Ann Bot 77:497–505
Brown PH, Shelp BJ (1997) Boron mobility in plants. Plant Soil 193:85–101
Brown PH, Bellaloui N, Hu H, Dandekar A (1999) Transgenically enhanced sorbitol synthesis facilitates phloem boron transport and increases tolerance of tobacco to boron deficiency. Plant Physiol 119:17–20
Camacho-Cristóbal JJ, Anzellotti D, González-Fontes A (2002) Changes in phenolic metabolism of tobacco plants during short-term boron deficiency. Plant Physiol Biochem 40:997–1002
Camacho-Cristóbal JJ, Rexach J, Herrera-Rodríguez MB, Navarro-Gochicoa MT, González-Fontes A (2011) Boron deficiency and transcript level changes. Plant Sci 181:85–89
Cara FA, Sánchez E, Ruiz JM, Romero L (2002) Is phenol oxidation responsible for the short-term effects of boron deficiency on plasma-membrane permeability and function in squash roots? Plant Physiol Biochem 40:853–858
Carmen Rodriguez- Hernandez M, Moreno DA, Carvajal M, Carmen–Martinez- Ballesta M (2013) Interactive effects of boron and NaCl stress on water and nutrient transport in two broccoli cultivars. Funct Plant Biol 40(7):739–748
Carpena-Artes O, Carpena-Ruiz RO (1987) Effect of boron in tomato plant. Leaf evaluations. Agrochimica 31:391–400
Coetzer LA, Robbertse PJ (1987) Pollination biology of Persea Americana Fuerte. S Afr Avocado Growers’ Assoc Yearb 10:43–45
Cosgrove DJ (1999) Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol 50:391–417
Crop Science Society of America (CSSA) (1992) Glossary of crop science terms. CSSA, Madison
Dannel F, Pfeffer H, Römheld V (2002) Update on boron in higher plant. Uptake, primary translocation and compartmentation. Plant Biol 4:193–204
Dave IC, Kannan S (1981) Influence of boron deficiency on micronutrients absorption by Phaseoulus vulgaris and protein contents in cotyledons. Acta Physiol Plant 3:27–32
Dear B, Lipsett J (1987) The effect of boron supply on the growth and seed production of subterranean clover (Trifloium subtereneum L). Aust J Agric Res 38:537–546
Domingues DS, Leite SMM, Farro APC, Coscrato VE, Mori ES, Furtado EL, Wilcken CF, Velini ED, Guerrini IA, Maia IG, Marino CL (2005) Boron transport in Eucalyptus. 2. Identification in silico of a putative boron transporter for xylem loading in eucalypt. Genet Mol Biol 28:625–629
Downton WJS, Hawkar JS (1980) Interaction of boron and chloride on growth and mineral composition of cabernet sauvignon vines. Am J Enol Vitic 31:277–282
Du Ying Q, Rong LX, Hua HJ, Zhiyao H (1999) Influence of B and/or Mo application on the growth and yield of peanut. Chin J Oil Crop Sci 21:61–66
El-Shintinawy F (1999) Structural and functional damage caused by boron deficiency in sunflower leaves. Photosynthetica 36:565–573
Ferrol N, Donaire JP (1992) Effect of boron on plasma membrane proton extrusion and redox activity in sunflower cells. Plant Sci 86:41–47
Fleischer A, O’Neill MA, Ehwald R (1999) The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol 121:829–838
Francosis LE (1986) Effect of excess boron on broccoli, cauliflower and radish. Am Soc Hortic Sci 111:494–498
Furlani ÂMC, Carvalho CP, de Freitas JG, Verdial MF (2003) Wheat cultivar tolerance to boron deficiency and toxicity in nutrient solution. Sci Agric 60:359–370
Ganie MZ, Akhter F, Bhat MA, Malik AR, Mihd Junaid J, Shah MA, Bhat AH, Bhat TA (2013) Boron- a critical nutrient element for plant growth and productivity with reference to temperate fruits. Curr Sci 104:76–85
Garg OK, Sharma AN, Kona GRSS (1979) Effect of boron on the pollen vitality and yield of rice plants (Oryza sativa L. var. Jaya). Plant Soil 52:575–578
Goldbach HE, Yu Q, Wingender R, Schulz M, Wimmer M, Findeklee P, Baluska F (2001) Rapid response reactions of roots to boron deprivation. J Plant Nutr Soil Sci 164:173–181
Goldberg S (1997) Reactions of boron with soil. Plant Soil 193:35–48
Gomez-Rodriguez MV, Gomez-Ortega M, Alvarez-Tinaut MC (1981) Boron, copper, iron, manganese and zinc content in leaves of flowering sunflower plants (Helianthus annuus L.) grown with different boron supplies. Plant Soil 62:461–464
Gupta U (1979) Boron nutrition of crops. Adv Agron 31:273–307
Hajiboland R, Rad B, Bastani S (2013) Phenolic metabolism in boron deficient tea (Camellia sinensis L. O. Kuntze) plants. Acta Biol Hung 64:196–206
Han S, Chen L, Jiang H, Smith BR, Yang L, Xie C (2008) Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J Plant Physiol 165:1331–1341
Harris HC, Brolmann JB (1966) Comparison of calcium and boron deficiencies of the peanut II. seed quality in relation to histology and viability. Agron J 58:578–582
Hossain AKMZ, Hossain MA (2004) Effects of aluminum and boron supply on growth of seedlings among 15 cultivars of wheat (Triticum aestivum L.) grown in Bangladesh. Soil Sci Plant Nutr 50:189–195
Hu HN, Brown PH (1997) Absorption of boron by plant roots. Plant Soil 193:49–58
Hu H, Penn SG, Lebrilla CB, Brown PH (1997) Isolation and characterization of soluble B complexes in higher plants. The mechanism of phloem mobility of boron. Plant Physiol 113:649–655
Huang L, Ye Z, Bell WR, Dell B (2005) Boron nutrition and chilling tolerance of warm climate crop species. Ann Bot 96:755–767
Hundt I, Schilling G, Fisher F, Bergmann W (1970) Investigations on the influence of the micronutrient boron on nucleic acid metabolism. Albrecht-Thaer-Arch 14:825–837
Kamali AR, Childers NF (1970) Growth and fruiting of peach in sand culture as affected by boron and fritted form of trace elements. J Am Soc Hortic Sci 95:652–656
Kanwal S, Rahmatullah Aziz T, Maqsood MA, Abbas N (2008) Critical ratio of calcium and boron in maize shoot for optimum growth. J Plant Nutr 31:1535–1542
Kastori R, Plesnicar M, Pankovic D, Sakac Z (1995) Photosynthesis, chlorophyll fluorescence and soluble carbohydrates in sunflower leaves as affected by boron deficiency. J Plant Nutr 18:1751–1763
Khan Towhid Osman (2013) Plant nutrients and soil fertility management. In: Soils: principles, properties and management. Springer, Dordrecht/Heidelberg/New York, pp 129–159
Kobayashi M, Matoh T, Azuma J (1996) Two chains of rhamnogalacturonan II are crosslinked by borate-diol ester bonds in higher plant cell walls. Plant Physiol 110:1017–1020
Kot FS (2009) Boron sources, speciation and its potential impact on health. Rev Environ Sci Biotechnol 8:3–28
Leece DR (1978) Effects of boron on the physiological activity of zinc in maize. Aust J Agric Res 29:739–749
Li B, Li H, Kui WH, Chao MC, Jern WS, Li HP, Chu WJ, Wang L (1978) Studies on cause of sterility of wheat. J Northeast Agric Coll 3:1–19
Lombin GL, Bates TE (1982) Comparative responses of peanut, alfalfa and soybeans to varying rates of boron and manganese on two calcareous Ontario soils. Can J Soil Sci 62:1–9
Loomis WD, Durst RW (1992) Chemistry and biology of boron. Biofactors 3:229–239
Malvi UR (2011) Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J Agric Sci 24:106–109
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, San Diego
Matoh T, Ochiai K (2005) Distribution and partitioning of newly taken-up boron in sunflower. Plant Soil 278:351–360
McIlrath WJ, Debruyn JA, Skok J (1960) Influence of boron supply on the micronutrients content of setaria shoots. Soil Sci 89:117–121
Miwa K, Fujiwara T (2010) Boron transport in plants: co-ordinated regulation of transporters. Ann Bot 105:1103–1108
Morsey MA, Taha EM (1986) Effect of boron, manganese and their combination on sugar beet under El-Minia conditions. 2: concentration and uptake of N, P, K, B and Mn. Ann Agric Sci Ain Shams Univ Cairo 31:1241–1259
Mozafar A (1989) Boron effect on mineral nutrients of maize. Agron J 81:285–290
Mukhopadhyaya M, Ghosh PD, Mondal TK (2013) Effect of boron deficiency on photosynthesis and antioxidant responses of young tea plantlets. Russ J Plant Physiol 60:633–639
Nable RO (1989) Effect of boron toxicity upon the mineral nutrient composition of barley and wheat cultivars. Div Rep Div Soils, CSIRO No. 104: 1–10
Nable RO, Bañuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 193:181–198
Nadia MA, Badran M, Abd El-Hamide AF (2006) Response of peanut to foliar spray with boron and/or rhizobium inoculum. J Appl Sci Res 2:1330–1337
Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T (2007) Cell-type specificity of the expression of OsBOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 19:2624–2635
Noppakoonwong RN, Rerkasem B, Bell RW, Dell B, Loner Gan JF (1997) Prognosis and diagnosis of boron deficiency in blackgram (Vigna mungo L. Hepper) in the field by using plant analysis. In: Bell RW, Rerksaem B (eds) Proceedings of boron in soil and plants. Kluwer Academic Publishers, Dordrecht, pp 89–93
North KA, Ehlting B, Kopriva A, Rennenberg H, Kopriva S (2009) Natural variation in Arabidopsis adaptation to growth at low nitrogen conditions. Plant Physiol Biochem 47:912–918
Oertli JJ, Richardson WF (1970) The mechanism of boron immobility in plants. Plant Physiol 23:108–116
Pal B, Jadaun SPS, Raghav CS (1989) Effect of phosphorous and boron application on dry matter yield and nutrient contents in berseem. J Indian Soc Soil Sci 37:579–581
Pandey N, Archana (2012) Effect of boron on seed germination and biochemical changes in linseed at seeling stage. Indian J Agric Biochem 25:167–170
Pandey DK, Pandey N (2008) Screening of wheat genotypes for their susceptibility to boron deficiency. Res Environ Life Sci 1:37–42
Parks RQ, Lyon CB, Hood SL (1944) Some effect of boron supply on the chemical composition of tomato leaflets. Plant Physiol 19:404–419
Patel MS, Golakia BA (1986) Effect of calcium carbonate and boron application on yield and nutrient uptake by groundnut. J Indian Soc Soil Sci 34:815–820
Pollard AS, Parr AJ, Loughman BC (1977) Boron in relation to membrane function in higher plants. J Exp Bot 28:831–834
Power PP, Woods WG (1997) The chemistry of boron and its speciation in plants. Plant Soil 193:1–13
Ruiz JM, Baghour M, Bretones G, Belakbir A, Romero L (1998) Nitrogen metabolism in tobacco plants (Nicotiana tabacum L.): role of boron as a possible regulatory factor. Int J Plant Sci 159:121–126
Ryden P, Sugimoto-Shirasu K, Smith AC, Findlay K, Reiter WD, McCann MC (2003) Tensile properties of arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol 132:1033–1040
Sathya S, Mahendran PP, Arulmozhiselven K (2013) Influence of soil and foliar application of borax on fractions of boron under tomato cultivation in boron deficient soil of typic Haplustalf. Afr J Agric Res 8:2567–2571
Sharma PN, Ramchandra T (1990) Water relations and photosynthesis in mustard plants subjected to boron deficiency. Indian J Plant Physiol 33:150–154
Shelp BJ, Shattuck VI (1987) Boron nutrition and mobility, and its relation to hollow stem and the elemental composition of greenhouse grown cauliflower. J Plant Nutr 10:143–162
Simojoki P (1972) Boron deficiency, pollen sterility and ergot disease of barley. Ann Agric Fenn 11:333–341
Singh AL (1994) Micronutrient nutrition and crop productivity in groundnut. In: Singh K, Purohit SS (eds) Plant productivity under environmental stress. Agro Botanical Publishers, Bikaner, pp 67–72
Singh V, Singh SP (1983) Effect of applied boron on the chemical composition of lentil plants. J Indian Soc Soil Sci 31:169–170
Singh JP, Dahia DJ, Narwal RP (1990) Boron uptake and toxicity in wheat in relation to zinc supply. Fert Res 24:105–110
Stangoulis JCR, Brown PH, Bellaloui N, Reid RJ, Graham RD (2001) The efficiency of boron utilization in canola. Aust J Plant Physiol 28:1109–1114
Steinburg RA, Specht AW, Roller EM (1955) Effect of micronutrient deficiency on mineral composition, nitrogen fraction, ascorbic acid and burn of tobacco grown flowering in water culture. Plant Physiol 30:123–129
Stewart WM, Dibb DW, Johnston AE, Smyth TJ (2005) The contribution of commercial fertilizer nutrients to food production. Agron J 97:1–6
Takano J, Yamagami M, Noguchi K, Hayashi H, Fujiwara T (2001) Preferential translocation of boron to young leaves in Arabidopsis thaliana regulated by the BOR1 gene. Soil Sci Plant Nutr 47:345–357
Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T (2002) Arabidopsis boron transporter for xylem loading. Nature 420:337–339
Tariq M (1997) Effect of boron supply on the availability of nutrients in soil and uptake by radish (Raphanus sativas L.) Ph.D. thesis, University of Reading, England
Tariq M, Mott CJB (2006) Effect of applied boron on the accumulation of cations and their ratios to boron in radish (Raphanus sativus L.). Soil Environ 25:40–47
Tariq M, Mott CJB (2007) Effect of boron on behavior of nutrients in soil-plant systems – a review. Asian J Plant Sci 6:195–202
Thurzo S, Szabo Z, Nyeki J, Silva AP, Nagy PT, Goncalves B (2010) Effect of boron and calcium sprays on photosynthetic pigments, total phenols and flavonoid content of sweet cherry (Prunus avium L.). Acta Hortic 868:457–461
Tolgyesi G, Kozma A (1974) Investigation on factors affecting boron uptake by grasses. Agrokemia es Talajatan 23:83–98
Touchton JT, Boswell FC (1975) Effects of boron on soyabean yield, chemical composition and related characteristics. Agron J 67:417–420
Vaughan AKF (1977) The relation between the concentration of boron in the reproductive and vegetative organ of maize plants and their development. Rhod J Agric Res 15:163–170
Wallace A, Romney EM, Alexander GV, Kinnear J (1977) Phytotoxic and some interactions of the essential metals iron, manganese, molybdenum, zinc, copper and boron. Commun Soil Sci Plant Anal 8:741–750
Wang ZY, Tang YL, Zhang FS, Wang H (1999) Effect of boron and low temperature on membrane integrity of cucumber leaves. J Plant Nutr 22:543–550
Warington K (1923) The effect of boric acid and borax on the broad bean and certain other plants. Ann Bot 37:629–672
Wimmer MA, Eichert T (2013) Review: mechanism for boron deficiency mediated changes in plant water relations. Plant Sci 203–204:25–32
Wimmer MA, Lochnit G, Bassil E, Mühling KH, Goldbach HE (2009) Membrane associated, boron-interacting proteins isolated by boronate affinity chromatography. Plant Cell Physiol 50:1292–1304
Woodbridge CG, Venegas A, Carndall PC (1971) The boron content of developing pear, apple and cherry flower buds. J Am Soc Hortic Sci 96:613–615
Woods WG (1994) An introduction to boron: history, sources, uses, and chemistry. Environ Health Perspect 102:5–11
Yadav OP, Manchanda HR (1979) Boron tolerance studies in gram and wheat grown on a sierozem sandy soil. J Indian Soc Soil Sci 27:174–180
Yang YH, Gu HJ (2004) Effects of boron on aluminum toxicity on seedlings of two soybean cultivars. Water Air Soil Pollut 154:239–248
Yang L, Zhang Q, Dou J, Li L, Guo L, Shi L, Xu F (2013) Characteristics of root boron nutrition confer high boron efficiency in Brassica napus cultivars. Plant Soil 371:95–104
Zhao D, Oosterhuis DM (2002) Cotton carbon exchange, non-structural carbohydrates, and boron distribution in tissues during development of boron deficiency. Field Crop Res 78:75–87
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bariya, H., Bagtharia, S., Patel, A. (2014). Boron: A Promising Nutrient for Increasing Growth and Yield of Plants. In: Hawkesford, M., Kopriva, S., De Kok, L. (eds) Nutrient Use Efficiency in Plants. Plant Ecophysiology, vol 10. Springer, Cham. https://doi.org/10.1007/978-3-319-10635-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-10635-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10634-2
Online ISBN: 978-3-319-10635-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)