Abstract
This research originates from a wider three-year hydrogeological study about Aosta Plain (Italy), from Villeneuve to Pontey municipalities (12 ground and surface waters sample surveys leading to the chemical analysis of more than 800 samples). The hydrogeochemical characterization of the unconfined aquifer shows that 80 % of the groundwater sampled from June 2006 to May 2009 has Ca-HCOs composition. Instead, 8.4 % of the samples shows sulphates concentration higher than carbonate and chlorides one. This predominant inversion is detected in few wells, located in a delimited area. Considering the absence of hazard sources connected to human activities, it is reasonable to introduce the hypothesis of natural occurrence of sulphates. Therefore, the delimited research area has been the object of geological, geomorphological and hydrochemical observations to trace the sulphates occurrence back to soluble lithotypes or to pyrite mineralization, both present in the area. Different data elaborations reveals that the predominant sulphates source is represented by gypsum formations normally associated with particular carbonate rocks.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
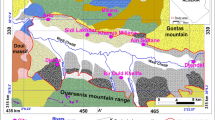
A three-year study, regarded mainly the plain portion and the first urbanised hill zone of the medium Aosta Valley (North-west of Italy) extending from Villeneuve to Pontey municipalities, was conducted from June 2006 to May 2009. The main results of this study induced to deepen the sulphates presence in a new research area (Fig. 260.l).
The hydrogeological characterization of the above defined area has led to interesting hydrodinamic and hydrochimical conclusions.
On the orographic right side, a strong contribution from the versant off the municipalities of Gressan and Charvensod is shown by piezometric lines nearly parallel to the versant and flow lines evidently following a South-North direction. Regarding the hydrochemical characterization, the analyses with a suitable ionic balance are 582 relating to 97 check points off the 103 initially sampled. They show that more than 80 % of the groundwater sampled has Ca-HCO3 composition (HCO3 −>SO4 2−>Cl− – Ca2+>Mg2+>Alk). Instead, 8.4 % of the samples shows sulphates concentrations higher than carbonate and chlorides ones, while cations remain exactly alike to the one of the most diffused waters (SO4 2−>HCO3 −>Cl− – Ca2+>Mg2+>Alk). The inversion sulphates-carbonates may be the symptom of a waters time of travel relatively long because the carbonates are less solvable than the sulphates and they are likely to precipitate before. In this case also an inversion magnesium-calcium would be recorded, the reason being the fact that the calcium precipitate together with the carbonates. In this kind of waters this inversion is not registered. Therefore, the composition of the waters can be attributed to an input of sulphates, which are reasonably of natural origin, considering the absence of hazard sources connected to human activities. This area is of small dimensions and include the portion of the plain in the municipalities of Aymavilles, Jovengan, Gressan and Charvensod.
From a pure hydrochemical point of view, the River Dora Baltea waters show a ratio Mg2+/Ca2+ where calcium is always the dominant element with a range value from 0.035 to 0.478 mg/l and an average of 0.267 ± 0.073 mg/l. Much wider is the range relative to the ratio HCO3 −/SO4 2− which fluctuates from 0.429 to 4.528 mg/l. This finding indicates that in some cases the sulphates are in a greater amount than the bicarbonates. However, this characteristic is peculiar of the waters sampled from the river at the above mentioned municipalities and after the confluence of the torrents Grand-Eyvia, Gressan and Clou-Neuf, with highly rich in sulphates waters (Graph 260.1).
2 Materials and Methods
The method followed to understand and describe the relative high concentration of sulphates in ground and surface waters consists in geological, petrographic, geo- morphological and hydrochemical observations and elaborations. Starting from the official Aosta Valley geological cartography (1:100,000 scale) and the existing geomorphology maps, the analysis developed with a series of investigations on the spot aimed to describe the outcrops of pyrite rich rocks and soluble rocks of the area, their mineralogical composition and the geomorphological evidences in surface of their presence in the deep layers. With reference to the results of chemical analysis carried out on samples of water during a comprehensive three-year study, it was decided to focus the hydrochemical study narrowing down the search to only the Gressan and surrounding area. Indeed, this area stands out for higher sulphate content in the groundwater and in the surface water and for a number of sources picked up and monitored by the municipality.
The torrent Gressan and some of its tributaries were sampled at seven spots. Same important springs and two wells in the plain were sampled too to define the relationships between ground and surface water with the geological background. The geochemical modelling that has been used to evaluate the activities of the solution components of the mineral gypsum (CaSO4. 2H2O) is PHREEQC code (Parkhurst and Appelo 1999).
3 Results
In the multi-strata system of the Western Alps, in which the area of study is located, there are two kinds of soluble lithotypes: the carbonate and sulphatic ones. They are gypsum-carbonate breccias, gypsum and anhydrite rocks. Along the cut of the torrent Gressan the soluble rocks outcrops show sub-vertical faces, the surface of which appears intensively altered and characterized by the presence, at their base, of significant deposits connected to breaking up processes. These breccias are composed of carbonate centimetre and subcentimetre-size clasts, which are white and saccharoid, in a finer-grained clastic sulphatic and carbonate matrix, pale yellow that becomes yellow ocher at the contact of the clasts.
On the orographic right side of Valnontey, the main tributary valley of Cogne Valley, it can be documented a huge mass of sulphate rocks of a yellow colour and powdery aspect, porous and sometimes cavernous.
Sulphate rocks with associated marble that follow the important thrust of the Piedmont Zone over the Falda of Gran Paradiso (Pennidic System) are present on the left side of the same valley. The observation of the dust produced from a sampled rock under the optical microscope demonstrates the presence of carbonates, gypsum and anhydrite, tabular shaped albite, green emerald phengite, knee- twinned amphibole with elongate prismatic habit, rutile, chromite, chlorite and opaque minerals.
The presence of sulphate rocks in the deep layers can have geomorphologic evidences in surface. In particular, where this kind of phenomenon acts, it is possible to recognize closed depressions, collapse zones, sinkholes and “collapsed” slopes. In the area of study, in the territory of Villeneuve, a sinkhole has been described from Alberto et al. 2004.
Moreover, some Deep-Seated Gravitational Slope Deformations (DSGSD), recognizable in the area of Jovengan, Gressan and Charvensod, seem to be connected to the presence of this kind of rocks and to their deep dissolution. Evidences of their activity have been recognized (trenches perpendicular to the versant and hollows aligned parallel to the slopes).
The hydrochemical study has been focused to show if the oxidation of pyrite can be the main responsible process of relatively high values of SO42 in ground and surface waters or if the sulphates amount is due to triassic rocks solution. With regard to surface water, the torrent Gressan and some of its tributaries were sampled at seven spots. The electrical conductivity showed an increase in its value with the decreasing of altitude. The concentration of sulphates is characterized by a comparable trend. The same trend is also verified by the samples of groundwater from some springs. This finding seems to bring the presence of sulphates to an input related to the dissolution of gypsum found in Carniola rocks. To have another evidences of this thesis, the activities of some water samples components are evaluated. After having inserted the values of anions and cations obtained from chemical analysis and the physical parameters required by the PHREEQC code and having obtained the activities, the values log (a Ca2+) versus −log(a SO4 2−), indicated as pCa2+ and pSO 24 respectively, are plotted on a second graph Fig. 260.2. The graph shows the line for pK gypsum, where (pCa2 +) (p SO4 2−) = 4, 6 where the mineral equilibrium condition is verified. This line splits the graph area in two fields. Water samples that plot to the left field are undersaturated, while those that plot to the right are oversaturated with respect to gypsum. This kind of data representation is useful to evaluate the chemical evolution of the groundwater. In fact, by taking samples along the flow path and by plotting their values of (pCa2+) (p SO4 2−), it is possible to make some considerations about the origin of sulphate. Plotting the values of (pCa2+) (p SO4 2−) of groundwater two springs (spring 1 and spring 2), one piezometer and one well sampled along the versant and in the plain from higher to lower elevations, the samples take place in the under saturated field and along the red line drawn in the graph (Fig. 260.2).
4 Conclusions
Along their way downstream, waters, extremely aggressive towards calcium and sulphates, come into contact with the soluble Triassic formations enriching themselves of both elements. This hypothesis is underpinned by the assessment and the comparison of the activities of calcium and sulphates in the samples of groundwater considered. In fact, the samples take place in the under saturated field and along the red line drawn in the graph Fig. 260.2 showing a trend directly proportional to the altitude and inversely proportional to the concentration of the two elements. This situation reveals that there are sources of both calcium and sulphate in the groundwater along its flow path. This perfect correspondence between the activities of Ca2+ and SO4 2− can be related to an input from gypsum dissolution. On the contrary, the oxidation of pyrite as the reason for the concentration of SO42− can be excluded. Indeed, in this case the points would line up parallel to the y (p SO42−), showing a source of sulphates into the water but no additional input of calcium, whose activity stays constant.
References
Alberto W, Carraro F, Giardino M, Tiranti D (2004) Segnalazione di sinkholes a vari stadi di evoluzione nelle Alpi Occidentali, 37–52. In: Atti del Primo Seminario Nazionale: Stato dell’arte sullo studio dei fenomeni di sinkholes e ruolo delle amministrazioni statali e locali nel governo del territorio, 20–21 Maggio 2004, Roma, Italy
Alberto W, Carraro F, Giardino M, Tiranti D (2007) Genesis and evolution of “pseudocarniole”: preliminary observations from the Susa Valley (Western Italy). In: Schreiber BC, Lugli S, Babel M (eds), Evaporites through space and time. Geological Society, London, Special Publication, 285, pp 155–168
Debenedetti A, Turi B (1975) Carniole della Valle d’Aosta, studio isotopico ed ipotesi genetica. Bollettino della Societa Geologica Italiana 94:1883–1894
Deutsch WJ (1997) Groundwater geochemistry, foundamentals and applications to cantamination. Lewis Publishers, USA, 221p
Lodi LP (2010) Dallo studio idrogeologico della piana di Aosta alla redazio- ne di linee guida per la caratterizzazione idrogeologica dei contesti alpini di fon- dovalle. Unpublished Ph.D. thesis
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2)—a computer program for speciation, reaction-path, ID-transport, and inverse geochemical calculations. U.S. Geological Survey Water Resources Investigations Report 994259, 312 pp
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
De Maio, M., Lodi, L.P., Suozzi, E. (2015). Natural Occurrence of Sulphates in Ground and Surface Waters in Aosta Plain (Italy). In: Lollino, G., Manconi, A., Guzzetti, F., Culshaw, M., Bobrowsky, P., Luino, F. (eds) Engineering Geology for Society and Territory - Volume 5. Springer, Cham. https://doi.org/10.1007/978-3-319-09048-1_260
Download citation
DOI: https://doi.org/10.1007/978-3-319-09048-1_260
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09047-4
Online ISBN: 978-3-319-09048-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)