Abstract
The class Holothuroidea (sea cucumbers) belonging to the phylum Echinodermata is characterized by the production of triterpene glycosides with sulfate groups attached to the monosaccharide residues in a great majority of the saponins isolated so far. Due to their toxicity and membranotropic action, these polar compounds have attracted the attention of chemists and pharmacologists and a wide spectrum of biological activities has been found for these secondary metabolites. The purpose of this communication is to review the structural characteristics and the cytotoxic properties of triterpene glycosides isolated from sea cucumbers in the last five years, focusing on structure-activity correlations and research on their mechanisms of action.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
24.1 Introduction
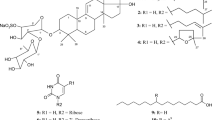
Echinoderms belonging to the class Holothuroidea (sea cucumbers) produce complex mixtures of triterpene glycosides (holothurins) that are responsible for their general toxicity and may play a defensive role due to their membranotropic action [1, 2]. Most of these saponins contain an aglycone based on a “holostanol” skeleton [3β,20S-dihydroxy-5α-lanostano-18,20-lactone] (1) (Fig. 24.1) and a sugar chain of two to six monosaccharide units linked to the C-3 of the aglycone. Two main series of aglycones can be distinguished in their structures: glycosides based on a 3β-hydroxy-holost-9(11)-ene aglycone and those containing a 3β-hydroxy-holost-7-ene skeleton. Usually aglycones that have a Δ9,11 double bond are characteristic of sea cucumbers belonging to the order Aspidochirota, while those with a Δ7 unsaturation were generally isolated from animals of the order Dendrochirotida.
Sea cucumber triterpene glycosides differ in the composition and number of the sugar units, the number and positions of the sulfate groups and the structural characteristics of the aglycone. Some examples of holothurins having non-holostane aglycones have been found in seven species of sea cucumbers belonging to the order Dendrochirotida. The majority are monosulfated at the glucose or xylose units [1].
Most of the triterpene glycosides are tetra- or pentaglycosides. Carbohydrate units include quinovose, glucose, 3-O-methylglucose, xylose and sometimes 3-O-methylxylose, 3-O-methylquinovose, 3-O-methylglucuronic acid and 6-O-acetylglucose. The first monosaccharide unit is always xylose, while 3-O-methylglucose and 3-O-methylxylose are always terminal. Sixty percent of the triterpene glycosides isolated so far from holothurians have sulfate groups linked to the monosaccharide units of the oligosaccharide chain. Although most of them are monosulfated oligoglycosides, a number of di- and trisulfated glycosides have been isolated, mainly from the order Dendrochirotida. Most tetra- and pentasaccharides are sulfated at C-4 of the xylose unit. Additional sulfate groups at C-6 of the 3-O-Me-glucose and glucose units have been found in trisulfated tetraglycosides. The majority of tetrasaccharides show a linear chain with the most common 3-O-Me-Glc-(1 → 3)-Glc-(1 → 4)-Qui-(1 → 2)-Xyl structure. The few disaccharides that have been isolated show a Qui-(1 → 2)-4-OSO3Na-Xyl chain attached to C-3 of the triterpenoid aglycone. Some hexasaccharides have been isolated from sea cucumbers of the order Aspidochirota: Stichopus japonica, Stichopus chloronotus, Parastichopus californius and Bohadschia bivittata [1]. They are non-sulfated glycosides with a linear 3-O-Me-Glc-(1 → 3)-Glc-(1 → 4)-Xyl chain and a branching of a linear trisaccharide at C-2 of the xylose unit.
Several holothurins are specific for different taxonomic groups of sea cucumbers and structural characteristics of triterpene glycosides have been used to resolve taxonomic problems in the class Holothuroidea [3].
Triterpene glycosides are produced in the skin and in the Cuvier’s tubules of sea cucumbers and are ejected when the animals are disturbed. This behavior may be associated to a defensive function due to the ability of holothurins to form complexes with cholesterol and other ∆ 5-sterols that lead to the development of single ion channels and larger pores which cause significant changes in the physico-chemical properties of cell membranes [4 –6]. Sea cucumbers are resistant to their own toxins due to the presence of ∆7-, 14α-methyl- and 14α-dimethyl-∆9,11-sterols as well as their conjugated forms such as steryl sulfates and steryl xylosides [7].
Sea cucumbers are important as human food source and they have long been utilized in folk medicine in Asia. Their triterpene glycosides exhibit a wide spectrum of biological effects: antifungal, cytotoxic, hemolytic, cytostatic and immunomodulatory activities [5]. These biological activities are a consequence of their membranotropic action against any cellular membrane containing ∆5-sterols.
Several monographs concerning the structures and biological activities of holothurins have been published [1–3, 5, 8–10]. The aim of the present review is to report the most recent findings in the field, focusing on the structural characteristics and cytotoxic activities of these glycosides from 2009 to 2013.
24.2 Chemical Structure and Cytotoxic Activity
24.2.1 Glycosides with 3β-hydroxy-holost-9,11-ene Aglycones
Two new triterpene glycosides with hydroxyl groups at positions 12α and 17α of the holostanol skeleton, scabrasides A (2) and B (3) (Fig. 24.2 ), were isolated from the sea cucumber Holothuria scabra [11]. This holothurian is widely distributed in the South China Sea and is used as a tonic in China. The in vitro cytotoxicity of both glycosides was evaluated against human leukemia (HL-60, MOLT-4), human lung cancer (A-549), and human hepatoma (BEL-7402) cells. Compounds 2 and 3 showed high activities towards HL-60 and MOLT-4 with IC50 values of 0.05 and 0.09 μM (for compound 2) and of 0.25 and 0.08 μM (for compound 3), respectively. Both compounds displayed lower activities towards A-549 and BEL-7402 cells.
The new sulfated triterpene glycoside scabraside D (4) (Fig. 24.2) showed significant cytotoxicity against A-549 (IC50 = 1.72 μM), mouse leukemic cell (P-388) (IC50 = 0.96 μM), gastric cancer cell (MKN-28) (IC50 = 1.27 μM), human colorectal cancer cell (HCT-116) (IC50 = 1.72 μM), and human breast cancer cell (MCF-7) (IC50 = 1.80 μM) [12].
Two sulfated tetraglycosides, hemoiedemosides A (5) and B (6), (Fig. 24.3) isolated from the Patagonian sea cucumber Hemioedema spectabilis [13] were evaluated for in vitro cytotoxicity and antiproliferative activity on human lung cancer (A-549) and cervical cancer (HeLa) cell lines [14]. Compounds 5 and 6 differ only in the degree of sulfation of the oligosaccharide chain. Trisulfated glycoside 6 contains an additional sulfate group at C-6 of the terminal 3-O-methyl glucose unit. Glycoside 6 exhibited high antiproliferative activity towards Hela and A-549 with IC50 values of 2.15 and 3.16 μM, respectively. Compound 5 was less active than 6 with IC50 values of 7.43 μM in A-549 and 9.95 μM in HeLa cell lines. Besides, the higher cytotoxicity of glycoside 6 on both cell lines with CC50 values of 2.80 μM for Hela and 5.96 μM for A-549 may be related to the presence of a third sulfate group in the carbohydrate chain. Similar results were observed for okhotosides B1, B2 and B3. Monosulfated okhotoside B1 showed lower cytotoxicity than the disulfated analogs B2 and B3 in HeLa cell line [15].
One new sulfated diglycoside, leucospilotaside B (7) (Fig. 24.4) was isolated from the sea cucumber Holothuria leucospilota [16]. Glycoside 7 exhibited significant cytotoxicity against four tumor cell lines (lung cancer A-549, leukocythemia HL-60 and MOLT-4, and liver cancer BEL-7402) with IC50 values ranging from 0.44–2.62 μg/ml.
Two new disulfated holostane glycosides with an acetyl group at position 16β of the aglycone, pentactasides B (8) and C (9) (Fig. 24.5), were isolated from the sea cucumber Pentacta quadrangularis [17]. Both saponins differ only in the side chain of the triterpene aglycone. The in vitro cytotoxicity of both glycosides was evaluated against five human tumor cell lines (P-388, A-549, HCT-116, MCF-7 and MKN-28). Compounds 8 and 9 showed significant cytotoxicities against all tumor cell lines with IC50 values between 0.09 and 2.30 μM. Interestingly, the activity of 8 against HCT-116 (IC50 = 0.09 μM) and of 9 against A-549 (IC50 = 0.58 μM) was significantly higher than that of the positive control, 10-hydroxycamptothecine (IC50 of 0.14 and 0.74 μM, respectively).
Two sulfated triterpene glycosides , holothurin A1 (10) and 24-dehydroechinoside A (11) (Fig. 24.6), isolated from the sea cucumber Pearsonothuria graeffei, possess an identical carbohydrate chain and differ in their side chains [18]. Glycoside 10 has a hydroxyl group at C-22, while 11 possesses a 24(25)-double bond. Both glycosides were evaluated for their effects on metastasis in vitro and in vivo. Both compounds strongly inhibit tumor metastasis but 10 had more potent antimetastatic activity than 11, suggesting that the 24(25)-double bond in the side chain of 11 could promote antimetastasic activity. In addition, only glycoside 10 markedly suppressed the expression of NF-κB indicating that a hydroxyl group at C-21 may relate to the targeting of NF-κB in tumor metastasis. Recently, 24-dehydroechinoside A (11) was isolated from the sea cucumber Holothuria scabra [12] and showed significant cytotoxicity (IC50 = 0.41–1.21 μM) against five tumor cell lines (P-388, A-549, MKN-28, HCT-116 and MCF-7).
Ds-echinoside A (12) (Fig. 24.7), a non-sulfated triterpene glycoside, was isolated for the first time as a natural product from the sea cucumber P. graeffei [37]. Previously, it had been obtained by desulfation of echinoside A [19]. Compound 12 inhibited the proliferation of human hepatocellular liver carcinoma cells HepG2 (IC50 = 2.65 μM) and suppressed HepG2 cell adhesion, migration and invasion in a dose-dependent manner. It exhibited a significant antimetastatic activity through the specific inhibition of NF-kB-dependent matrix metalloproteinase-9 (MMP-9) and vascular endothelial growth factor (VEGF) expressions.
Recently, echinoside A (13) (Fig. 24.7) was isolated from P. graeffei [21]. Glycoside 13 and its desulfated analog 12 exhibited marked anti-cancer activity in HepG2 cells by blocking cell-cycle progression and inducing apoptosis through the mitocondrial pathway.
24.2.2 Glycosides with 3β-hydroxy-holost-7-ene Aglycones
Two monosulfated glycosides (14 and 15) (Fig. 24.8) with an oxygenated function at C-16 and differences in the third unit of the carbohydrate chain were evaluated for their effects on caspase activation and apoptosis of human leukemia (HL-60) cells [22]. Frondoside A (14), a major triterpene glycoside isolated from sea cucumber Cucumaria frondosa, has an acetoxy group at C-16 of the aglycon and xylose as the third monosaccharide residue [23], while cucumarioside A2-2 (15), isolated from Cucumaria japonica, depicts a 16-keto group and a glucose residue as the third monosaccharide unit in the carbohydrate chain [24].
Both glycosides strongly induced apoptosis of HL-60 cells, but frondoside A (14) had more potent effects than cucumarioside A2-2 (15) on cytotoxicity, cell cycle changes and apoptosis . This study indicated that the acetyl group at C-16 of the aglycon in frondoside A may play a significant role in frondoside’s cytotoxicity and caspase activation since glycoside 14 led to no caspase activation before early apoptosis while cucumarioside A2-2-induced apoptosis was caspase-dependent. New studies on cucumarioside A2-2 have shown that this glycoside affects mouse cell immunity in vitro, which is reflected in significant changes in immune cell morphology and behavior [25]. A new immunomodulatory lead named cumaside was created on the basis of a mixture of monosulfated triterpene glycosides isolated from C. japonica (mainly cucumarioside A2-2) with cholesterol in an approximate molar ratio of 1:2. Cumaside showed antitumor activity against different forms of experimental mouse Ehrlich carcinoma in vivo both independently and in combination with cytostatics [26].
Recently, it has been demonstrated that frondoside A inhibited the migration of human breast cancer cell line MDA-MB-231 in a wound healing assay [27] as well as the metastasis in vivo from a gland-implanted tumor [28]. Frondanol-A5P, a parent compound containing C. frondosa-derived glycosides, inhibited growth of S2013 and AsPC-1 pancreatic cancer cells [29].
Four new triterpene glycosides , cucumariosides H5 (16), H6 (17), H7 (18) and H8 (19) along with the known cucumarioside H (20) (Fig. 24.9) were isolated from the Far Eastern sea cucumber Eupentacta fraudatrix. These glycosides have a rare branched pentasaccharide carbohydrate moiety with one sulfate group at C-4 of the first xylose unit and 3-O-methyl-D-xylose as the terminal monosaccharide unit. Glycosides 16–18 and 20 differ from each other in the side chains of the aglycones, while cucumarioside H8 (19) has a novel aglycone with an unprecedented 16(22)-epoxy group [30]. The cytotoxic activities of glycosides 16–18 and 20 against mouse spleen lymphocytes and hemolytic activity against mouse erythrocytes were studied. Glycoside 17 having a 24(25)-double bond in the aglycone side was less active, while cucumariosides H (20) and H5 (16) having diene-systems and H7 (18) with a saturated side chain in the aglycone moiety showed a more potent cytotoxic and hemolytic activities. The new cucumarioside H2 (21) with a 25-hydroxyl group in the side chain showed low activity against mouse spleen lymphocytes and Ehrlich carcinoma cells [31]. Evaluation of the cytotoxicity of new minor cucumariosides A1 (22), A3 (23), A4 (24), A5 (25), A6 (26), A12 (27) and A15 (28) showed that glycosides 22 and 26 were the most active [32]. These results demonstrate that the structure of aglycone side chains influences significantly the cytotoxic action of these glycosides.
Patagonicoside A (29), the major triterpene glycoside of the sea cucumber Psolus patagonicus [33], and its desulfated analog (30) (Fig. 24.10) were tested for their antiproliferative activity in human hepatocellular (Hep3B), human mammary (MDA-MB231), and human lung (A-549) carcinoma cells. Both compounds were able to suppress the growth of the three tumor cell lines. Compound 30 exhibited a slightly stronger antiproliferative activity for Hep3B (IC50 = 5.09 μM) and MDA-MB231 (IC50 = 5.39 μM) while the effect for A-549 was similar to that of Patagonicoside A with IC50 values of 16.53 and 15.04 μM, respectively. Studies on the structure-activity relationship for sea cucumber glycosides revealed that their biological activities depend on both the aglycone and the carbohydrate structures. An 18(20)-lactone in the aglycone moiety is an important requirement for membranotropic action, together with the presence of a linear tetrasaccharide fragment in the carbohydrate chain. Besides, glycosides containing quinovose as the second monosaccharide unit are the most active. Patagonicoside A (29) and its desulfated analog (30) contain these favorable structural features. Nevertheless, both glycosides showed low hemolytic activity (82 and 87 μM, respectively) in comparison with sea cucumber triterpene glycosides containing a linear tetrasaccharide chain [5]. This could be assessed to the uncommon presence of two 12α- and 17α-hydroxyl groups and a Δ7 double bond in the aglycone moiety and may be related to their lower level of cytotoxicity. In order to approach to the mechanism of antiproliferative action of these compounds, the effect of both glycosides on nuclear factor-κB (NF-κB) activation was studied. Patagonicoside A and its desulfated analog promoted NF-κB translocation to the nucleus of tumor cells A-549, in absence and in presence of the tumor necrosis factor-α (TNF-α). Hence, their antiproliferative action would be related to their membranolytic activity [34].
Chemical examination of the sea cucumber Pseudocnus dubiosus leoninus led to the isolation of a new disulfated tetraglycoside Pseudocnoside A (31) (Fig. 24.11) [14]. Compound 31 was evaluated for in vitro cytotoxicity and antiproliferative activity on two human tumor cell lines (A-549 and HeLa) and these activities were compared to those of two structurally related triterpene glycosides, patagonicosides B (32) and C (33) (Fig. 24.11) isolated from Psolus patagonicus [35]. The antiproliferative activity of 31 in A549 cells (IC50 = 14.53 μM) was considerably higher than in HeLa cells (IC50 = 57.36 μM). Patagonicosides B (32) and C (33) exhibited similar antiproliferative and cytotoxic activities in HeLa and A549 with IC50 values of 7.94 and 9.73 μM (for compound 32) and of 3.57 and 5.56 μM (for compound 33), respectively. This study indicated that hydroxyls at C-12α and C-17α may play an important role in the cytotoxicity of these glycosides in comparison to a keto group at C-16 as in compound 31.
Stichoposide C (34) (Fig. 24.12) is a quinovose-containing hexaoside that was isolated from the sea cucumbers Stichoposus chloronotus [36] and Thelenota ananas [37].
Glycoside 34 induced apoptosis in mouse CT-26 subcutaneous tumor and HL-60 leukemia cells in a dose-dependent manner and increased ceramide generation in vivo through activation of acid and neutral sphingomyelinases [38].
24.3 Concluding Remarks
During the last five years many new examples of sea cucumber glycosides were isolated, in particular those containing a 3β-hydroxy-holost-7-ene aglycone. Notably, the sea cucumber Eupentacta fraudratrix is a rich source of triterpene glycosides containing this type of aglycone and displaying differences in their side chains. An increased interest in the evaluation of the cytotoxicities of these polar metabolites has allowed establish structure-activity correlations as well as investigation on their mechanisms of action. Frequently, different tumour cell lines exhibit a differential sensitivity to the cytotoxic effects of sea cucumber glycosides, which can be related to their chemical structures.
Usually, sea cucumber triterpene glycosides occur as complex mixtures of structurally related compounds that are present in low amounts in the organisms. Therefore, their isolation requires a combination of chromatographic procedures to obtain the pure compounds. Recently, new strategies based on the isolation of partially purified mixtures of bioactive triterpene glycosides and their admixture with cholesterol, such as cumaside, pose new alternatives to the development of pharmaceutical drugs. The growth of sea cucumbers by aquaculture would contribute to the supply of triterpene glycosides preventing the overexplotation of these echinoderms.
References
Chludil HD, Murray AP, Seldes AM et al (2003) Biologically active triterpene glycosides from sea cucumbers. In: Ur-Rahman A (ed) Studies in natural products chemistry, vol 28. Elsevier, Amsterdam, pp 587–616
Maier MS, Murray AP (2006) Secondary metabolites of biological significance from echinoderms. In: Fingerman M (ed) Biomaterials from aquatic and terrestrial organisms. Science Publishers, Enfield, pp 559–593
Kalinin VI, Silchenko AS, Avilov SA et al (2005) Sea cucumber triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem Rev 4:221–236
Kalinin VI (2000) System-theoretical (Holistic) approach to the modelling of structural– functional relationships of biomolecules and their evolution: an example of triterpene glycosides from sea cucumbers (Echinodermata, Holothurioidea). J Theor Biol 206:151–168
Kalinin VI, Aminin DL, Avilov SA et al (2008) Triterpene glycosides from sea cucumbers (Holothuroidea, Echinodermata). Biological activities and functions. In: Ur-Rahman A (ed) Studies in natural products chemistry, vol 35. Elsevier, Amsterdam, pp 135–196
Van Dyck S, Caulier, Todesco M et al (2011) The triterpene glycosides of Holothuria forskali: usefulness and efficiency as a chemical defense mechanism against predatory fish. J Exp Biol 214:1347–1356
Ponomarenko LP, Kalinovsky AI, Moiseenko OP et al (2001) Free sterols from the holothuriansSynapta maculata, Cladolabes bifurcates and Cucumaria sp. Biochem Physiol 128B:53–62
Maier MS (2008) Biological activities of sulfated glycosides from echinoderms. In: Ur-RahmanA (ed) Studies in natural products chemistry, vol 35. Elsevier, Amsterdam, pp 311–354
Bordbar S, Anwar F, Saari N (2011) High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs 9:1761–1805
Kim S, Himaya SWA (2012) Triterpene glycosides from sea cucumbers and their biological activities. In: Kim SK (ed) Advances in food and nutrition research, vol 65. Elsevier, Amsterdam, pp 297–319
Han H, Yi Y, Xuet Q et al (2009) Two new cytotoxic triterpene glycosides from the sea cucumberHolothuria scabra. Planta Medica 75:1608–1612
Han H, Ling LI, Yi YH et al (2012) Triterpene glycosides from sea cucumber Holothuriascabra with cytotoxic activity. Chin Herb Med 7:158–167
Chludil HD, Muniain C, Seldes AM et al (2002) Cytotoxic and antifungal triterpene glycosidesfrom the Patagonian sea sucumber Hemoiedema spectabilis. J Nat Prod 65:860–865
Careaga V, Bueno C, Muniain C et al (2014) Pseudocnoside A, a new cytotoxic and antiproliferativetriterpene glycoside from the sea cucumber Pseudocnus dubiosus leoninus. Nat Prod Res 28:213–220
Silchenko AS, Avilov SA, Kalinin VI et al (2008) Constituents of the sea cucumber Cucumariaokhotensis. Structures of okhotosides B1-B3 and cytotoxic activities of some glycosidesof this species. J Nat Prod 71:351–356
Han H, Zhang W, Yi YH et al (2010a) A novel sulfated holostane glycoside from sea cucumberHolothuria leucospilota. Chem Biodiversity 7:1764–1769
Han H, Xu QZ, Yi YH et al (2010b) Two new cytotoxic disulfated holostane glycosides fromthe sea cucumber Pentacta quadrangularis. Chem Biodiversity 4:183–188
Zhao Q, Xue Y, Liu, ZD et al (2010) Differential effects of sulfated triterpene glycosides,holothurin A1, and 24-dehydroechinoside A, on antimetastasic activity via regulation of theMMP-9 signal pathway. J Food Sci 75:280–288
Zhao Q, Liu ZD, Xue Y et al (2011) Ds-echinoside A, a new triterpene glycoside derivedfrom sea cucumber, exhibits antimetastasic activity via the inhibition of NF-ï«B-dependentMMP-9 and VEGF expressions. Biomed Biotechnol 12:534–544
Kitagawa I, Inamoto T, Fuchida M et al (1980) Structures of echinoside A and B, two antifungaloligoglycosides from the sea cucumber Actinopyga echinites (Jaeger). Chem Pharm Bull28:1651–1653
Zhao Q, Xue Y, Wang JF et al (2012) In vitro and in vivo anti-tumor activities of echinosideA and ds-echinoside A from Pearsonothuria graeffei. J Sci Food Agric 92:965–974
Jin J, Shatina VV, Shin SW et al (2009) Differential effects of triterpene glycosides, frondosideA and cucumarioside A2–2 isolated from sea cucumbers on caspase activation andapoptosis of human leukemia cells. FEBS Lett 583:697–702
Girard M, Belanger J, ApSimon JW et al (1990) Frondoside A, novel triterpene glycosidefrom the holothurian Cucumaria frondosa. Can J Chem 68:11–18
Aminin DL, Agafonova IG, Berdyshev EV et al (2001) Immunomodulatory properties ofcucumariosides from the edible Far-Eastern holothurian Cucumaria japonica. J Med Food4:127–135
Aminin DL, Gorpenchenko TY, Bulgakov VP et al (2011) Triterpene glycoside CucumariosideA2-2 from sea cucumber stimulates mouse immune cell adhesion, spreading and motility.J Med Food 14:594–600
Aminin DL, Chaykina EL, Agafonova IG et al (2010) Antitumor activity of the immunomodulatorylead Cumaside. Intern Immunopharm 10:648–654
Marzouqi NA, Iratni R, Nemmar A et al (2011) Frondoside A inhibits human breast cancercell survival, migration, invasion and the growth of breast tumor xenografts. Eur J Pharmacol668:25–34
Ma X, Kundu N, Collin PD et al (2012) Frondoside A inhibits breast cancer metastasis andantagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Res Treat 123:1001–1008
Roginsky AB, Ding XZ, Woodward C et al (2010) Anti-pancreatic cancer effects of a polarextract from the edible sea cucumber, Cucumaria frondosa. Pancreas 39:646–652
Silchenko AS, Kalinovsky AI, Avilov SA et al (2011) Structure of cucumariosides H5, H6,H7 and H8, triterpene glycosides from the sea cucumber Eupentacta fraudatrix and unprecedentedaglycone with 16,22-epoxy-group. Nat Prod Commun 6:1075–1082
Silchenko AS, Kalinovsky AI, Avilov SA et al (2012a) Structures and cytotoxic properties ofcucumariosides H2, H3 and H4 from the sea cucumber Eupentacta fraudatrix. Nat Prod Res26:1765–1774
Silchenko AS, Kalinovsky AI, Avilov SA et al (2012b) Triterpene glycosides from the sea cucumberEupentacta fraudatrix. Structure and biological action of cucumariosides A1, A3, A4,A5, A6, A12 and A15, seven new minor non-sulfated tetraosides and unprecedented 25-keto,27-norholostane aglycone. Nat Prod Commun 7:517–525
Murray AP, Muniain C, Seldes AM et al (2001). Patagonicoside A: a novel antifungal disulfatedtriterpene glycoside from the sea cucumber Psolus patagonicus. Tetrahedron 57:9563–9568
Careaga VP, Bueno C, Muniain C et al (2009) Antiproliferative, cytotoxic and hemolyticactivities of a triterpene glycoside from Psolus patagonicus and its desulfated analog. Chemotherapy55:60–68
Careaga V, Munian C, Maier MS (2014) Patagonicosides B and C, two antifungal sulfated triterpeneglycosides from the sea cucumber Psolus patagonicus. Chem Biodiversity 8:467–475
Kitagawa I, Kobayashi M, Inamoto T et al (1981) Stichlorosides A1, A2, B1, B2, C1 and C2from the sea cucumber Stichopus chloronotus (Brandt). Chem Pharm Bull 29:2387–2391
Stonik VA, Maltsev II, Elyakov GB (1982) Structures of thelenotoside-A and thelenoside-B from the sea cucumber Thelenota ananas. Chem Nat Prod 624–627
Yun SH, Park ES, Shin SW et al (2012) Stichoposide C induces apoptosis through the generation of ceramide in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin Cancer Res 18:5934–5948
Acknowledgments
The authors gratefully acknowledge grants from the University of Buenos Aires and the National Research Council of Argentina (CONICET). M. S. M. is a Research Member of CONICET.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Careaga, V., Maier, M. (2015). Cytotoxic Triterpene Glycosides from Sea Cucumbers. In: Kim, SK. (eds) Handbook of Anticancer Drugs from Marine Origin. Springer, Cham. https://doi.org/10.1007/978-3-319-07145-9_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-07145-9_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-07144-2
Online ISBN: 978-3-319-07145-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)