Abstract
Cardiovascular disease (CVD) has become the leading cause of death for women. The incidence of CVD in women increases substantially with aging, probably because menopause diminishes the gender protection contributing to an adverse impact on cardiovascular risk variables. In this setting, the menopausal transition (MT) must be considered a critical period in women’s life because it leads to several adverse changes, especially with regard to lipid profile and modifies cardiovascular risk factors. Thus, it may be necessary to encourage lifestyle measures and therapeutic interventions such as hormone replacement therapy (HRT) throughout the MT to counteract or prevent these events. HRT for postmenopausal women has been available for more than 60 years. Ever since, HRT has been the subject of discussion and debate regarding its safety and efficacy; nowadays, it is well established that timing in the initiation as well as the type, dose and route of HRT are crucial for the success and benefit of HRT. Furthermore, a new study, published by the British Medical Journal, recently added interesting evidence to this debate, proving that menopausal hormone therapy saves lives and reduces disability.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 The Menopausal Transition: Definitions
As defined by the Stages of Reproductive Aging Workshop (STRAW) held in July 2001 and updated in 2011, the menopausal transition (MT) begins with variations in menstrual cycle length associated with a decrease in inhibin B and anti-Müllerian hormone (AMH) concentration and a rise in follicle-stimulating hormone (FSH); it ends with the final menstrual period (FMP), classically confirmed only when followed by 12 months of amenorrhea [1]. Typically, the MT starts in the late 40s or early 50s and continues for about 4–7 years.
The original STRAW staging system is widely considered to be the gold standard for characterizing reproductive aging throughout menopause using menstrual patterns, endocrinological markers, and ovarian imaging. Moreover, the STRAW + 10 criteria update the 2001 STRAW criteria by providing greater clarity for menstrual patterns, including duration of phases and stages. This system classifies reproductive and postreproductive life into seven stages, with the MT accounting for two of those stages. The main event is the FMP; five stages come before FMP and two stages follow it (Fig. 17.1).
The Stages of Reproductive Aging Workshop (STRAW) +10 staging system (Adapted from Harlow et al. [1])
2 Impact of the Menopause Transition on a Woman’s Life
Menopausal transition is a physiological event in every woman’s life; nevertheless, it can profoundly affect quality of life. In fact during this period, women complain about new and unexpected annoying symptoms and their bodies are at the mercy of intense hormonal and physical changes. The clinical signs associated with this period are vasomotor symptoms, sleep disruption, mood alteration, urogenital complaints, and sexual dysfunction. Among these, the most frequent are vasomotor symptoms, which afflict 75 % of women and usually disappear within 1–5 years.
Moreover, the years around the menopause are associated with weight gain, increased central adiposity and waist circumference. The annual rate of weight gain, estimated to be about 0.5 kg, is independent of menopausal status, while it is consistently related to chronological aging [2]. In contrast, adverse changes in either body fat distribution, from a more gynoid pattern to a more android pattern, or body composition with increased fat mass and decreased lean mass may be due to hormonal changes occurring during the menopausal transition [3]. The deleterious change in body fat distribution consists of an increase in abdominal adiposity owing to the selective accumulation of fat in the intra-abdominal compartment [4], independent of age and total body fat mass [5]. Specifically, increased abdominal adiposity is known to increase the risk of developing CVD.
Data from the Women’s Healthy Lifestyle Project provide clear evidence that weight gain and increased waist circumference, along with elevations in lipid levels and other risk factors for coronary heart disease (CHD), are preventable through use of lifestyle intervention in healthy menopausal women. In fact, although these changes are inevitable with age and menopause, physical activity may attenuate the impact of both events. Thus, weight gain prevention should be recognized as an important health goal for women before they approach menopause and women should do regular physical activity [6].
As hormonal changes during the MT directly or indirectly adversely affect quality of life, body composition, and risk of cardiovascular disease (CVD), the maintenance of health parameters in the premenopausal years is crucial for a healthy postmenopausal life.
3 Estrogens and Cardiovascular Risk
Estrogens and the other sex hormones regulate some of the fundamental cardiovascular functions including blood pressure, blood flow, vasodilatation and vasoconstriction, vascular inflammation and remodeling, and atherosclerosis [7]. The actions of endogenous estrogens on the cardiovascular system can be mediated directly on the vessels or indirectly through the modulation of cardiovascular risk factors.
Estrogen exerts pleiotropic functions on the cardiovascular system through both genomic and nongenomic effects [8, 9]. Traditionally, estrogen receptors (ERs) act as transcription factors regulating the expression of target genes by directly binding to specific DNA sequences, the estrogen response element (ERE). Nongenomic effects are rapid responses that occur too quickly to be mediated by gene transcription, instead involving modulation of membrane and cytoplasmic proteins.
At this level, estrogen triggers rapid vasodilatation, exerts anti-inflammatory effects, regulates vascular cell growth and migration, leading to a protective action on vessels [10]. These rapid and nongenomic effects are reached by complex interactions with membrane-associated signaling ERs, leading to the activation of downstream cascades such as mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-OH kinase (PI3K). These cascades are responsible for the important cardiovascular actions of estrogen, for instance, the activation of nitric oxide (NO) synthesis or the remodeling of the endothelial actin cytoskeleton. Moreover, these cascades play crucial roles in regulating the expression of target proteins implicated in cell proliferation, apoptosis, differentiation, movement, and homeostasis [11].
Furthermore, estrogens also have systemic effects that may have an influence on cardiovascular risk, altering the serum lipid concentrations, the coagulation and fibrinolytic systems, and the antioxidant system [7]. In fact, through ER estrogens regulate hepatic expression of apoprotein genes and several coagulation and fibrinolytic proteins. The net effect of these changes is to improve the lipid profile and to promote vasodilatation and antioxidant activities. By contrast, the menopause leads to an overturning of all these effects, increasing the cardiovascular risk.
Recent advancements in the characterization of the molecular basis of the actions of estrogen help us to understand its biological functions and would be beneficial in elucidating current controversies on its clinical efficacy in the cardiovascular system.
4 The Menopausal Transition and Cardiovascular Risk
Cardiovascular diseases are the number one cause of death globally, both for men and women. It is well documented that morbidity and mortality rates from CVD are higher in men than in women; however, this gender gap narrows after the menopause, suggesting that female sex hormones and aging might play a role [12]. In fact, the incidence of CVD in women increases substantially with aging, probably because the menopause diminishes the gender protection contributing to an adverse impact on cardiovascular risk variables [7]. Nevertheless, whether this higher cardiovascular risk is a function of aging or a consequence of the loss of endogenous estrogen due to the menopause, or both, has been debated in the literature for many years [13, 14].
In this respect, it is worth mentioning the results of the Study of Women’s Health Across the Nation (SWAN). SWAN is a multicenter, multi-ethnic longitudinal study designed to characterize the physiological and psychosocial changes that occur during the menopausal transition and to observe their effects on subsequent health and risk factors for age-related diseases. A total of 3,302 women were enrolled at seven clinical sites between 1996 and 1997. At the time of enrollment, women were premenopausal, not taking hormones and between 42 and 52 years of age. Participants self-identified as African–American (28 %), Caucasian (47 %), Chinese (8 %), Hispanic (8 %), or Japanese (9 %). SWAN has a multidisciplinary focus and thus has repeated measures of bone health, cardiovascular risk factors, psychosocial factors, and ovarian hormones [15].
In this setting, Matthews et al. [16] evaluated the change in CHD risk factors in relation to a very particular and critical period of a woman’s life, the FMP. Women who experienced a natural menopause (1,054 out of the total) were analyzed independent of age and other confounders. The results showed significant increases in total cholesterol, LDL-C, and Apo B within a year of the FMP; importantly, the rate of change relative to the FMP did not vary by ethnicity, suggesting that the menopause might have a uniform influence on lipids. The other risk factors changed in a linear pattern consistent with chronological aging: triglycerides, lipoprotein (a), insulin, factor VIIc, and systolic blood pressure increased; diastolic blood pressure, tissue plasminogen activator antigen, fibrinogen, and high-sensitivity C-reactive protein did not change.
In conclusion, the year immediately around the FMP has been identified as the critical time period of changes in the lipid profile leading to an increased risk of CVD in the post-menopausal years. Although a significant portion of this increased risk is likely due to changes in plasma lipid–lipoprotein levels secondary to estrogen deficiency, a number of other hormonal and physiological changes that occur during the menopause transition may also contribute. Therefore, during the menopausal transition several adverse changes take place and modify cardiovascular risk factors. It may thus be necessary to encourage lifestyle measures and therapeutic interventions such as hormone replacement therapy throughout the menopausal transition to counteract or prevent these events.
5 Hormone Therapy: A Debate Still Open
Hormone replacement therapy (HRT) for postmenopausal women has been available for more than 60 years. Since that moment, HRT has been the subject of discussion and debate. In the early 1990s, the effects of HRT were thought to be beneficial because of a reduction of 30–50 % in the risk of CVD and osteoporosis. These effects were confirmed by a large number of observational studies [17, 18]; thus, in 1992 the American College of Physicians published guidelines strongly advising a preventative use of HRT [19]. In those years an escalation in the use of HRT was registered across the world [20].
However, in the late 1990s, a number of studies started questioning the protective role and the safety of HRT. Randomized clinical trials (RCTs) overturned the previous hypothesis about the advantage of HRT, showing no evidence of cardiovascular benefit, but instead a negative effect on women’s health. Among these, the Women’s Health Initiative (WHI) trial had a great impact. Over 16,000 menopausal women between the ages of 50and 79 (mean age 63 years) were enrolled across 40 clinical centers in the USA. After a mean of 5.2 years of follow-up the study was stopped because of an increased risk for cancer and adverse effects on the cardiovascular system [21, 22]. The consequent release in 2002 of the principal findings from the WHI was associated with a substantial decline in use by postmenopausal women [23].
Nevertheless, more recent subgroup analyses suggested that the lack of benefit or increase in CVR observed in the WHI might result from the harmful effect of HRT in older women well after the menopause. It is clear that the characteristics of women selected for RCTs were markedly different than those of women studied from the general population in observational studies from which the estrogen cardioprotective hypothesis was generated. Therefore, these secondary analyses pointed out that the benefit of HRT may be dependent upon both the age of the women at the time of HRT initiation and the timing of the initiation of hormone exposure. Not only the role of timing, but also the role of the type, dose, and route of HRT should be analyzed. These hypotheses have encouraged two new RCTs: the Kronos Early Estrogen Prevention Study (KEEPS) and the Early vs Late Intervention Trial with Estradiol (ELITE). KEEPS is a randomized clinical and controlled study with the objective of clarifying the controversy about the effects of HRT on the progression of the atherosclerosis risk–benefit factor with the use of estrogens in postmenopausal women. Healthy women aged 42–58 years who are within 36 months of their last menstrual period have been recruited to receive either oral estrogens or estradiol patches; in addition, both groups are given oral micronized progesterone for 11 days of each month. Outcomes will be carotid–intima media thickness and the accrual of coronary calcium. Preliminary reports on this study have been distributed, but a full publication is still awaited. On the other hand, ELITE randomized women based on the number of years since menopause (less than 6 years or more) to receive either estradiol or placebo. As in KEEPS, the primary endpoint is the change in carotid intima-media thickness. The results from these studies will help us to clarify some of the aspects that are still unsolved.
The most recent Position Statement of the North American Menopause Society on hormone therapy published in early 2012 clarifies the existing data and provides easy-to-follow recommendations for menopause management [24]. HRT should be given to healthy and chronologically young perimenopausal women within 10 years of the onset of menopause in the lower dose regimens [25]. Based on the available evidence, those women with these characteristics who carry on the use of HRT for several years experience a significant decrease in their risk of developing a heart attack.
6 The Window of Opportunity
Over the last decade, a large quantity of data have strongly supported the notion that the presence of a window of therapeutic opportunity for the use of estrogens in healthy women with recent menopause appears to be the key to successful prevention and amelioration of any further damage [26]. Timing of the initiation is important because hormone replacement is effective before tissue damage due to aging becomes too extensive [27]. The major clinical implication is that whereas HRT is not recommended for reduction of CVD risk by the WHI and other RCTs, its use for other indications should not be hampered by the fear of increasing CVD in younger, newly menopausal women [28].
These hypotheses could have a biological basis. In fact, around the time of menopause, women still have healthy arteries, allowing a “window” for HRT to produce cardiovascular benefit. However, aging arteries become less responsive to the beneficial effects of estrogens. Indeed, the menopause is associated with endothelial dysfunction, decreased NO-dependent relaxation and intimal thickening; moreover, age-related changes in ER amount, distribution or affinity may also contribute to cardiovascular risk [29].
It is well established that estrogen may have a different and controversial effect on the atherosclerotic process, depending on its stage. Estrogen inhibits early development of atherosclerosis by preventing the accumulation of foam cells in the endothelium, whereas it may increase the cardiovascular risk once atherosclerosis has been established, causing disruption of the fibrous cap and rupture of the plaque by increasing expression of matrix metalloproteinases (MMPs) [30]. Thus, the preexisting cardiovascular condition could affect the outcome of HRT.
7 Rethinking Menopausal Hormone Therapy
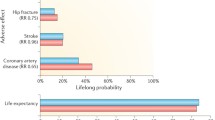
A new study, published by the British Medical Journal, is now adding interesting evidence to the debate. The paper, authored by Schierbeck et al. [31], reports the long-term follow-up of a cohort of postmenopausal women originally enrolled in the Danish Osteoporosis Prevention Study (DOPS), a randomized, open-label trial investigating the effect of hormone intervention with estradiol or a combination of estradiol and a synthetic progestin on osteoporotic fractures. The study enrolled 1,006 patients aged 45–58 who were recently menopausal or had perimenopausal symptoms between 1990 and 1993, with a planned duration of 20 years; both the 502 women who received HRT and the 504 who received no treatment were followed for nearly 16 years (10 years of treatment and 6 years of follow-up). The intention-to-treat analysis on the cohort of more than 1,000 provides an interesting set of results, which were awaited by many in the field.
The key result is that women receiving hormone replacement had a significantly lower incidence of CHD. The magnitude of this reduction was around50 % and applied both to the period of active treatment and to the later observational phase. In addition, no differences in stroke, venous thromboembolism or breast cancer were recorded throughout the study. There are at least two key differences between this study and the ones that have been available so far: the age of the women enrolled and the characteristics of the medication. In fact, this trial looks at women who started hormone replacement very close to the menopausal transition. By contrast, the WHI [32], enrolled nearly 75 % of patients over 60 years, and less than 5 % of the entire population were close to the menopause. Moreover, women were treated with 17β-estradiol and norethisterone acetate instead of conjugated equine estrogens and medroxyprogesterone acetate, emphasizing that the use of a progestin during hormone replacement, particularly medroxyprogesterone acetate, is associated with a higher risk of CVD [22] compared with estrogen-only therapies. It was also found in this study, that women who did not receive the progestin had a trend toward having a lower incidence of cardiovascular events [31].
Nevertheless, after nearly 20 years of debate, the never-ending contradiction between studies showing the benefit of HRT and guidelines stating that HRT should not be used to prevent chronic disorders still exists. The last and most recent one published by the US Preventive Services Task Force [33] in 2012 explicitly advises against the use of either estrogens or of combinations of estrogens and progestins for the prevention of chronic conditions.
It is established that HRT is generally initiated in the real clinical world because women suffer from hot flashes or vulvo-vaginal atrophy, with the only aim of counteracting these symptoms. However, this study should raise awareness in the media, the regulatory authorities, and the general population that menopausal hormone therapy saves lives and reduces disability. This is not trivial and a shift in communication and clinical attitude could lead to enormous benefits for women and health systems throughout the world.
References
Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW et al (2012) Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 97(4):1159–1168
Wang Q, Hassager C, Ravn P, Wang S, Christiansen C (1994) Total and regional body-composition changes in early postmenopausal women: age-related or menopause-related? Am J Clin Nutr 60(6):843–848
Sternfeld B, Dugan S (2011) Physical activity and health during the menopausal transition. Obstet Gynecol Clin North Am 38(3):537–566
Toth MJ, Tchernof A, Sites CK, Poehlman ET (2000) Menopause-related changes in body fat distribution. Ann N Y Acad Sci 904:502–506
Toth MJ, Tchernof A, Sites CK, Poehlman ET (2000) Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord 24(2):226–231
Simkin-Silverman LR, Wing RR (2000) Weight gain during menopause. Is it inevitable or can it be prevented? Postgrad Med 108(3):47–50
Mendelsohn ME, Karas RH (1999) The protective effects of estrogen on the cardiovascular system. N Engl J Med 340(23):1801–1811
Fu XD, Simoncini T (2007) Non-genomic sex steroid actions in the vascular system. Semin Reprod Med 25(3):178–186
Simoncini T, Genazzani AR (2003) Non-genomic actions of sex steroid hormones. Eur J Endocrinol 148(3):281–292
Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR (2004) Genomic and non-genomic effects of estrogens on endothelial cells. Steroids 69(8–9):537–542
Simoncini T (2009) Mechanisms of action of estrogen receptors in vascular cells: relevance for menopause and aging. Climacteric 12(Suppl 1):6–11
Lerner DJ, Kannel WB (1986) Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J 111(2):383–390
Tracy RE (1966) Sex difference in coronary disease: two opposing views. J Chronic Dis 19(11):1245–1251
Tunstall-Pedoe H (1998) Myth and paradox of coronary risk and the menopause. Lancet 351(9113):1425–1427
Santoro N, Sutton-Tyrrell K (2011) The SWAN song: Study of Women’s Health Across the Nation’s recurring themes. Obstet Gynecol Clin North Am 38(3):417–423
Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B et al (2009) Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 54(25):2366–2373
Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B et al (1992) Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 117(12):1016–1037
Henderson BE, Paganini-Hill A, Ross RK (1991) Decreased mortality in users of estrogen replacement therapy. Arch Intern Med 151(1):75–78
Guidelines for counseling postmenopausal women about preventive hormone therapy. American College of Physicians (1992). http://www.ncbi.nlm.nih.gov/pubmed/1443972. Ann Intern Med 117(12):1038–1041
Keating NL, Cleary PD, Rossi AS, Zaslavsky AM, Ayanian JZ (1999) Use of hormone replacement therapy by postmenopausal women in the United States. Ann Intern Med 130(7):545–553
Yager JD, Davidson NE (2006) Estrogen carcinogenesis in breast cancer. N Engl J Med 354(3):270–282
Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL et al (2003) Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349(6):523–534
Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K (2004) Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 140(3):184–188
Gass ML, Heights M, Manson JE, Cosman F, Hayes H, Grodstein F, et al (2012) North American Menopause Society. The 2012 hormone therapy position statement of: the North American Menopause Society. http://www.ncbi.nlm.nih.gov/pubmed/22367731. Menopause 19(3):257–271
Ghazal S, Pal L (2013) Perspective on hormone therapy 10 years after the WHI. Maturitas 76(3):208–212
Hodis HN, Mack WJ (2011) A “window of opportunity:” the reduction of coronary heart disease and total mortality with menopausal therapies is age- and time-dependent. Brain Res 1379:244–252
Genazzani AR, Simoncini T (2007) Timing is everything. Gynecol Endocrinol 23(1):1–4
Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD et al (2010) Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab 95(7 Suppl 1):s1–s66
Smiley DA, Khalil RA (2009) Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels. Curr Med Chem 16(15):1863–1887
Khalil RA (2013) Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol 86(12):1627–1642
Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L et al (2012) Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 345:e6409
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288(3):321–333
Moyer VA (2013) Menopausal hormone therapy for the primary prevention of chronic conditions: U.S. Preventive services task force recommendation statement. Ann Intern Med 158:47–54
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 International Society of Gynecological Endocrinology
About this chapter
Cite this chapter
Spina, S., Bernacchi, G., Cecchi, E., Genazzani, A.R., Simoncini, T. (2015). Cardiovascular Prevention at the Menopausal Transition: Role of Hormonal Therapies. In: Fauser, B.C.J.M., Genazzani, A.R. (eds) Frontiers in Gynecological Endocrinology. ISGE Series. Springer, Cham. https://doi.org/10.1007/978-3-319-09662-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-09662-9_17
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09661-2
Online ISBN: 978-3-319-09662-9
eBook Packages: MedicineMedicine (R0)