Abstract
Chronic kidney disease (CKD) is one of the major causes of morbidity and mortality worldwide and it is closely related to cardiovascular disease. The most important causes of CKD in developed countries are diabetic nephropathy and renovascular disease/hypertension. Despite a proven favorable effect of RAAS blockade on short-term parameters (blood pressure, proteinuria) as well as long term outcome (slower decline of GFR), progression of chronic kidney disease to end stage renal disease still occurs in many patients. Mineralocorticoid receptor activation inhibition is currently being studied as a new therapeutic approach to CKD. Next to the classic anti-diuretic and potassium wasting effects, aldosterone has been shown to directly impact the heart, central nervous system, vasculature and kidneys, promoting inflammation, fibrosis and tissue remodeling, independently of its effect on sodium status and blood pressure. In this chapter we will describe the non-conventional renal and extrarenal effects of aldosterone, and review the effects of mineralocorticoid receptor antagonists in cardiovascular diseases and chronic kidney disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic Kidney Disease
- Diabetic Nephropathy
- Angiotensin Converting Enzyme Inhibitor
- Chronic Kidney Disease Patient
- Mineralocorticoid Receptor
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Clinical Case ScenarioA 54 year old man suffering from CKD (eGFR 58 ml/min) was treated with the maximum dose of an ACE inhibitor. He had no primary glomerular disorder necessitating specific immunosuppressive treatment. However, proteinuria (4.8 g/day, target <1.0 g/day) and ambulatory blood pressure (155/96 mmHg) were insufficiently controlled. He was sent to a dietician and instructed to adhere to a moderate sodium restriction. He successfully changed his habits, reflected by a decrease in urinary sodium excretion from 245 mmol/day to 150 mmol/day, and a concomitant improvement of proteinuria (2.6 g/day) and blood pressure (145/88 mmHg), both typical for the amount of sodium restriction. As proteinuria and blood pressure were still above target, the decision was then made to add the MRA inhibitor spironolactone 25 mg once daily. The patient was extensively instructed to stop the drug in any circumstance of excessive water/sodium loss. The proteinuria (1.1 mmol/day) and blood pressure (132/81 mmHg) further decreased. There was an acceptable decrease in eGFR from 58 to 51 ml/min (reflecting the reversible effect of treatment on glomerular pressure) and renal function remained stable in three subsequent years. Based on the 24 h urine collections, he was reinforced to keep his sodium restriction of a few occasions. In the next summer he suffered from a severe gastroenteritis after having a barbecue. He immediately stopped spironolactone and promptly contacted the outpatient clinic for blood collection. The potassium concentration remained within acceptable limits. After recovery of his illness, spironolactone was started again.
Introduction
Chronic kidney disease (CKD) is characterized by prolonged (≥3 months) structural and functional abnormalities of the kidneys. CKD is defined by a decreased renal function, that is glomerular filtration rate (GFR) <60 mL/min/1.73 m2, and/or urinary loss of protein. The latter, proteinuria, may also be measured as albuminuria.
CKD is one of the major causes of morbidity and mortality worldwide and it is closely related to cardiovascular disease. CKD prevalence has been steadily increasing over the last decades, leading to a large global disease burden. The most important causes of CKD in developed countries are diabetic nephropathy and renovascular disease/hypertension. Deterioration of renal function into end stage renal disease (ESRD) leads to a requirement for renal replacement therapy, i.e. dialysis or kidney transplantation. Unfortunately, the possibility for transplantation is limited by donor shortage, and dialysis is associated with a poor quality of life, partly caused by an extremely increased risk of cardiovascular complications. Prevention of renal function loss in the earlier stages of CKD is therefore of utmost importance.
The so-called renoprotective treatment aims to halt kidney damage and thus progressive renal function loss with blood pressure and proteinuria as important intermediate outcome measures. Therapy of choice is blockade of the renin-angiotensin-aldosterone system (RAAS), with either angiotensin converting enzyme inhibitors (ACEi), or angiotensin II receptor blockers (ARB). Despite a proven favorable effect of RAAS blockade on short-term parameters (blood pressure, proteinuria) as well as long term outcome (slower decline of GFR), progression of CKD to ESRD still occurs in many patients. In the search for additional treatment options, combination therapy by ACEi and ARB, or combination of either ACEi or ARB with direct renin-inhibitor, unfortunately do not improve long term renal outcome, and may even worsen it.
Mineralocorticoid receptor activation (MRA) inhibition is currently being studied as a new therapeutic approach to CKD. Although this therapy is not new, there is new attention, fuelled by striking new insights that have been obtained in the understanding of aldosterone and its (patho) physiological effects. Next to the classic anti-diuretic and potassium wasting effects, aldosterone has been shown to directly impact the heart, central nervous system, vasculature and kidneys, promoting inflammation, fibrosis and tissue remodeling, independently of its effect on sodium status and blood pressure. Furthermore, it has been shown that during prolonged RAAS blockade treatment, aldosterone levels return to normal only in 10–50 % of cases, suggesting a window of opportunity for intervention. Here we will review the role of MRA inhibition as an added treatment option in the management of chronic kidney disease.
Aldosterone
Classic Effects and Regulation of Aldosterone Release
Aldosterone, primarily synthesized in the zona glomerulosa of the adrenal cortex, is a steroid hormone with mineral corticoid activity. In addition, aldosterone is also locally synthesized in the blood vessels, brain, heart and adipocytes. Adrenal release of aldosterone is stimulated by an increase in angiotensin II, by hyperkalemia and by the adrenocorticotropic hormone (ACTH). Circulating aldosterone levels are higher in men than in women, but the clinical significance of this difference is so far unknown.
The classic role of aldosterone is homeostatic volume control by promoting sodium and fluid reabsorption in the kidneys, thus providing a main contribution to the body’s defense against sodium/volume depletion. Furthermore in hyperkalemia aldosterone promotes potassium excretion in the kidneys and colon. These classical effects are achieved by binding to the mineralocorticoid receptor (MR), which is located in the cortical collecting ducts in the distal nephron. The MR is a cytosolic receptor which migrates to the cell nucleus when ligand-activated. Here it attaches to the hormone regulatory part of target genes, enhancing transcription and translation of these genes, and thus stimulating the synthesis of aldosterone-induced proteins. The subsequent molecular pathway leads to an increased expression of the Na+/K+ pump in the apical epithelial membrane of the cell, increasing sodium reabsorption and potassium wasting. Furthermore, it increases expression of the basolateral Na+/K+ ATPase, stimulating sodium extrusion out of the cell and potassium entry into the cell. Lastly it increases the expression of apical renal outer medullary potassium channels which are involved in passive excretion of potassium. The MR has equal affinity to both mineral corticosteroids and glucocorticosteroids. While glucocorticoid plasma levels greatly exceed aldosterone plasma levels, the enzyme 11-beta-hydroxysteroid dehydrogenase-2 metabolizes intracellular glucocorticosteroid levels from 100 to 10-fold that of aldosterone, rendering aldosterone the major activator of the MR.
Non-classic Effects of Aldosterone
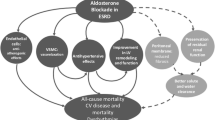
In the last decades new renal and extra-renal effects of aldosterone have been found, which can be reversed by MRA inhibition (Fig. 5.1). It has become known that aldosterone not only leads to genomic effects through activation of the MR, but can also lead to rapid non-genomic effects which do not require the transcriptional pathway described earlier [1, 2]. These non-genomic effects consist primarily of pro-fibrotic and pro-inflammatory changes and are mediated through (an interplay of) the cytosolic MR and aldosterone receptors in the cell membrane (Fig. 5.2). Aldosterone exerts these effects on not only renal targets, such as podocytes, mesangial cells and renal vasculature, but also on extra-renal targets where the MR has been found, primarily cardiomyocytes, endothelial cells, vascular smooth muscle cells, adipocytes, and macrophages [3].
Stimulation of cytosolic and membrane MRs by aldosterone in combination with stimulation of aldosterone receptors in the cell membrane lead to pro-inflammatory and pro-fibrotic effects [2]. In the mitochondria NADPH oxidase activity is increased, while G6DP activity is reduced, stimulating ROS generation [3]. Furthermore aldosterone upregulates pro-inflammatory and pro-fibrotic cytokines, and induces macrophage infiltration [4]. Ultimately these changes lead to tissue inflammation and fibrosis. MR mineralocorticoid receptor, Ald aldosterone, AR aldosterone receptor, G6PD glucose-6-phosphate dehydrogenase, ROS reactive oxygen species; IL-6 interleukin; TNF-α tumor necrosis factor, MCP-1 monocyte chemoattractant protein, ICAM-1 intercellular adhesion molecule, CTGF connective tissue growth factor, PAI-1 plasminogen activator inhibitor, TGF-b transforming growth factor
Aldosterone is pathophysiologically involved in kidney damage through multiple mechanisms in addition to the effects of elevated systemic blood pressure [4, 5] (Fig. 5.2). Firstly, glomerular damage is induced by the increased production of reactive oxygen species (ROS) by mitochondria [6, 7]. Furthermore, aldosterone increases expression of the pro-inflammatory serine/threonine-protein kinase and activates NFkB, which ultimately lead to mesangial inflammation, fibrosis, and glomerular injury [8]. Also macrophage infiltration into the renal cortex is stimulated by aldosterone, which promotes inflammation [9]. Lastly increased local renal aldosterone production has been shown to induce apoptosis in podocytes, through mechanisms which are not completely elucidated [10]. These local effects were larger in rats with diabetes mellitus, which was associated with increased MR and aldosterone levels. All the mechanisms listed above may contribute to renal damage and consequent deterioration of kidney function. Indeed, an increased proteinuria has been found in patients with excess aldosteronemia, independent of blood pressure [4, 5]. As proteinuria is a bad prognostic sign for CKD, this adds to the rationale for aldosterone inhibition as a new promising approach in halting disease progression.
The heart was the first extra-renal organ where mineralocorticoid receptors were found. Here aldosterone has been shown to promote electrophysiological remodeling, leading to atrial fibrillation and ventricular arrhythmias [3]. Remodeling takes place through modulation of T-type, potassium L-type and ryanodine receptor calcium channel activity [11–14]. Furthermore through pro-inflammatory processes cardiac hypertrophy and cardiac fibrosis are propagated, resulting in a reduced cardiac function. The blood vessels are a target for aldosterone through MRs in the vascular smooth muscle cells and endothelial cells. Here too hyperaldosteronism can lead to tissue inflammation, remodeling and fibrosis. MRA leads to endothelial swelling and stiffness by promoting the insertion of the epithelial sodium channel (ENaC) into the cell membrane [15]. Due to a simultaneous decreased ability to form nitric oxide, vasodilatation is limited. There is evidence that this is a main pathophysiological event leading to hypertension caused by hyperaldosteronism, rather than excess fluid retention.
Excess aldosterone levels are associated with the cardiometabolic syndrome, which is characterized by insulin resistance, central obesity, dyslipidemia and hypertension [3, 16]. Aldosterone impairs pancreatic beta cell function by promoting inflammation and oxidative stress in the islets of Langerhans [17, 18]. Also aldosterone degrades insulin receptor substrate proteins, reducing insulin sensitivity and glucose-uptake [19]. Furthermore, aldosterone promotes the release of inflammatory cytokines from adipose tissues resulting in systemic inflammation and impaired glucose tolerance [18]. The MR is found in adipocytes and perivascular adipose tissue (PVAT). Adipocytes and PVAT can produce local levels of aldosterone which exert paracrine and autocrine effects, influencing not only adipose tissues but also the vasculature and thus promoting vascular remodeling. In line, aldosterone blockade has been shown to be particularly effective to reduce blood pressure in obesity–related hypertension [20].
The Interaction Between Salt Status and Aldosterone Effects
The activity of the RAAS depends on what is denoted to as the “salt status”, the RAAS being suppressed in state of sodium/fluid excess and being activated in a state of sodium depletion. Interestingly, an interaction between salt status and the pro-fibrotic, pro-inflammatory effects of aldosterone has been described, which is independent of blood pressure [21]. While in rats on a high or normal salt diet, high levels of aldosterone are associated with development of substantial end organ damage, high levels of aldosterone do not have such target organ effects during a low salt diet. Furthermore, the hypertrophic effects observed in high-salt, high-aldosterone rats can be completely reversed by the addition of MRA inhibitors, underlining the causal role of the mineralocorticoid receptor in the pathophysiological process. Salt excess has a role in sensitizing cardiovascular tissue for damage caused by an excess in aldosterone through mechanisms currently unknown. Interestingly new research shows that in CKD patients urinary salt excess is a significant predictor of urinary excretion of the mineralcorticoid metabolites tetrahydroaldosterone and tetrahydrocorticosterone, suggesting an alternative regulatory mechanism for aldosterone [22]. Whereas this novel insight may provide a missing link between CKD, high salt-status, and increased target organ damage, further research regarding this topic is warranted.
MRA Inhibition
MRA Inhibition in Heart Failure
After publication of the results from the large randomized controlled trials RALES and EPHESUS, MRA inhibition has been adopted as part of the standard treatment of chronic heart failure [23, 24]. These studies showed that MRA inhibition with spironolactone or eplerenone reduces morbidity and mortality in patients with severe chronic heart failure (NYHA functional class III and IV; Table 5.1). The EMPHASIS-HF study showed that eplerenone was also effective in patients with mild symptoms of heart failure (NYHA functional class II) [25]. This is supported by a meta-review published in 2009 which showed a reduction in all-cause mortality and an increase in ejection fraction in patients with left ventricular failure on spironolactone therapy [30]. However, in a recent observational study spironolactone was shown not to have a significant effect on risk of hospitalization and death, suggesting that the benefits of MRA inhibition that has been found in randomized trials may not be true in clinical practice [26]. However, in this study outcome was not adjusted for baseline disease severity and spironolactone use may have been more often applied in patients with a worse clinical condition. Currently the ACCF/AHA recommends MRA inhibition therapy in patients with NYHA class II to IV heart failure and a left ventricular ejection fraction of ≤35 %, unless contraindicated [31].
The Role of MRA Inhibition in CKD Treatment
Current treatment of CKD aims to prevent progressive renal function loss and its associated cardiovascular complications. Treatment of hypertension and proteinuria is the cornerstone of renoprotection. Inhibition of the RAAS by either ACEi or ARB has been proven to be an effective treatment option in CKD, reducing proteinuria as well as the rate of renal function loss. However, additional treatment options are necessary as progression of kidney disease still occurs in many patients. Combined blockade of the RAAS at different levels has been tested in several combinations, mostly ACEi combined with ARB [32]. Whereas dual blockade, with either ACEi plus ARB, or ACEI plus a renin-inhibitor is associated with a better efficacy on short term (i.e. proteinuria), hard outcome studies show that dual RAAS blockade does not confer better reno- and cardio-protection, but to the contrary, is associated with worse outcome [33].
Another strategy to improve the efficacy of RAAS-blockade, is in manipulating the salt status: correction of the volume overload in patients on monotherapy RAAS-blockade, by either diuretics, a low sodium diet or their combination, considerably potentiates the efficacy of monotherapy RAAS-blockade [34, 35, 36]. Post-hoc analyses suggest that moderate dietary sodium restriction is also associated with better long-term outcome of RAAS-blockade [37], but prospective studies, so far, are unfortunately lacking. Observational data have shown a worse long term outcome in subjects that consume very low (<5 g/day) amounts of salt [38]. Whereas this might be related to underlying conditions associated with poor intake and malnutrition, it has also been pointed out that reactive hyperreninemic hyperaldosteronism might exert an adverse effect in such subjects [39] (Fig. 5.3). Of note, despite interference with the renin-angiotensin axis, a low sodium diet during ACEi or ARB is associated with secondary hyperreninemic aldosteronism that is particularly marked during the combination of diuretics and low sodium, demonstrating that the feedback loop between volume status and aldosterone is not disrupted by the current modes of inhibition of the RAAS by ACEi or ARB.
Effects of enalapril on PRA and PAC (mean ± SEM) on liberal (broken lines) and low (continuous lines) sodium diet. PRA plasma renin activity, PAC plasma aldosterone concentration (Reprinted by permission from Macmillan Publishers Ltd: Navis et al. [39])
It has long been known that while under RAAS inhibition treatment with ACEi or ARB, aldosterone levels initially decrease, but can thereafter gradually increase to pretreatment, or even exceed pretreatment levels, a phenomenon that has been called “aldosterone breakthrough” or “aldosterone escape” [40] (Fig. 5.3). Whereas this so-called escape is usually associated with long term treatment, detailed assessment of the early effects of RAAS-blockade on aldosterone levels demonstrate that, actually, aldosterone displays an early partial return towards its baseline values even during the first week of treatment, possibly in response to the negative sodium balance induced by the RAAS-blockade, again demonstrating the preservation of aldosterone’s role in volume homeostasis, despite ACE-inhibition [41].
Keeping in mind the deleterious effects of aldosterone on the kidneys, MRA inhibition is a promising new therapeutic approach in CKD. Studies in experimental animals, in proteinuric models and in CKD patients support a renoprotective effect of MRA inhibition. As monotherapy RAAS-blockade is therapy of choice for CKD, in particular the added effects of MRA to ACEi or ARB are of clinical interest. These could be due to interference with classic effects of aldosterone (i.e. a diuretic effect of MRA) as well as due to interference with the non-classic effects of aldosterone. A Cochrane review dating from 2009 has shown that MRA antagonism in addition to an ACEi or ARB, can in small doses significantly reduce proteinuria while only slightly influencing renal function or blood pressure, with the adverse effect of an increased incidence of hyperkalemia (Table 5.1) [27]. Long term clinical endpoints such as cardiovascular events or renal function were not available in the included studies. A recent systematic review concerning the effect of MRA inhibition in addition to RAAS inhibition on diabetic nephropathy confirmed these effects on proteinuria, blood pressure, glomerular filtration rate and potassium levels, however data on long term cardiovascular and renal outcome were still not available [28]. In non-diabetic patients with CKD, long term effects of MRA inhibition in addition to RAAS inhibition are also unavailable, while short term effects are comparable to that in patients with diabetic nephropathy [29]. In patients with mild CKD, treatment with MRA inhibitors was shown to have a beneficial effect on left ventricular mass and arterial stiffness [42]. In summary, the short-term effects of spironolactone and eplerenone are beneficial for CKD patients and these agents are also safe, provided that potassium levels are being monitored. However, there is a lack of data concerning the long term clinical outcome of dual treatment with an ACE-inhibitor or ARB and a MRA inhibitor, as well as lack of data on the mechanism of the added effect of MRA to ACEi or ARB. The treatment can be considered in individual patients who otherwise have an unacceptable poor (renal) prognosis, given that these patients are extremely well instructed with regards to stopping the MRA inhibitor in circumstances of volume/sodium depletion. It is obvious that more research is needed to determine the definite role of MRA inhibition in CKD treatment, in particular whether the risks outweigh te benefits.
Aldosterone Breakthrough and Sodium Status Intervention in CKD
The importance of aldosterone breakthrough during RAAS blockade treatment on clinical endpoints is currently unknown. While a low salt diet is associated with higher aldosterone levels, the deleterious effects of aldosterone on end organs are generally absent during low salt-status. In contrast, in patients with a high salt intake, however, aldosterone breakthrough may be associated with an increase in target organ damage. Better elucidation of the interrelationships between salt status and the renoprotective effects of aldosterone blockade in CKD is therefore needed. This could also contribute to a better understanding of the mechanisms of the added effects of MRA to ACE-inhibition or ARB. In a best-case scenario MRA inhibition could be an add-on therapy that allows to combine ACE-inhibition or ARB with low sodium diet potentiating its renoprotective effects, without the possible adverse effects of a reactive rise in aldosterone. Several studies on the renoprotective effects of MRA inhibition are currently ongoing, including long term intervention for prevention of diabetic nephropathy (PRIORITY) [43] and a short-term intervention analyzing the role of sodium status, and comparison with conventional diuretic during RAAS-blockade (http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2133). These results may provide insight in the complex interplay between aldosterone breakthrough, salt status and MRA inhibition and thus delineate the optimal use of MRA for renoprotection.
Effects on Vascular Fibrosis and Hypertension
Spironolactone and eplerenone are anti-hypertensive agents used in patients with resistant essential hypertension. The rationale behind the use of MRA inhibitors for treating hypertension is derived from the classical genomic effects of aldosterone. By blocking the MR, slow onset volume and sodium loss occurs, and thusly blood pressure is lowered. However, in the absence of activation of the MR in vascular tissues, also arterial stiffness induced by aldosterone can be prevented. In 2008 a study has shown that treatment with eplerenone can reduce the media collagen/elastin ratio, reducing vascular stiffness [44]. Also, in low-renin hypertensive patients eplerenone was shown to be more effective than the ARB losartan in lowering blood pressure [45]. Possibly, in a low-renin state the MRA inhibitor is more efficient in reducing the non-classical effects of aldosterone than ARB. However, in diabetic patients spironolactone was shown not to influence endothelial function, while effectively reducing blood pressure [46]. While MRA inhibitors are effective in reducing blood pressure, more research is needed to clear up the physiological mechanisms.
MRA Inhibition and PTH
Primary hyperparathyroidism is a known risk factor for developing cardiovascular disease. Recent findings have suggested a bilateral interplay between aldosterone and parathyroid hormone levels. Aldosterone secretion in the zona glomerulosa of the adrenal gland is regulated by Ca2+ channels [47]. There is evidence PTH levels can up-regulate aldosterone by increasing plasma calcium [48]. On the other hand, relative hyperaldosteronism stimulates urinary and fecal Ca2+ excretion in the presence of excess dietary salt intake, and thusly stimulates PTH secretion [49]. Recently the MR was also identified in parathyroid cells [50]. It is unknown, however, whether activation of these MRs also lead to increased PTH excretion. The combination of relative hyperaldosteronism and elevated PTH levels can increase target organ damage, as well as have deleterious skeletal effects. Research has shown that patients with congestive heart failure treated with spironolactone have a reduced fracture risk compared with patients without MRA inhibition treatment [51]. Recently the relationship between the RAAS and the PTH level in humans, without primary hyperaldosteronism has been studied, and it was shown that spironolactone treatment can slightly but statistically significantly reduce plasma PTH levels [52]. However in this study aldosterone infusion did not directly increase PTH levels, suggesting a more long term gradual effect of aldosterone on PTH levels. As secondary hyperparathyroidism often occurs in both CKD and congestive heart failure patients, the interplay between PTH and aldosterone may be relevant [53]. Future research will have to determine the role of PTH on end organ damage, and the effect of MRA inhibitors on PTH and calcium metabolism.
Adverse Effects of MRA Inhibition
The most important adverse events associated with MRA inhibition therapy are hyperkalemia and hemodynamically mediated renal function loss. Most trials concerning MRA inhibitor usage show an increase in hyperkalemic events in patients treated with mineralocorticoid receptor antagonists, however, this was not associated with an increased risk of hospitalization or death in patients with heart failure or CKD [26, 54]. Furthermore, significant or irreversible renal function loss was not seen. Still, long-term studies on the effect of MRA inhibition in CKD patients on renal function and cardiovascular events are currently lacking.
Conclusion
New insights in the non-genomic effects of aldosterone on multiple target organs render MRA inhibition a promising approach in CKD treatment. The role of aldosterone breakthrough during RAAS blockade therapy is under investigation. While salt restriction is associated with higher aldosterone levels during ACEi or ARB, low salt intake could prevent the non-classical pro-fibrotic pro-inflammatory effects of aldosterone. Future studies will have to answer the question whether there is a role for MRA inhibition in CKD treatment in addition to current evidence-based renoprotective treatment (RAAS blockade with ACEi or ARB, salt restriction). Furthermore, upcoming data on the efficacy of MRA inhibition as compared to conventional diuretics, and the optimal combination with dietary salt targeting in CKD will allow the understanding needed to use MRA inhibition for optimal therapeutic benefit in CKD patients.
References
Shavit L, Lifschitz MD, Epstein M. Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: current concepts and emerging treatment paradigms. Kidney Int. 2012;81(10):955–68.
Williams JS. Evolving research in nongenomic actions of aldosterone. Curr Opin Endocrinol Diabetes Obes. 2013;20(3):198–203.
Nguyen Dinh Cat A, Jaisser F. Extrarenal effects of aldosterone. Curr Opin Nephrol Hypertens. 2012;21(2):147–56.
Ueda K, Nagase M. Mineralocorticoid receptor activation as an etiological factor in kidney diseases. Clin Exp Nephrol. 2014;18(1):16–23.
Ritz E, Tomaschitz A. Aldosterone and kidney: a rapidly moving frontier (an update). Nephrol Dial Transplant. 2013.
Zhu C, Huang S, Yuan Y, Ding G, Chen R, Liu B, et al. Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARgamma. Am J Pathol. 2011;178(5):2020–31.
Terada Y, Ueda S, Hamada K, Shimamura Y, Ogata K, Inoue K, et al. Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoid-inducible protein kinase-1. Clin Exp Nephrol. 2012;16(1):81–8.
Leroy V, De Seigneux S, Agassiz V, Hasler U, Rafestin-Oblin ME, Vinciguerra M, et al. Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol. 2009;20(1):131–44.
Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59(2):324–30.
Lee SH, Yoo TH, Nam BY, Kim DK, Li JJ, Jung DS, et al. Activation of local aldosterone system within podocytes is involved in apoptosis under diabetic conditions. Am J Physiol Renal Physiol. 2009;297(5):F1381–90.
Lalevee N, Rebsamen MC, Barrere-Lemaire S, Perrier E, Nargeot J, Benitah JP, et al. Aldosterone increases T-type calcium channel expression and in vitro beating frequency in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;67(2):216–24.
Perrier R, Richard S, Sainte-Marie Y, Rossier BC, Jaisser F, Hummler E, et al. A direct relationship between plasma aldosterone and cardiac L-type Ca2+ current in mice. J Physiol. 2005;569(Pt 1):153–62.
Gomez AM, Rueda A, Sainte-Marie Y, Pereira L, Zissimopoulos S, Zhu X, et al. Mineralocorticoid modulation of cardiac ryanodine receptor activity is associated with downregulation of FK506-binding proteins. Circulation. 2009;119(16):2179–87.
Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, Perrier R, Soukaseum C, Nguyen Dinh Cat A, et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 2005;111(23):3025–33.
Hillebrand U, Schillers H, Riethmuller C, Stock C, Wilhelmi M, Oberleithner H, et al. Dose-dependent endothelial cell growth and stiffening by aldosterone: endothelial protection by eplerenone. J Hypertens. 2007;25(3):639–47.
Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62(2):313–9.
Mosso LM, Carvajal CA, Maiz A, Ortiz EH, Castillo CR, Artigas RA, et al. A possible association between primary aldosteronism and a lower beta-cell function. J Hypertens. 2007;25(10):2125–30.
Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150(11):776–83.
Wada T, Ohshima S, Fujisawa E, Koya D, Tsuneki H, Sasaoka T. Aldosterone inhibits insulin-induced glucose uptake by degradation of insulin receptor substrate (IRS) 1 and IRS2 via a reactive oxygen species-mediated pathway in 3T3-L1 adipocytes. Endocrinology. 2009;150(4):1662–9.
Costa MB, Andrade Ezequiel DG, Morais Lovis JC, Oliveira MM, Baumgratz de Paula R. Aldosterone antagonist decreases blood pressure and improves metabolic parameters in obese patients with the metabolic syndrome. J Clin Hypertens (Greenwich). 2010;12(9):753–5.
Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6(5):261–73.
McQuarrie EP, Freel EM, Mark PB, Fraser R, Connell JM, Jardine AG. Urinary sodium excretion is the main determinant of mineralocorticoid excretion rates in patients with chronic kidney disease. Nephrol Dial Transplant. 2013;28(6):1526–32.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21.
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
Lee KK, Shilane D, Hlatky MA, Yang J, Steimle AE, Go AS. Effectiveness and safety of spironolactone for systolic heart failure. Am J Cardiol. 2013;112(9):1427–32.
Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009;(3):CD007004.
Mavrakanas TA, Gariani K, Martin PY. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy: a systematic review. Eur J Intern Med. 2014;25(2):173–6.
Boesby L, Elung-Jensen T, Klausen TW, Strandgaard S, Kamper AL. Moderate antiproteinuric effect of add-on aldosterone blockade with eplerenone in non-diabetic chronic kidney disease. A randomized cross-over study. PLoS One. 2011;6(11):e26904.
Ezekowitz JA, McAlister FA. Aldosterone blockade and left ventricular dysfunction: a systematic review of randomized clinical trials. Eur Heart J. 2009;30(4):469–77.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey Jr DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239.
Vidt DG. Telmisartan, ramipril, or both in patients at high risk for vascular events. Curr Hypertens Rep. 2008;10(5):343–4.
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–13.
Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol. 2008;19(5):999–1007.
Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ. 2011;343:d4366.
Kwakernaak AJ, Krikken JA, Binnenmars SH, Visser FW, Hemmelder MH, Woittiez AJ, et al. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2(5):385–395.
Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23(1):165–73.
Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, MacIsaac RJ, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34(3):703–9.
Navis G, de Jong PE, Donker AJ, van der Hem GK, de Zeeuw D. Moderate sodium restriction in hypertensive subjects: renal effects of ACE-inhibition. Kidney Int. 1987;31(3):815–9. doi:10.1038/ki.1987.71.
Lijnen P, Staessen J, Fagard R, Amery A. Increase in plasma aldosterone during prolonged captopril treatment. Am J Cardiol. 1982;49(6):1561–3.
Navis G, de Jong P, Donker AJ, van der Hem GK, de Zeeuw D. Diuretic effects of angiotensin-converting enzyme inhibition: comparison of low and liberal sodium diet in hypertensive patients. J Cardiovasc Pharmacol. 1987;9(6):743–8.
Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54(6):505–12.
Siwy J, Schanstra JP, Argiles A, Bakker SJ, Beige J, Boucek P, et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant. 2014;29(8):1563–70.
Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension. 2008;51(2):432–9.
Weinberger MH, White WB, Ruilope LM, MacDonald TM, Davidson RC, Roniker B, et al. Effects of eplerenone versus losartan in patients with low-renin hypertension. Am Heart J. 2005;150(3):426–33.
Swaminathan K, Davies J, George J, Rajendra NS, Morris AD, Struthers AD. Spironolactone for poorly controlled hypertension in type 2 diabetes: conflicting effects on blood pressure, endothelial function, glycaemic control and hormonal profiles. Diabetologia. 2008;51(5):762–8.
Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84(2):489–539.
Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014;63(1):20–31.
McCarron DA, Rankin LI, Bennett WM, Krutzik S, McClung MR, Luft FC. Urinary calcium excretion at extremes of sodium intake in normal man. Am J Nephrol. 1981;1(2):84–90.
Maniero C, Fassina A, Guzzardo V, Lenzini L, Amadori G, Pelizzo MR, et al. Primary hyperparathyroidism with concurrent primary aldosteronism. Hypertension. 2011;58(3):341–6.
Carbone LD, Cross JD, Raza SH, Bush AJ, Sepanski RJ, Dhawan S, et al. Fracture risk in men with congestive heart failure risk reduction with spironolactone. J Am Coll Cardiol. 2008;52(2):135–8.
Brown JM, Williams JS, Luther JM, Garg R, Garza AE, Pojoga LH, et al. Human interventions to characterize novel relationships between the renin-angiotensin-aldosterone system and parathyroid hormone. Hypertension. 2014;63(2):273–80.
Wu C, Kato TS, Pronschinske K, Qiu S, Naka Y, Takayama H, et al. Dynamics of bone turnover markers in patients with heart failure and following haemodynamic improvement through ventricular assist device implantation. Eur J Heart Fail. 2012;14(12):1356–65.
Edwards NC, Steeds RP, Chue CD, Stewart PM, Ferro CJ, Townend JN. The safety and tolerability of spironolactone in patients with mild to moderate chronic kidney disease. Br J Clin Pharmacol. 2012;73(3):447–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gant, C., Laverman, G.D., Navis, G. (2015). MRA Inhibition in CKD: More Than Salt and Water. In: Goldsmith, D., Covic, A., Spaak, J. (eds) Cardio-Renal Clinical Challenges. Springer, Cham. https://doi.org/10.1007/978-3-319-09162-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-09162-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09161-7
Online ISBN: 978-3-319-09162-4
eBook Packages: MedicineMedicine (R0)