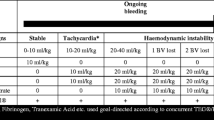
Abstract
Transfusion in trauma has undergone a paradigm shift in the past decade. Previous resuscitation strategies for trauma haemorrhage featured early administration of large volumes of crystalloids with subsequent delivery of plasma to treat a gradually evolving coagulopathy due to haemodilution, hypothermia and acidosis. However, the identification of an acute endogenous coagulopathy in trauma victims triggered a re-evaluation of this strategy. Acute traumatic coagulopathy (ATC) occurs rapidly after severe injury as a product of combined tissue damage and hypoperfusion. A series of retrospective observational studies have identified that trauma patients receiving early (and high-dose) administration of haemostatic blood products (including plasma, fibrinogen and platelets), rather than crystalloids, may have better survival with reduced morbidity. This has been attributed to better prevention and/or treatment of ATC. Most western trauma centres now utilise a massive haemorrhage protocol to guide rapid delivery of these blood products in prespecified ratios. However, the re-emergence of thromboelastography is offering promise to refine this formulaic approach and replace it with patient-tailored algorithms. This chapter will describe the recent evolution in trauma transfusion with focus on our developing understanding of the coagulopathy driving these changes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- International Normalise Ratio
- Trauma Patient
- Injury Severity Score
- Disseminate Intravascular Coagulation
- Tranexamic Acid
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
The past decade has seen a paradigm shift in the mode of trauma-haemorrhage resuscitation. This was driven by the recognition of an acute traumatic coagulopathy (ATC) in severely injured victims and the potential for improved survival with earlier administration of haemostatic blood products to treat it. This chapter will initially summarise the conventional concepts of traumatic coagulopathy, which have been understood and accepted for decades. They are generally iatrogenic in origin and develop gradually over time as a consequence of suboptimal resuscitation practices. It will then introduce and describe the aetiology and pathophysiology of ATC. This newly discovered entity develops rapidly after injury as a consequence of combined tissue disruption and systemic hypoperfusion. It is endogenous in origin and has led to a shift in our transfusion practices. These changes and potential future developments will then be discussed.
11.2 Conventional Concepts of Trauma-Induced Coagulopathy
Impairments of haemostasis after trauma result in difficult control of haemorrhage, increased transfusion requirements and worse mortality. Acute blood loss proceeds at a greater rate in the presence of systemic coagulopathy. Oozing of blood from blunt tissue injuries, vascular access sites and surgical incisions is not amenable to surgical control and may threaten viability. This uncontrollable haemorrhage may force the early termination of operations and result in the sacrifice of organs or limbs in order to preserve life. It also worsens outcomes from traumatic brain injury by an increased potential for intracranial haemorrhage and secondary neuronal damage.
Coagulopathy associated with trauma has been recognised for decades and is a constituent of the ‘triad of death’, together with hypothermia and acidosis. Classically it has been understood as due to loss, dilution or dysfunction of the coagulation proteases. Loss is explained as being due to bleeding or consumption, dilution from fluid administration and massive transfusion, whilst protease dysfunction results from hypothermia and the effect of acidosis on enzyme function.
Consumption of coagulation factors after trauma may not be limited to the location of tissue injury; trauma has frequently been cited as a disease that can precipitate disseminated intravascular coagulation (DIC). Whether trauma is a primary cause of DIC, as well as the mechanism through which DIC develops, is a matter of some scientific debate [1, 2]. Although systemic markers of coagulation activation (fibrin degradation products) are often elevated in trauma-induced coagulopathy (TIC), histological studies have failed to identify fibrin deposition, the hallmark of DIC. Whilst some bleeding trauma patients may be diagnosed and their prognosis categorised with DIC scoring systems, this is of limited clinical use because their specificity is poor and treatment is directed at the underlying pathology.
Until the most recent edition, the Advanced Trauma Life Support (ATLS) manual has recommended treating or preventing haemorrhagic shock by the initial administration of up to 2 l of crystalloid fluid. These synthetic products are capable of increasing intravascular volume and supporting tissue perfusion, at least temporarily. However, they have no haemostatic content, and diluting residual circulating coagulation factors produces an iatrogenic coagulopathy in a dose-response fashion [3].
Mild hypothermia is very common in trauma patients and probably has minimal clinical impact. Contemporary studies of clinically important hypothermia (<35 °C) have reported an incidence rate of 1.5–8 %, and this is dependent upon the characteristics of the trauma cohort, their environment and the timing of the sample measurement [4–6]. The cause of hypothermia is multifactorial. From the time of injury, trauma itself alters the normal central thermoregulation and blocks the shivering response. Bleeding with subsequent systemic hypoperfusion creates an oxygen deficit and impairs heat-producing respiration, particularly in the large cutaneous and skeletal muscle beds. Trauma patients, who are frequently under the influence of alcohol, are then invariably immobilised with consequent loss of muscle activity and undressed to facilitate inspection and management of wounds.
Acidosis is a common event in trauma, typically produced by low-flow shock states and excess ionic chloride administered during resuscitation. When pH was reduced from 7.4 to 7.1 in pigs, Martini et al. showed that thrombin generation decreased to 47 % of control values, whilst fibrinogen concentration decreased by 18 % [7]. The depletion of fibrinogen was attributable to accelerated degradation rather than impaired synthesis [8]. These decreases were also associated with a 47 % increase in splenic bleeding time. Although acidosis can be corrected by the administration of buffer solutions, this does not correct the coagulopathy [9, 10], implying that the acid effect is more than simply a physical reduction in protease activity.
The traditional description of TIC depicts it as developing late after injury and as a consequence of continued haemorrhage and subsequent medical therapy. Figure 11.1 illustrates the putative unidirectional nature of this pathology. However, in the past decade, a series of research studies have demonstrated that some trauma patients arrive in the emergency department with a coagulopathy already established. These patients have generally had short transfers with little time to become hypothermic or receive significant exogenous fluid resuscitation. The impairment of their haemostatic system occurs early, develops endogenously and subsequently becomes exacerbated by the conventional causes of coagulopathy.
11.3 Acute Traumatic Coagulopathy
The first landmark report of the existence of acute traumatic coagulopathy (ATC) was in a retrospective study of the admission coagulation results of 1,088 trauma patients transferred to the Royal London Hospital by air ambulance [11]. Twenty-four per cent arrived in the emergency department with a clinically significant coagulopathy already established, and they were four times more likely to die than those presenting with normal clotting parameters. Subsequent studies performed by independent research groups on a total of over 45,000 patients have confirmed the existence of this early coagulopathy [12–17]. All of these studies reported a strong association between acute coagulopathy and mortality and identified it as an independent risk factor for death [12]. It has also been associated with longer intensive care and hospital stays. Patients are more likely to develop acute renal injury [15] and multiple organ failure [14] and have fewer ventilator-free days [13, 14], and there is a trend towards an increased incidence of acute lung injury [14].
Injury severity is closely associated with the degree of acute coagulopathy seen after trauma. In the London study, only 10.8 % of patients with an injury severity score (ISS) of 15 or below had a coagulopathy, compared with 33.1 % of those with an ISS over 15 [11]. This figure increased to 61.7 % for those with an ISS over 45. In a larger German study, a coagulopathy was evident in 26 % of patients with an ISS 16–24, in 42 % of patients with an ISS 25–49 and in 70 % of patients with an ISS > 50, respectively [14]. However, not all patients who are severely injured present with a coagulopathy; tissue trauma alone does not appear sufficient.
Shock with tissue hypoperfusion is a strong independent risk factor for poor outcomes in trauma [18, 19] and is central to the aetiology of ATC. One study of acute coagulopathy found that no patient with a normal base deficit (BD) had prolonged prothrombin time (PT) or activated partial thromboplastin time (aPTT), regardless of injury severity or the amount of thrombin generated [13]. In contrast, there was a dose-dependent prolongation of clotting times with increasing systemic hypoperfusion. Only 2 % of patients with a BD under 6 mmol/l had prolonged clotting times, compared with 20 % of patients with a BD over 6 mmol/l. The synergistic relationship between tissue injury, systemic hypoperfusion and severity of ATC has been characterised in an observational study of over 5,000 trauma patients [20] (Fig. 11.2).
The relationship between injury severity and shock. Mortality of patients grouped according to ISS and BD. *p < 0.001 compared with ISS < 16, BD ≤ 0 [20]
Whilst it is accepted that tissue injury and systemic hypoperfusion are the principal aetiological drivers of ATC, the pathophysiology remains to be fully elucidated. Advocates of the DIC hypothesis propose that there is a rapid universal depletion of procoagulant factors caused by systemic consumption. However, contemporary evidence would indicate this is not the case. Functional activity of most coagulation factors required for thrombin activation is maintained at levels adequate for haemostasis. A few studies of severely injured trauma patients have identified a pronounced and isolated reduction of factor V down to activities around 30 %, a level consistent with prolongation of clotting times [21–23]. This might be of pathophysiological importance given that activated factor V is a target substrate for lysis by activated protein C. However, endogenous thrombin-generating potential has been reported to be normal or even enhanced in subjects with ATC [24].
Further downstream, clotting factor 1 (fibrinogen) is consistently reported as depleted early in trauma studies [25]. Concentrations below 1 g/dl acutely after injury have been reported, and thromboelastometric analysis frequently identifies an impairment of fibrin polymerisation. Unfortunately, at present, there are no rapidly available point of care tests available to measure circulating fibrinogen concentrations, and the optimal concentration necessary for effective haemostasis is unknown; contemporary guidelines recommend a plasma fibrinogen level of 1.5–2.0 g/dl [26].
Historically, TIC has been understood to develop exclusively in response to impairment or loss of procoagulant factors. The identification of ATC led to recognition of the pathological role of anticoagulant and fibrinolytic systems in response to trauma haemorrhage. Brohi et al. first identified a correlation between residual protein C depletion, assumed to be secondary to upregulated activation, and severity of ATC [13]. Subsequent clinical studies have confirmed the early activation of protein C after injury and its association with ATC, increased morbidity and mortality [23, 27, 28]. In a murine model, ATC could be blocked by inhibition of protein C activation using both pharmacological and gene-modulating methods [29]. Interventional clinical studies are required to determine the magnitude of influence this pathway, and other endogenous anticoagulants, has on ATC in humans.
Fibrinolysis is clearly a functional component of ATC. The impressive survival benefit associated with administration of tranexamic acid for traumatic haemorrhage highlights the pathological role of this pathway after injury [30]. Recent prospective clinical studies have better defined the incidence and clinical importance of this entity. Thromboelastometric analysis of 334 major trauma patients (ISS > 15) upon admission to the emergency room identified hyperfibrinolysis in 23, an incidence of 6.8 % [31]. In 14 cases, hyperfibrinolysis was considered fulminant with a complete breakdown of the clot observed within 60 min. A reduction of clot firmness between 16 and 35 % was observed in another nine patients. The mortality rate in patients with fulminant hyperfibrinolysis was 85.7 %, compared with 11.1 % in low-grade fibrinolysis. Patients with hyperfibrinolysis had higher ISS, lower Glasgow Coma Scale (GCS), lower systolic blood pressure and higher lactates than patients without hyperfibrinolysis. Amongst the majority of trauma victims that do not demonstrate hyperfibrinolysis on thromboelastometry, a significant proportion will experience ‘occult’ fibrinolysis with high D-dimers, which correlates strongly with poor outcomes [32].
Platelet counts are mildly reduced by trauma and this appears to associate with poor outcomes. A retrospective cohort study of 389 massively transfused trauma patients reported that in a logistic regression model controlling for ISS, GCS and admission BD, the odds of death at 24 h decreased by 12 % for every 50 × 109/l increase in platelet count [33]. However, in most contemporary studies, they do not decline to levels that may be expected to contribute significantly to coagulopathy [27, 34]. Nevertheless, a few reports have identified that a high ratio of platelets to packed red blood cells is associated with improved outcomes [35]. This may lead us to conclude that the primary platelet impairment provoked by injury and/or haemorrhagic shock is functional. A study of 163 trauma patients has reported a minor, but significant, difference in platelet aggregometry parameters (ADTtest and TRAPtest) between survivors and non-survivors [36].
Vascular endothelium is an active participant in the pathophysiology of ATC. Large capillary beds host thrombomodulin and endothelial protein C receptors anchored through their luminal surface that capture thrombin and accelerate protein C activation 1,000-fold [37]. In addition to inactivating coagulation factors Va and VIIIa, aPC also consumes plasminogen activator inhibitor-1 (PAI-1), the major antagonist of tissue-type plasminogen activator tissue type plasminogen activator (t-PA). Consequently, traumatic haemorrhage with tissue hypoperfusion leads to overwhelming release of t-PA from vascular endothelial cells and subsequent hyperfibrinolysis [13].
Fascinating data is emerging, demonstrating an association between tissue hypoperfusion, neurohormonal activation and markers of endothelial disruption [38, 39]. In a prospective study of 75 adult trauma patients, circulating adrenaline levels were elevated in subjects with higher ISS, higher lactate and lower systolic blood pressure. This correlated positively and independently with the incidence of ATC as well as levels of syndecan-1, histone-complexed DNA, high-mobility group box 1, soluble thrombomodulin, t-PA and D-dimers. Trauma haemorrhage has been shown experimentally in rats to cause shedding of the endothelial glycocalyx. Interestingly, this can be prevented by resuscitation with plasma, but not crystalloids or colloids [40]. Endothelial glycocalyx degradation is capable of triggering thrombin generation, protein C activation and hyperfibrinolysis. This is important because it indicates another potential mechanism by which tissue injury and shock mediate systemic anticoagulation early after injury.
11.4 Diagnosing TIC
Trauma-induced coagulopathy has conventionally been diagnosed as a prolongation of the international normalised ratio (INR) or aPTT greater than 1.5 times the normal value. However, the evidence for this diagnostic threshold is weak. More recently, the clinical relevance of different magnitudes of TIC has been elucidated (Figs. 11.3 and 11.4), and a prothrombin time ratio (PTr) or INR > 1.2 is now an accepted diagnostic definition of ATC [20].
The relationship between admission PTr and hospital mortality. *p < 0.001 compared with PTr = 1 [20]
The relationship between admission PTr and blood products transfused. *p < 0.001 compared with PTr = 1. +p < 0.001 compared with PTr = 1 [20]
Prothrombin time and aPTT are screening tests that report the time taken for initial fibrin polymerisation of platelet poor plasma, at 37 °C, in response to exogenous stimulation of coagulation. As such they neglect the pivotal role of platelets, do not measure clot strength and may not reflect haemostasis in vivo [41, 42]. Further, they are not reported expeditiously enough to accurately guide transfusion in the acute setting.
Recently there has been a renewed interest in the use of thromboelastometry for trauma care. These machines (ROTEM and TEG) employ a rotating pin or cup to measure the viscoelastic properties of ex vivo whole blood as it clots inside a small cuvette. They provide an assessment of the speed and strength of clot formation as well as fibrinolytic degradation over time. Research studies suggest their superiority to routine tests of coagulation for diagnosing haemostatic impairments after injury [43, 44]. Trauma patients with ATC (defined as PT ratio >1.2) have a ‘signature’ thromboelastogram (Fig. 11.5). Compared to patients with PT ratio ≤1.2, the ATC trace is characterised by a reduction in clot strength with much smaller changes in clotting times and can be identified within 5 min – threshold of clot amplitude at 5 min (CA5) ≤35 mm (Fig. 11.6) [46]. Future studies using these machines will attempt to identify trace configurations associated with specific coagulopathy phenotypes. Further, they undoubtedly have a potential role in guiding transfusion in real time, and evidence-based treatment algorithms are required to determine our clinical responses to different trace patterns.
The ‘signature’ thromboelastogram of trauma patients with and without ATC. Note clotting times (time to initiation of the curves) are minimally effected. The maximal amplitude (strength of the clot) is the principal difference between the cohorts [46]
Although few patients with ATC demonstrate early depletion of platelets, monitoring the platelet count is useful to identify an emerging dilutional coagulopathy. However, these tests suffer from the same logistical problems as PT and aPTT tests and offer no assessment of platelet function. Platelet aggregometry is not widely available and is too slow to be of use in clinical care. Developments are occurring with viscoelastic tests to enable them to offer a point of care assessment of platelet responsiveness and contribution to clot strength. Given the increased use of platelet inhibitors within the population, this will be beneficial by helping clinicians tailor platelet therapy to individual patient haemostatic function rather than simple counts of platelet number.
11.5 Treating ATC
11.5.1 Conventional Treatment
Conventional trauma-haemorrhage transfusion protocols were developed during the latter half of the twentieth century to guide the delivery of different fractionated allogenic blood products. These guidelines most commonly recommended the administration of packed red blood cells to target a haemoglobin concentration of greater than 10 g/dl after trauma. Fresh frozen plasma (FFP) was not deemed required until the INR had exceeded 1.5 and platelets when their number dropped below 50,000 cells/mm3 [47–49]. Hence, it was not considered necessary to replace clotting factors until a dilutional coagulopathy was already established.
An increasing body of evidence developed to indicate that these protocols cannot and do not achieve normal haemostasis in trauma patients requiring a massive transfusion. A study from Houston examined the effectiveness of their pre-intensive care unit (ICU) massive transfusion protocol at correcting coagulopathy in severely injured and shocked trauma victims [50]. Ninety-seven patients receiving 10 or more units of packed red blood cells (PRBC) during hospital day 1 had a mean admission INR of 1.8 ± 0.2. Adherence to their massive transfusion protocol with administration of 5 units of FFP (commenced after the 6th unit of PRBC) together with 12 units of PRBC failed to correct this coagulopathy despite good management of hypothermia and acidosis. By the time of ICU admission 6.8 ± 0.3 h later, coagulopathy persisted (INR 1.6 ± 0.1), and this was identified as an independent predictor of mortality in this cohort.
11.5.2 Earlier and More FFP
During the first decade of the twenty-first century, data emerged from computer models and observational studies of trauma resuscitation protocols indicating that earlier and more aggressive treatment of coagulopathy with plasma and platelets could improve survival of trauma victims [3, 51]. A landmark retrospective study of the United States Army Joint Theater Trauma Registry analysed the effect of different plasma to red blood cell unit ratios on the mortality of trauma patients admitted to combat support hospitals in Iraq between November 2003 and September 2005 [52]. Two hundred and forty-six patients received 10 or more units of red blood cells (packed red blood cells or fresh whole blood), and they were grouped according to the ratio of plasma to red blood cells transfused. The low ratio group median was 1:8, the medium ratio group median was 1:2.5, and the high ratio group median was 1:1.4. Overall mortality rates were 65, 34 and 19 %, and haemorrhage mortality rates were 92.5, 78 and 37 %, respectively (Fig. 11.7). Upon logistic regression, plasma to red blood cell ratio was independently associated with survival (odds ratio 8.6; CI, 2.1–35.2).
The association between ratios of plasma received by injured soldiers during resuscitation and their risk of death [52]
This remarkable finding prompted clinicians to study their trauma databases to determine whether similar associations were present in the civilian sphere. Multiple retrospective reports flowed in the literature, mostly reporting a positive correlation between the ratio of FFP to PRBC delivered and survival. The first of these was a report by Duchesne et al. in 2008 from New Orleans, which divided 135 massively transfused trauma patients admitted between 2002 and 2006 into those that received a FFP to PRBC ratio of 1:1 and those that received a ratio 1:4 [53]. When the amount of PRBCs was greater than twice the number of units of FFP, a ratio of 1:4 was designated. A FFP:PRBC ratio of 1:1 was assigned to patients who received <2 units of PRBCs per unit of FFP given. Massive transfusion is associated with high mortality and 75 of the 135 cohorts (55.5 %) died. There was no difference in age, male gender, incidence of penetrating injuries, initial systolic pressure and ISS between the two cohorts. A significant difference in mortality was noted in patients with FFP:PRBC ratio of 1:4, 56 of 64 (87.5 %) versus 19 of 71(26 %), p = 0.0001. Patients in the 1:1 group died from ongoing bleeding, 3 of 19 (15.7 %) early after ICU admission within the first day and 16 of 19 (84.2 %) died of multiple organ failure at day 18 ± 12 . In the 1:4 group patients died from ongoing bleeding, 11 of 56 (19.6 %) early after ICU admission and 45 of 56 (80.3 %) died of multiple organ failure at day 16 ± 8. The authors noted that whilst their resuscitation practices addressed the ‘acidosis’ and ‘hypothermia’ components of the lethal triad, it neglected the third factor, coagulopathy. The title of their paper ‘Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years?’ rightly questioned whether the practice of component blood therapy, which is logistically easier than delivering whole blood, was as clinically effective.
Most published studies originated from North American trauma centres, but a few similar reports emanated from Europe and Australia [54, 55]. Not all studies reported data consistent with improved survival from high-dose FFP. For example, a prospective study performed at the R Adams Cowley Shock Trauma Center in Baltimore selected 81 patients receiving a massive transfusion within 24 h (out of 806 trauma admissions) and calculated a logistic regression analysis to determine the effect of FFP:PRBC [56]. No significant effect on mortality was identified for either the PRBC to FFP ratio as a continuous variable (odds ratio (OR), 1.49; 95 % CI, 0.63–3.53; p = 0.37) or 1:1 ratio as a binary variable (OR, 0.60; 95 % CI, 0.21–1.75; p = 0.35) when controlling for age, gender, ISS, closed head injury, laparotomy status and ICU length of stay.
11.5.3 The Optimal Blood Product Ratio
There is a good potential rationale for administering high doses of plasma to patients receiving massive transfusion, as these are the subjects most likely to develop a dilutional coagulopathy. Reports of positive survival benefit with a high FFP:PRBC ratio in studies of non-massively transfused trauma patients are less numerous, perhaps because their procoagulant capacity never becomes diluted below a threshold necessary for satisfactory haemostasis. Further, results of some studies suggest that ‘1:1’ resuscitation is unnecessary and a lower ratio, such as 2:3 or 1:2, could optimise haemostatic support whilst reducing harmful plasma side effects [45, 57]. Regardless, several meta-analyses performed on pooled data have concluded that a high ratio of FFP:PRBC is associated with a significant survival benefit for victims of trauma [58–60].
Prompted by these findings, several groups have performed similar studies looking at the effect of high platelet and/or fibrinogen administration and obtained broadly similar positive results [35, 61]. However, there is inherent survivorship bias in these observational studies that raises doubt on the validity of their conclusions. That is, fresh frozen plasma, which is the predominant source of plasma for most institutions, requires 30–45 min thawing and has conventionally been associated with a delay to administration compared with PRBCs. Therefore, patients with massive and ongoing haemorrhage often die during the early hours of hospital admission before receiving substantial quantities of FFP and thus are included in the low ratio cohort, whereas patients surviving long enough to receive sufficient FFP feature in the high ratio cohort. In other words, perhaps survivors receive plasma rather than plasma producing survivors.
Authors subsequently employed various strategies attempting to eliminate or minimise survivorship bias in their studies. For example, some only included patients who had survived the initial few hours when FFP administration typically lags. However, this strategy sometimes resulted in bias against FFP because early hospital death is frequently caused by rapid haemorrhage and this is the patient group who might be expected to benefit most from plasma. Another method used was to model the relationship between mortality and FFP:PRBC ratio over time and treat the ratio as a time-dependent covariate. Snyder et al. compared mortality of patients who had a high ratio at the end of 24 h with those who had a low ratio and found the former to be associated with increased survival [62]. This advantage became statistically insignificant when they divided the 24 h study period into 0.5–6 h subintervals and calculated the ratio and the mortality rates of both groups within each subinterval.
Obtaining a conclusive answer to the question of optimal blood product ratios to treat coagulopathy using observational studies alone is not possible. A multicentre, randomised trial (PROPPR – Pragmatic, Randomized Optimal Platelet and Plasma Ratios) will compare different ratios of blood products given to trauma patients who are predicted to require massive transfusions. Recruited subjects will receive blood products based on a 1:1:1 or 1:1:2 ratio of platelets, plasma and red blood cells. A total of 580 patients will be enrolled into this study from 12 participating sites in the United States and Canada, and it is estimated to report findings in 2015.
11.5.4 Massive Transfusion Protocols
Some investigators have hypothesised that earlier administration of coagulation delivers survival benefit rather than the precise ratio of products given. Delivering higher volumes of blood transfusion products to rapidly haemorrhaging trauma patients places significant logistical demands upon the local pathology service. Integrated ‘massive transfusion’ or ‘major haemorrhage’ protocols (MHP) have been developed to facilitate and direct the delivery of these products. Riskin et al. performed a 4-year cohort study to assess the impact of implementing MHP, aiming to deliver a FFP:PRBC ratio of 1:1.5 [63]. For the 2 years before and after MHP initiation, there were 4,223 and 4,414 trauma activations, respectively. The FFP:PRBC ratios were identical, at 1:1.8 and 1:1.8 (p = 0.97). Despite no change in FFP:PRBC ratio, mortality decreased from 45 to 19 % (p = 0.02). This was associated with a significant decrease in mean time to first product: cross-matched RBCs (115 to 71 min; p = 0.02), FFP (254 to 169 min; p = 0.04) and platelets (418–241 min; p = 0.01). Effectively, catching up with coagulation support may not be as effective as preventing the impairment in the first place.
11.5.5 Other Haemostatic Agents
Whilst haemostatic resuscitation with a generic formula of blood products is the current practice in the United Kingdom and North America, it has not been implemented universally. Concerns regarding plasma transfusion-related side effects (e.g. immune-mediated adverse reactions, pathogen transmission, transfusion-related acute lung injury), better availability of coagulation factor concentrates and improved utilisation of point of care tests have combined to offer some European trauma centres an alternative, patient-tailored resuscitation solution. For example, in some Austrian centres, resuscitation commences with PRBCs and crystalloids. Recombinant fibrinogen concentrate is then given early to patients who demonstrate impaired fibrin polymerisation on their admission or subsequent viscoelastic test. Platelets are administered to subjects with continued clot weakness despite adequate fibrin function. Prothrombin complex concentrate (PCC, a mixture of recombinant vitamin K-dependent coagulation factors) is supplemented only to those who develop prolonged clotting times on viscoelastic testing (used as a surrogate measure of insufficient thrombin potential). Plasma is rarely indicated in this resuscitation protocol. Some retrospective cohort analyses have associated improved survival and reduced blood product use with this strategy [64].
Use of coagulation factor concentrates is logistically easier and targets specific defects in haemostasis. However, concentrates are expensive and their side-effect profile is not as well characterised as plasma. It is also not known whether focused replacement of coagulation factor deficits is more clinically effective than generalised support of the coagulation cascade. Finally, goal-directed management requires rapid results from validated tests of haemostasis using evidence-based algorithms. It is possible that point of care viscoelastic tests will be able to provide this service, but substantial further study is required before the next paradigm shift in trauma transfusion is adopted.
Whilst debate about optimal haemostatic resuscitation protocols is likely to persist for the foreseeable future, some clear and concise evidence regarding haemostatic adjuncts is now available. Firstly, recombinant factor VII appears to have no role in the resuscitation of haemorrhaging trauma victims after the early termination of the CONTROL trial of recombinant factor VII [65]. Although it had previously been shown to safely reduce blood loss from trauma in a Phase 2 trial, the Phase 3 trial was stopped prematurely after recruiting 573 of 1502 planned patients because the overall mortality was unexpectedly low and recombinant VII had no positive effect on survival [66]. Conversely, CRASH2 was a successful double-blind, prospective, randomised, placebo-controlled trial that enrolled 20, 211 adult trauma patients with or at risk of significant bleeding in 40 countries [30]. Patients were randomly given either a standard dose of tranexamic acid (n = 10,060) or matched placebo (n = 10,067) within 8 h of injury. All-cause mortality within 28 days was significantly reduced in the tranexamic acid group compared with the placebo group (risk ratio [RR] = 0.91; 95 % CI 0.85–0.97; p = .004). The risk of death caused by bleeding was also significantly reduced with tranexamic acid compared with placebo (RR = 0.85; 95 % CI 0.76–0.96; p = .008) and was particularly apparent for risk of death caused by bleeding on the day of randomisation (RR = 0.80; 95 % CI 0.68–0.93; p = .004). There was no increase in fatal vascular occlusive events or in deaths caused by multiorgan failure, head injury or others. Tranexamic acid is a cheap and easily administered medication. Research is ongoing in an attempt to refine the indication and patient population with greatest potential benefit from this drug. However, until better clinical efficacy is proven with improved inclusion/exclusion criteria, tranexamic acid should be administered as per the published clinical trial (bolus of 1 g within first 3 h of trauma, followed by continuous infusion of 1 g).
11.5.6 Blood Pressure Target
The principles of haemostatic resuscitation have recently been combined with a strategy of ‘permissive hypotension’ to create a modern concept termed ‘damage control resuscitation’ [67]. Permissive hypotension refers to resuscitation targeting a sub-physiological blood pressure in order to minimise haemorrhagic loss whilst maintaining adequate perfusion to essential organ beds. It is not a new concept; evidence of attempts to avoid ‘popping the clot’ is centuries old. However, it returned to scientific consciousness with the publication of a landmark (nonrandomised) trial conducted in 1994 that showed a statistically significant 8 % absolute reduction in mortality for hypotensive patients with penetrating torso trauma assigned to delayed (in the operating theatre) compared with prehospital or emergency room fluid resuscitation [68]. Two subsequent studies on trauma patients with blunt and/or penetrating injuries failed to confirm the clinical benefit of permissive hypotension [69, 70]. More recently, a prospective trial of 90 trauma patients in haemorrhagic shock randomised subjects to a minimum resuscitation target of 50 mmHg or 65 mmHg (mean arterial pressure). Although the authors could not demonstrate a survival difference for the two treatment strategies at day 30, 24 h postoperative death and coagulopathy were increased in the group with the higher target minimum pressure. One major drawback to this study was that no statistically significant difference between the actual mean arterial pressure was observed between the two groups for the duration of the study (64.4 mmHg vs. 68.5 mmHg, p = 0.15). Nevertheless, citing this evidence, current European guidelines recommend a target systolic blood pressure of 80–90 mmHg until major bleeding has been stopped in the initial phase following trauma without brain injury [26].
11.6 Summary
The discovery of ATC spurred significant leaps in our understanding of the haemostatic responses to injury and shock resuscitation. Acute traumatic coagulopathy is now recognised as a rapidly developing endogenous process that can be exacerbated by resuscitation with fluids devoid of coagulation factors. Conversely, potential for improved survival with reduced morbidity has been demonstrated by rapid administration of haemostatic blood products. The precise pathophysiology of ATC requires better characterisation, and this will direct the focus of our efforts to treat ATC. The haemostatic status of a trauma victim is constantly evolving in response to tissue damage, systemic hypoperfusion and medical attempts to support trauma. Future resuscitation paradigms will become more patient tailored and incorporate diagnostic tools that are capable of guiding therapy in a more responsive way.
References
Hess J, Brohi K, Dutton R, Hauser C, Holcomb J, Kluger Y, Mackway-Jones K, Parr M, Rizoli S, Yukioka T, Hoyt D, Bouillon B. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–54.
Gando S. Acute coagulopathy of trauma shock and coagulopathy of trauma: a rebuttal. You are now going down the wrong path. J Trauma. 2009;67(2):381–3.
Hirshberg A, Dugas M, Banez E, Scott B, Wall MJ, Mattox K. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma. 2003;54(3):454–63.
Beekley A, Watts D. Combat trauma experience with the United States Army 102nd Forward Surgical Team in Afghanistan. Am J Surg. 2004;187(5):652–4.
Martin R, Kilgo P, Miller P, Hoth J, Meredith J, Chang M. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24(2):114–8.
Wang H, Callaway C, Peitzman A, Tisherman S. Admission hypothermia and outcome after major trauma. Crit Care Med. 2005;33(6):1296–301.
Martini W, Pusateri A, Uscilowicz J, Delgado A, Holcomb J. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J Trauma. 2005;58(5):1002–9; discussion 1009–10.
Martini W, Holcomb J. Acidosis and coagulopathy: the differential effects on fibrinogen synthesis and breakdown in pigs. Ann Surg. 2007;246(5):831–5.
Martini W, Dubick M, Pusateri A, Park M, Ryan K, Holcomb J. Does bicarbonate correct coagulation function impaired by acidosis in swine? J Trauma. 2006;61(1):99–106.
Martini W, Dubick M, Wade C, Holcomb J. Evaluation of tris-hydroxymethylaminomethane on reversing coagulation abnormalities caused by acidosis in pigs. Crit Care Med. 2007;35(6):1568–74.
Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30.
MacLeod J, Lynn M, McKenney M, Cohn S, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44.
Brohi K, Cohen M, Ganter M, Matthay M, Mackersie R, Pittet J. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–8.
Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, Simanski C, Neugebauer E, Bouillon B. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38(3):298–304.
Rugeri L, Levrat A, David J, Delecroix E, Floccard B, Gros A, Allaouchiche B, Negrier C. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5(2):289–95.
Niles S, McLaughlin D, Perkins J, Wade C, Li Y, Spinella P, Holcomb J. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64(6):1459–63; discussion 1463–5.
Hess J, Lindell A, Stansbury L, Dutton R, Scalea T. The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion. 2009;49(1):34–9.
Siegel J, Rivkind A, Dalal S, Goodarzi S. Early physiologic predictors of injury severity and death in blunt multiple trauma. Arch Surg. 1990;125(4):498–508.
Rutherford E, Morris JJ, Reed G, Hall K. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33(3):417–23.
Frith D, Goslings J, Gaarder C, Maegele M, Cohen M, Allard S, Johansson P, Stanworth S, Thiemermann C, Brohi K. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8:1919–25.
Floccard B, Rugeri L, Faure A, Denis MS, Boyle EM, Peguet O, Levrat A, Guillaume C, Marcotte G, Vulliez A, Hautin E, David JS, Négrier C, Allaouchiche B. Early coagulopathy in trauma patients: an on-scene and hospital admission study. Injury. 2010;43:26–32.
Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, Jansen J, Tien HC. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011;71(5 Suppl 1):S427–34.
Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, Myers JG, Rahbar MH, Brasel KJ, Phelan HA, del Junco DJ, Fox EE, Wade CE, Holcomb JB, Cotton BA, Matijevic N, PROMMTT Study Group. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S40–7.
Dunbar NM, Chandler WL. Thrombin generation in trauma patients. Transfusion. 2009;49(12):2652–60.
Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10(7):1342–51.
Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76.
Johansson PI, Sorensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, Ostrowski SR. Disseminated intravascular coagulation or acute coagulopathy of trauma shock early after trauma? A prospective observational study. Crit Care. 2011;15(6):R272.
Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255(2):379–85.
Chesebro B, Rahn P, Carles M, Esmon C, Xu J, Brohi K, Frith D, Pittet J, Cohen M. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32(6):659–65.
Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejía-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yutthakasemsunt S, CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32.
Tauber H, Innerhofer P, Breitkopf R, Westermann I, Beer R, El Attal R, Strasak A, Mittermayr M. Prevalence and impact of abnormal ROTEM(R) assays in severe blunt trauma: results of the ‘Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br J Anaesth. 2011;107(3):378–87.
Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De’Ath HD, Allard S, Hart DP, Pasi KJ, Hunt BJ, Stanworth S, MacCallum PK, Brohi K. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11(2):307–14.
Brown LM, Call MS, Margaret Knudson M, Cohen MJ, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G, MacLeod J, Dutton RP, Hess JR, Duchesne JC, McSwain NE, Muskat P, Johannigamn J, Cryer HM, Tillou A, Pittet JF, De Moya MA, Schreiber MA, Tieu B, Brundage S, Napolitano LM, Brunsvold M, Beilman G, Peitzman AB, Zenait MS, Sperry J, Alarcon L, Croce MA, Minei JP, Kozar R, Gonzalez EA, Stewart RM, Cohn SM, Mickalek JE, Bulger EM, Cotton BA, Nunez TC, Ivatury R, Meredith JW, Miller P, Pomper GJ, Marin B, Trauma Outcomes Group. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma. 2011;71(2 Suppl 3):S337–42.
Borgman MA, Spinella PC, Holcomb JB, Blackbourne LH, Wade CE, Lefering R, Bouillon B, Maegele M. The effect of FFP:RBC ratio on morbidity and mortality in trauma patients based on transfusion prediction score. Vox Sang. 2011;101(1):44–54.
Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, Matijevic N, Dong JF, Pati S, Wade CE, Cotton BA, Brasel KJ, Vercruysse GA, MacLeod JB, Dutton RP, Hess JR, Duchesne JC, McSwain NE, Muskat PC, Johannigamn JA, Cryer HM, Tillou A, Cohen MJ, Pittet JF, Knudson P, DeMoya MA, Schreiber MA, Tieu BH, Brundage SI, Napolitano LM, Brunsvold ME, Sihler KC, Beilman GJ, Peitzman AB, Zenati MS, Sperry JL, Alarcon LH, Croce MA, Minei JP, Steward RM, Cohn SM, Bulger EM, Nunez TC, Ivatury RR, Meredith JW, Miller PR, Pomper GJ, Marin B, Trauma Outcomes Group. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–28.
Solomon C, Traintinger S, Ziegler B, Hanke A, Rahe-Meyer N, Voelckel W, Schöchl H. Platelet function following trauma. A Multiple Electrode Aggregometry study. Thromb Haemost. 2011;106(2):322–30.
Esmon C. The protein C pathway. Chest. 2003;124(3 Suppl):26S–32.
Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200.
Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. High circulating adrenaline levels at admission predict increased mortality after trauma. J Trauma Acute Care Surg. 2012;72:428–36.
Torres LN, Sondeen JL, Ji L, Dubick MA, Filho IT. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;75(5):759–66.
Segal J, Dzik W, Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45(9):1413–25.
Levi M, Opal S. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10(4):222.
Kheirabadi B, Crissey J, Deguzman R, Holcomb J. In vivo bleeding time and in vitro thrombelastography measurements are better indicators of dilutional hypothermic coagulopathy than prothrombin time. J Trauma. 2007;62(6):1352–9; discussion 1359–61.
Johansson P, Stissing T, Bochsen L, Ostrowski S. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17(1):45.
Davenport R, Curry N, Manson J, De’Ath H, Coates A, Rourke C, Pearse R, Stanworth S, Brohi K. Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma. 2011;70(1):90–5; discussion 95–6.
Davenport R, Manson J, De’ath H, Platton S, Coates A, Allard S, Hart D, Pearse R, Pasi KJ, Maccallum P, Stanworth S, Brohi K. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–8.
Lunberg, George D. Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. JAMA. 1994;271:777–81.
O’Shaughnessy D, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S, Williamson L, British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126(1):11–28.
American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105(1):198–208.
Gonzalez E, Moore F, Holcomb J, Miller C, Kozar R, Todd S, Cocanour C, Balldin B, McKinley B. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–9.
Ho A, Dion P, Cheng C, Karmakar M, Cheng G, Peng Z, Ng Y. A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing hemorrhage. Can J Surg. 2005;48(6):470–8.
Borgman M, Spinella P, Perkins J, Grathwohl K, Repine T, Beekley A, Sebesta J, Jenkins D, Wade C, Holcomb J. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13.
Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, Weintraub SE, Wright MJ, McSwain NE. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65(2):272–6; discussion 276–8.
Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B, Working Group on Polytrauma of the German Society of Trauma Surgery (DGU). Red blood cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft für Unfallchirurgie. Vox Sang. 2008;95(2):112–9.
Mitra B, Mori A, Cameron PA, Fitzgerald M, Paul E, Street A. Fresh frozen plasma (FFP) use during massive blood transfusion in trauma resuscitation. Injury. 2010;41(1):35–9.
Scalea TM, Bochicchio KM, Lumpkins K, Hess JR, Dutton R, Pyle A, Bochicchio GV. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248(4):578–84.
Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB, Moore EE, Inflammation the Host Response to Injury Investigators. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986–93.
Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, Montori VM, Roback JD. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion. 2010;50(6):1370–83.
Johansson PI, Oliveri RS, Ostrowski SR. Hemostatic resuscitation with plasma and platelets in trauma. J Emerg Trauma Shock. 2012;5(2):120–5.
Bhangu A, Nepogodiev D, Doughty H, Bowley DM. Meta-analysis of plasma to red blood cell ratios and mortality in massive blood transfusions for trauma. Injury. 2013;44(12):1693–9.
Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, Hess JR, Dubick MA, Simon CD, Beekley AC, Wolf SE, Wade CE, Holcomb JB. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64(2 Suppl):S79–85; discussion S85.
Snyder CW, Weinberg JA, McGwin G, Melton SM, George RL, Reiff DA, Cross JM, Hubbard-Brown J, Rue LW, Kerby JD. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66(2):358–62; discussion 362–4.
Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, Spain DA, Brundage SI. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209(2):198–205.
Schöchl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14(2):R55.
Hauser C, Boffard K, Dutton R, Bernard G, Croce M, Holcomb J, Leppaniemi A, Parr M, Vincent J, Tortella B, Dimsits J, Bouillon B, CONTROL Study Group. Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500.
Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y, NovoSeven Trauma Study Group. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59(1):8–15; discussion 15–8.
Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–10.
Bickell WH, Wall MJ, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–9.
Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol Assess. 2000;4(31):1–57.
Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52(6):1141–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Frith, D., Brohi, K. (2015). Massive Transfusion in Trauma. In: Juffermans, N., Walsh, T. (eds) Transfusion in the Intensive Care Unit. Springer, Cham. https://doi.org/10.1007/978-3-319-08735-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-08735-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08734-4
Online ISBN: 978-3-319-08735-1
eBook Packages: MedicineMedicine (R0)