Abstract
High carbon steel is characterized by good toughness and high hardness, especially after an appropriate heat treatment. In this work heat treatment of 0.75 % carbon steel with 4.50 % Mo, including heating to a different austenitic phase (850, 900 and 950 °C) and holding for different times (30 min and 1 h), then cooling by different media is considered. The results show no noticeable increase in the hardness at 850 °C, but at 900 °C changing in hardness values can be observed clearly with oil and water quenched specimens, at 950 °C there is a significant changing in hardness values in all quenching media even at air and furnace cooling. As Austenization temperature increase, the hardness of the samples increases especially for samples cooled with oil and water at 950 °C. Tests showed that the microstructures contain carbides, that carbides did not completely melted even at the temperature of 1,050 °C due to percentage of molybdenum which produces stable carbides until melting.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Steel is an alloy of iron and carbon, where other elements are present in quantities very small compared to the major constituents. The other alloying elements allowed in plain-carbon steel are manganese (1.65 % max) and silicon (0.60 % max) [1]. Steel with low carbon content has the same properties as iron, soft but easily formed. As carbon content rises, the metal becomes harder and strong but less ductility and more difficult to weld. Higher carbon content lowers the steel melting point and its temperature resistance in general [2]. Heat treatment involves the application of heat, to material to obtain desired material properties (e.g. mechanical, corrosion, electrical, magnetic, etc.). During the heat treatment process, the material usually undergoes phase microstructural and crystallographic changes [3]. The purpose of heat treating carbon steel is to change the mechanical properties of steel, usually ductility, hardness, yield strength, tensile strength and impact resistance. The electrical, corrosion and thermal conductivity are also slightly altered during the heat treatment process [4]. If a sample of plain carbon steel in the austenitic condition is rapidly cooled to room temperature by quenching in water, its structures will be changed from austenite to martensite. Martensite in plain carbon steels is a metastable phase consisting of a super saturated interstitial solid solution of carbon in body centered cubic iron or body centered tetragonal iron [5].
Molybdenum is a relatively expensive alloying element, has a limited solubility in austenite and ferrite iron, and is a strong carbide former. Molybdenum has a strong effect on hardenability and, like chromium, increases the high-temperature hardness and strength of steels. Steel containing molybdenum are less susceptible to temper brittleness than other alloy steel [6]. The transformation of austenite to martensite by diffusionless shear type transformation in quenching is also responsible for higher hardness obtained and this property is attributed to the effectiveness of the interstitial carbon in hindering the dislocation motion [7]. When the steel has reached the hardening temperature it is austenitic provided that the temperatures has been correctly chosen. The time of holding at the hardening temperature depends on the desired degree of carbide dissolution and acceptable grain size. Since the amount of carbide is different for different types of steel the time of holding is also dependant on the grade of steel. However, the holding time is not only dependent on the hardening temperature but also on the rate of heating. With very slow heating, hypoeutectoid steels completely austenitic immediately above Ac3, and the holding time should not be necessary. With more rapid heating some holding time should be required to ensure temperature equalization carbide dissolution. Alternatively, a higher temperature might be used. Plain carbon and low alloy structural steels which contain easily dissolved carbides require only a few minutes holding time after they have reached the hardening temperature. In order to make certain that there has been sufficient carbide dissolution, holding time 5–15 min is quite sufficient [8].
2 Experimental Work
The material used in this study is high carbon, with the chemical composition of the material as shown in Table 1.
2.1 Treatment Processes
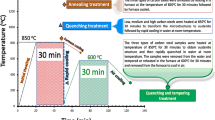
The steel samples were divided to six groups, first three groups, the samples were heated to the austenizing temperature of 850, 900 and 950 °C, soaked for 30 min for each austenizing temperature, then all samples subjected to annealing, normalizing processes, quenched in oil and quenched in water. Second three groups, the samples were heated at same austenizing temperatures but soaking time was 60 min and subjected to annealing, normalizing processes, quenched in oil and quenched in water.
2.2 Strength Test
The hardness values of the samples were determined using a digital hardness testing machine. The surfaces were thoroughly polished before samples were tested. The various hardness values were recorded in HRC. Tensile test was measured using a universal testing machine.
2.3 Corrosion Rate
The corrosion rates of the samples were determined using the weight loss method, samples were immersed in HCL solution (concentration 8 %).
2.4 Metallographic Examination
The samples were ground on a water lubricated hand grinding set-up of abrasive chapters, progressing through from the coarsest to the finest grit size. The 240, 320, 400, 500 and 800 grades were used in the order. Polishing was carried out on a rotating disc of synthetic velvet polishing cloth impregnated with 0.5 micron alumna paste. The specimens were then etched with the standard 2 % nital. The optical microscopic examination were carried out on a metallurgical microscope at a magnification of 100 X.
3 Results and Discussion
Hardness: The results of the hardness of the steel specimens after various heat treatment temperatures and different heat treatment processes are shown in Figs. 1, 2 and 3.
Corrosion Rate: The result of the corrosion rate investigations is shown in Fig. 4.
Microstructures analysis: The results of the microstructure analysis is shown in Figs. 5, 6 and 7.
4 Discussion
Figures 1, 2 and 3 shows the hardness value for steel specimens after various heat treatment temperatures and different heat treatment processes. Specimens heated to 850 °C with both different soaking time at austenite phase and cooled in different medium shows no noticeable increase in the hardness. But at 900 °C changing in hardness values can be observed clearly with oil and water quenched specimens, from 20 to 24 HRC in oil coolant and from 23 to 32 HRC at soaking time 30 and 60 min respectively. At Fig. 3 there is a significant changing in hardness values in all quenching media even at air and furnace cooling, the hardness values were changing from 35 to 42 HRC at slow cooling rate at furnace cooling, from 38 to 45 HRC at air cooling, from 40 to 49 HRC at oil quenching and from 46 to 56 HRC at water quenching due to clear stable carbides formation and with increasing temperature and soaking time the carbides particles become smaller in size and increasing hardness values which is also manifested in the microstructure. Figure 4 shows the corrosion rate of the annealing and normalizing process is a lower than the corrosion rate at quenching due to less of stressed inside the samples due to martensite structure formation, but the corrosion rate will be increases with quenching process after heat treatment do to formation of spheroidal graphite particles that contain a strong two phase with martensite. Figures 5, 6 and 7 show the microstructures of specimen for three different heating at 60 min soaking time. The structure consists of spheroidal carbides particles in a martensite matrix, also it is clear with increasing heating temperature the spheroidal carbides particles become smaller and this leads to an increase in hardness values.
5 Conclusion
The austenizing temperature is an important factor during heat treatment specially with high carbon steel to reach homogeneous austenite. The clear effect of the austenizing temperature and soaking time are effective with increases of the heat treatment temperature in coincidence of the soaking time specially with high carbon content in the steel and presence of the carbide former metal alloy, that carbides are stable even at high temperature and leads to significant increasing in hardness. When stable carbides are formed it will be decreasing in size with increasing the austenizing temperature and soaking time. With advantage of increasing the hardness and strength of samples, disadvantage is will be more susceptible to corrosion due to carbide formation.
References
Oberg, E. et al.: Machinery’s Handbook (25th ed), Industrial press Inc, New York (1996)
Smith, W., Hashemi, J.: Foundations of Material science and Engineering, (4th edn) p. 28–36. McGraw’s—Hill Book, Boston (2006)
Rajan, T.V., Sharma, C.P., Sharma, A.: Heat Treatment Principles and Techniques, pp. 36–58. PHI, Delhi (1989)
Mamoru, O., Yukito, T. Hitoshi, K, and Yuji, F.: Development of New Steel Plates for Building Structural use, Nippon Steel Technical Report, No.44, p. 5–18 (1990)
Smith, W.F.: Foundations of Materials Science and Engineering, (3rd edn), McGRAW-HILL, New York (2004)
Avner S.H.: Introduction to Physical Metallurgy, (2nd edn) pp. 60–361. McGRAW-HILL. New York (1974)
Callister Jr.W.O.: Material Science and Engineering An introduction, pp.322–328 Willey, USA (2003)
Smith J.L. Heat Treatment of Metals, (1st edn) pp. 211–212. CBS. New Delhi. Bangalore, Pune (2008)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gebril, M.A., Aldlemey, M.S., Kablan, A.F. (2014). Effect of Austenization Temperatures and Times on Hardness, Microstructure and Corrosion Rate of High Carbon Steel. In: Öchsner, A., Altenbach, H. (eds) Design and Computation of Modern Engineering Materials. Advanced Structured Materials, vol 54. Springer, Cham. https://doi.org/10.1007/978-3-319-07383-5_30
Download citation
DOI: https://doi.org/10.1007/978-3-319-07383-5_30
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-07382-8
Online ISBN: 978-3-319-07383-5
eBook Packages: EngineeringEngineering (R0)