Abstract
Marine capture fisheries in Mexico are dominated by sardine, shrimp, and tuna, representing as a whole 60 % of the total catch. However, shrimp and tuna are the most important fishery resources in terms of economic value. Capture shrimp fishery in Mexico has exhibited stagnating catches (around 65 thousand tonnes) since the last two decades, and shrimp stocks have been clearly depleted in some parts of the country. Conversely, shrimp (Litopenaeus vannamei) aquaculture has shown an exponential growth in the number of farms and shrimp production since mid-1980s. As a result, currently, shrimp aquaculture production has almost doubled the total production of the capture shrimp fishery. On the other hand, total catch of tuna by Mexican tuna fleet has fluctuated around 130 thousand tonnes during the last 5 years (2007–2011). Contribution of farmed tuna (Thunnus orientalis) has been negligible in terms of volume; however, the price of 1 tonne of farmed tuna is about 7–13 times that of tuna caught by the fishing fleet, making it an attractive alternative as source of employment and income. The case studies presented here are indicative of the potential value of aquaculture as a complementary productive activity to meet the growing human demand for food from the sea. This is especially relevant in terms of global fisheries production because the maximum fisheries catch potential from the oceans around the world has apparently been reached. However, there are still concerns associated with aquaculture impacts on the environment that must be addressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The fundamental purpose of aquaculture and fishing is providing seafood products for human consumption. Wild capture fishing is a much older activity than aquaculture, which is referred as the farming of aquatic animals and plants (FAO 2012). Fishing has been practiced by humanity since the earliest stages of human evolution as a source of food supply (Gartside and Kirkegaard 2009). This practice allowed many ancient cultures around the world to settle in the coastal areas, obtaining seafood products from the intertidal zone and from shallow waters along the seashore (Squires 2009).
Apparently, fishing in the ancient world did not only play a marginal role in the economy, since there is archeological evidence of fish processing on a commercial scale along the Mediterranean, Atlantic coast and in the Black Sea (Bekker-Nielsen 2005). However, during the last century the prominent intensification of fishing effort, and the fisheries industrialization in general, made possible a pronounced increase of total landings as well as of the diversity of catches, including the capture of new species that had not been exploited before, such as pelagic and deep sea organisms. However, while global fishing production and the total number of fishing species captured underwent a meaningful increase during the last decades, on the other hand, the intensification of the fishing effort caused the depletion of fishing stocks due to overfishing, overstressing the marine systems, which is considered to be largely responsible for the current global fisheries crisis (Pauly et al. 2002).
Total landings of worldwide marine fisheries have fluctuated from 16.8 million tonnes in 1950 up to 86.4 million tonnes in 1996, stabilizing at about 80 million tonnes during the following years; with a slight decrease in 2010, when the global marine capture fisheries was of 77.4 million tonnes. Stabilization of global fisheries production over the past recent years might suggest that the maximum fisheries catch potential from the oceans around the world has probably been reached. Actually, according to recent data from FAO (2012) 14.1 % of the world marine fishery stocks are underexploited or moderately exploited, 57.3 % are fully exploited, 13.7 % are overexploited and 7.6 % are depleted or in recovering. Additionally to the impacts of fishing per se, it has been pointed out that capture fisheries are also affected by climate-related threats, including climate change (Brander 2007).
Under this scenario there is apparently little chance to increase the production of marine resources based solely on captured fisheries production, and thus seafood supply as a source of animal protein for human consumption could be seriously compromised in the following years. Moreover, the world human population has increased exponentially, from 2.53 billion in 1950 to 7.16 billion in 2013 (United Nations 2013) and, as a result, the human population demand for seafood is continuously growing year after year. In this regard, during the last years, aquaculture has been playing an increasingly important role in contributing to the production of marine resources, and, although fisheries production remains stable, aquaculture production is still expanding globally and diversifying (FAO 2012). Aquaculture is the fastest growing sector of the world food economy, and its production is projected to overtake that of other sectors of animal food production. Indeed, since 2011 world farmed fish production topped beef production (Larsen and Roney 2013).
Aquaculture was probably first practiced in China more than 4,000 years ago, and particularly in Asia, this activity has been traditionally important as source of food (Rabanal 1988). In fact, Asian countries have historically been the major producers of cultured aquatic organisms in the world (FAO 2012). The contributions of capture fisheries and the aquaculture industry to the world’s production of marine resources for human consumption have evolved to reach total production levels of 148 million tonnes of fish in 2010 (FAO 2012). The combined contribution of fisheries and aquaculture might be critical to guarantee the supply of nutritious food and animal protein to the escalating demands of a growing world population. However, the future trends of this interaction might not be easily predicted, in part because fisheries catch is primarily dependent on the natural boundaries of the environment, whereas aquaculture productivity is mostly limited by water quality and biotechnological aspects of the aquatic species of interest. However, it is clear that contribution of aquaculture to seafood production is increasingly significant in many countries around the world.
Mexico is a country that has 8,475 km of coastline along the Pacific Ocean and 3,118 km along the Gulf of Mexico and the Caribbean Sea (Cifuentes-Lemus et al. 1995) (Fig. 5.1). Seafood production in Mexico was mostly based on capture fisheries, but since the mid-1980s aquaculture has become a growing contributor for the production of marine resources for human consumption. Even in certain regions of the country the relative contribution of fisheries and aquaculture production has been reversed in the last years, generating higher production of seafood by aquaculture than by fishing; such is the case of shrimp production along the Mexican Pacific coast.
In this chapter, we analyze the historical interactions of capture fisheries and aquaculture as complementary sectors in the production of marine resources for human consumption in Mexico. Advances and perspectives regarding the interaction between fishing and aquaculture activities of the most important fishery resources in the country (shrimp and tuna) are analyzed considering their production levels, inherent characteristics and concerns.
2 Inherent Characteristics and Global Situation of Marine Fisheries and Aquaculture
World marine fisheries are based on the capture and removal of marine organisms from the natural environment. This activity can be view as a form of hunting of wild animals in the marine ecosystem. The productivity and sustainability of fisheries are mostly dependent on natural constraints such as the population size and fish stocks regeneration, environmental variability and ecosystems health, but also of the fishery management practices. Most fisheries in the world are characterized by an open access regime and are impacted by additional stressors such as global climate, leading to fishing stocks to be overharvested and in some cases overexploited to the point of collapse (Pauly et al. 2005). Apparently, more than 3,000 years of scientific understanding of the phenomena, theirs causes, and suitable mitigation measures, are not sufficient to prevent the destruction of natural resources, as stated by Ludwig et al. (1993).
The global marine catch in 2009 was mostly comprised of pelagic species (41 %) such as herrings, sardines, anchovies (small pelagics) as well as tunas, bonitos, and billfishes (large pelagics) (Ye and Cochrane 2011). It has been pointed out that as part of the global marine fisheries crisis, a decrease in the mean trophic level of species groups in landings from 1950 to 1994 has been experienced, a phenomenon known as “fishing down marine food webs”. This situation not only denotes the gradual transition in catch composition (from long-lived, high trophic level piscivorous bottom fish toward short-lived, low trophic level invertebrates and planktivorous pelagic fish) but also implies that the exploitation rates of fishing marine stocks are unsustainable (Pauly et al. 1998). Moreover, from 1950 to 1989 global marine catches increased continuously at an average rate of 1.6 million tonnes per year, however, a shift in this trend has been observed from 1989 to 2011 exhibiting a phase of stagnating catches stabilizing at about 80 million tonnes (Fig. 5.2), suggesting that its maximum productive potential has probably been reached. Irrespective of the global fisheries crisis, human demand for fish and seafood will inevitably continue to grow; therefore, additional production of seafood is necessary to meet the global demand for food from the continuously growing world human population. Under this scenario, aquaculture production might be an important complementary source of seafood for human consumption.
Aquaculture is the farming of aquatic organisms (e.g. fish, mollusks, crustaceans, aquatic plants) in a controlled environment, which implies some form of intervention such as rearing, feeding and protecting from predators (FAO 2011). Aquaculture of marine species usually occurs in ponds or tanks (land based aquaculture) as well as in existing water bodies utilizing some type of artificial enclosure, like cages or pens, for containing the aquatic organisms (water based aquaculture).
Composition of farmed marine species varies among countries and is dependent of the culture environment (brackish or marine water). According to FAO data from 2011 (http://www.fao.org/fishery/statistics/en), freshwater aquaculture is dominated by the farming of carps and cichlids. However, aquaculture production of brackish and marine species is mainly characterized by mollusks (clams, oysters, mussels) representing about 61 % of the production, followed by crustaceans (shrimps, prawns, crabs) representing 15 %, and marine fishes (miscellaneous coastal and pelagic fishes) representing 8 % of the total. Aquaculture by volume is dominated by Asian countries; which all together account for about 80 % of world aquaculture production in brackish and marine environments.
In contrast with the marine fisheries crisis, aquaculture of marine and brackish species has experienced a continued growth, increasing its production from 340 thousand tonnes in 1950 to 23 million tonnes in 2011 (Fig. 5.2). In fact, when comparing marine fisheries and aquaculture production from 1993 to 2011, a significant negative trend is observed for capture fisheries whereas a significant positive pattern is evident for aquaculture production of brackish and marine species (Fig. 5.3). It is clear that fisheries alone cannot fill the global demand for fish and seafood; however, aquaculture production combined with wild fisheries production might be a plausible alternative.
3 Contribution of Fisheries and Aquaculture to Seafood Production in Mexico
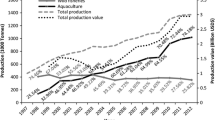
Commercial landings from marine capture fisheries in Mexico are dominated by sardines, shrimp, and tuna, which as a whole represent about 60 % of the total catch in the country. However, the most economically important species or species groups are shrimp and tuna accounting for 43 % and 7 %, respectively, of the total value of fishery production in the country (CONAPESCA 2011). According to fisheries and aquaculture statistics in Mexico, capture fisheries increased from 118,043 tonnes in 1954 to a maximum of 1.5 million tonnes in 1981; nevertheless, during the following years catches have fluctuated around 1.2–1.4 million tonnes (Fig. 5.4). Temporal trends of fishery landings in Mexico could be the result of different extractive phases of the fishery stocks over time, as described for Latin American benthic shell fisheries (Castilla and Defeo 2001). In this regard, an initial exploitation phase occurred during 1954 to early 1970s; afterward an expansive extraction phase began from mid-1970s extending to early 1980s. Finally, a trend toward stabilization of catches is observed from mid-1980s to the present.
With regard to aquaculture production in Mexico, its first record in official statistics was 109,061 tonnes in 1983, reaching 262,855 tonnes in 2011 (Fig. 5.4). To date, the contribution of aquaculture to the national fisheries production is about 16 % by volume. Nonetheless, aquaculture production accounts for 40 % in terms of economic value of the national fisheries production, which could be explained by the growing industry of shrimp farming, the most economically important species in Mexico (CONAPESCA 2011).
4 Trends and Advances on the Interaction of Fishing and Aquaculture for Shrimp Production
4.1 Situation of the Shrimp Fishery in Mexico
Shrimp is the most important marine resource in terms of economic value in Mexico, representing about 550 million dollars in 2011; its fishery occurs along the Gulf of Mexico and Caribbean coasts as well as the Pacific coast. Shrimps are captured as juveniles by an artisanal fleet in coastal lagoons, whereas the industrial fleet offshore captured adult shrimps, resulting in a sequential fishery. In the Gulf of Mexico and the Caribbean Sea the main species captured by the shrimp fishery are Farfantepenaeus aztecus, F. duorarum, Litopenaeus setiferus and F. brasiliensis. On the other hand, catches of Mexican Pacific shrimp fishery are mainly conformed by F. californiensis, Litopenaeus stylirostris and L. vannamei (INAPESCA 2006).
Shrimp catches in the Gulf of Mexico have shown an overall decreasing trend from 27 thousand tonnes in 1980 to 20 thousand tonnes in 2011, exhibiting remarkable differences in historical landings according to the region (Fig. 5.5). For instance, shrimp captures in the Campeche Sound (Southern Mexico) declined sharply from 14 thousand tonnes in 1979 to 2.6 thousand tonnes in 2006, with a slightly increase to 7.5 thousand tonnes in 2011 (Fig. 5.5). The shrimp stocks in this region of the Gulf of Mexico are depleted, particularly the pink shrimp (F. duorarum). During the 1960s this region accounted for about 90 % of total shrimp catches in the Gulf of Mexico and the Caribbean Sea; however over recent years its contribution has been usually lower than 20 % (Fig. 5.5). The decreasing trend in shrimp catches has been related to recruitment failures; however, the future scenario of the shrimp fishery in the Campeche Sound is not promissory because if the current levels of fishing intensity are maintained, then the stock density will continue to decrease (Ramírez-Rodríguez et al. 2000) aggravating the present situation of fishery depletion.
Conversely, the trend in shrimp catches in the Tamaulipas coast (Northeastern Mexico) has shown an increasing trend during the years 1950–1990, reaching an apparent stabilization of catches at around 12–13 thousand tonnes since 1992. It is worth noting that since 1990 the shrimp catches in Tamaulipas have been higher than catches from the Campeche Sound (Fig. 5.5). In fact, during the last years the shrimp fishery of Tamaulipas has provided annually more than 50 % of the total shrimp catch from the Gulf of Mexico, while Campeche usually contributes with no more than 20 %, except for the period of 2009–2011 where it was from 25 to 38 % (Fig. 5.5), which might be mostly due to the increase in captures of other shrimp species such as the Atlantic seabob (Xiphopenaeus kroyeri) and rock shrimp (Sicyonia brevirostris) from the Caribbean waters.
On the other hand, captures in the Mexican Pacific shrimp fishery have fluctuated around 45 thousand tonnes during the last years, representing the most important shrimp fishery in Mexico in terms of volume, contributing in average about 70 % of the national shrimp catch, whereas the Gulf of Mexico shrimp fishery accounts for the remaining 30 %. Historically, higher shrimp catches have been obtained on the west side coast of Mexico (Fig. 5.6). Therefore, national trends in shrimp catch follow the same pattern of the Mexican Pacific shrimp fishery, being the states of Sonora and Sinaloa the most productive for this fishery. Contrary to the declining trend of shrimp catch in the Gulf of Mexico, the Mexican Pacific shrimp fishery has exhibited an apparent stabilization since the early 1990s; this pattern is also mirrored in the national trend (Fig. 5.6). According to the above, it is clear that the shrimp fisheries in Mexico are at their maximum catch potential and indeed in some particular regions the shrimp stocks have declined steadily; and the most severe decline has been experienced by the pink shrimp (F. duorarum) in the Campeche Sound, Gulf of Mexico (Ramírez-Rodríguez et al. 2000).
Production of farmed shrimp in Mexico has been recorded in official statistics since 1985 with 35 tonnes (Secretaría de Pesca 1993). However, shrimp farming has grown exponentially since then, reaching an annual production of 133 thousand tonnes in 2009 and about 110 thousand tonnes in 2011 (Fig. 5.7). Moreover, since 2003 shrimp farming production exceeded the total national production of the capture shrimp fishery, and nowadays aquaculture production of shrimp nearly doubles the capture shrimp fishery production in Mexico. Farmed shrimp has made it possible to reach a national production of 170 to almost 200 thousand tonnes of shrimp during recent years (Fig. 5.7).
Along with the substantial increase in aquaculture production of shrimp, there has been an increase in the number of shrimp farms, particularly along the Mexican Pacific; while in contrast, a continuous reduction in the number of shrimp vessels has been observed since 2004. Afterward, in 2011 the number of shrimp vessels and shrimp farms was almost the same (Fig. 5.8). Although there has been a fast increment of farmed shrimp production in the Mexican Pacific during the last two decades, shrimp aquaculture has remained incipient in states located along the Mexican coasts of the Gulf of Mexico and consequently most shrimp production, from the fishery and aquaculture sectors, comes from the Mexican Pacific (Fig. 5.9). In this regard, according to official statistics of fisheries and aquaculture production in Mexico provided by CONAPESCA (2011), the Mexican Pacific accounts for 73 % of the national capture shrimp fishery production and 97 % of shrimp farming production in the country (Fig. 5.10).
4.2 Aquaculture of Shrimp in Mexico: Technological and Biological Aspects
Global production of farmed marine shrimp was around 3.5 million tonnes in 2011, being dominated by the culture of L. vannamei (white leg shrimp) and Penaeus monodon (giant tiger prawn) (Fig. 5.11). In the case of Mexico, the main shrimp species utilized in aquaculture industry is the Western white shrimp or white leg shrimp (L. vannamei). The culture of L. vannamei in Mexico began in the early 1980s because of the viral disease problems with L. stylirostris that until then used to be the most widely exploited in shrimp farming along the Mexican Pacific (SAGARPA 2012).
World aquaculture production of principal shrimp species (FAO 2012)
Production of the white shrimp (L. vannamei) represents about 75 % of the world shrimp production and it is the main species used in shrimp farms of America (including Mexico), Asia, and Africa (FAO 2012); being the main introduced species for aquaculture purposes in the last two mentioned continents.
This shrimp species reaches a maximum total length of a 23 cm, and shows a uniform growth rate. The white shrimp tolerates salinities from 15 to 40, and some experimental evidence suggests the possibility for farming shrimp in freshwater areas with special acclimation (Araneda et al. 2008, 2013; Miranda et al. 2010; Ortega-Salas and Rendón 2013). Even, some commercial farms in Mexico, particularly in the Northeastern region, operate under very low salinity to freshwater conditions.
Before the 1980s, a high number of shrimp farms used to obtain post larvae directly from the wild environment or brood stock collected from natural habitats for reproduction purposes in production laboratories (maturation and hatcheries). During the period of mid-1980s to the early 1990s, the traditional method to obtain the post larvae and brood stock from wild habitats changed to obtain organisms from shrimp farms, which support the use of quality control measures against shrimp diseases (Martínez-Córdova 1999; Walker and Winton 2010). In this period, all Mexican farms employed specific pathogen free (SPF) or specific pathogen resistant (SPR) post larvae. At same time, the collection of wild post larvae was prohibited in order to prevent dispersion of shrimp diseases and as an environmental protection measure for wild shrimp populations that support shrimp fisheries (Páez-Osuna 2001; Lightner 2005; Walker and Winton 2010). Nowadays, the brood stock individuals are collected from special shrimp ponds where SPF of SPR organisms are maintained under low density, suitable feeding, and a high control of water quality (Lightner 2005). Collected organisms are analyzed in certificated aquatic animal health laboratories (Ortega 2012) before being transported to maturation tanks.
Maturation and reproduction of brood stock shrimp is carried out in tanks under controlled conditions in a maturation laboratory (Treece 2000). Shrimp females with attached spermatophore are transferred to the spawning area for spawning. Afterward, shrimp females are returned to their specific maturation tank. The spawned eggs are maintained in the spawning area until hatching. The nauplii are collected, washed, and evaluated for quality (Treece 2000). Later, they are cultured under standard conditions to protozoea (I–III) and mysis stages (I–III) before assuming the body plan of the adult as a post larva (Treece 2000).
In 2012, Mexico had 58 official companies of post larvae production (commonly named as production laboratories) with 10,000–15,000 million of post larvae production of L. vannamei and/or L. stylirostris distributed in the Mexican Pacific (states of Baja California, Baja California Sur, Nayarit, Sinaloa, and Sonora) and the Gulf of Mexico (Tamaulipas and Yucatan). The majority of these laboratories (75 %) are located in the states of Sinaloa and Sonora; in fact, these states from the Mexican Pacific are the largest producers of farmed shrimp in the country and have the largest number of shrimp farms.
Extensive shrimp farming was the first shrimp production system installed in Mexico, before private investment in the aquaculture industry was allowed by the government (Martínez-Córdova 1999; DeWalt et al. 2002). As in other countries, extensive production of shrimp was initially based on the capture of wild post larvae; however, presently post larvae are supplied from controlled farms with SPF or SPR organisms (Lightner 2005; Walker and Winton 2010). Table 5.1 displays the general characteristics of the main shrimp farming systems in Mexico; the number of shrimp farms and the extension in hectares used for shrimp aquaculture in 2011 is also reported. Extensive shrimp culture systems are disappearing compared to intensive and semi-intensive systems. The historical transition from extensive to intensive systems is evident; in 1995 there were 71 extensive shrimp farms (2,884 ha) whereas there were 147 semi-intensive (10,872 ha), and 13 intensive (546 ha) production systems (SEMARNAP 1996). Afterward, by 2011 the number of extensive shrimp farms decreased to only two farms (3 ha); however, there was a notable increase of semi-intensive shrimp farms reaching the amount of 1,367 farms (71,057 ha), while the number of intensive shrimp production units did not change, remaining at 13 farms (383 ha) (CONAPESCA 2011).
In general, a few farms have continued using the extensive, which require relatively low levels of technology and investment. The production of shrimp depends on the natural productivity of the enclosed body water which is enhanced by fertilization (organic and inorganic); additionally, shrimp are fed a commercial feed close to harvest time, in order to increase shrimp size (Treece 2001; Martínez-Córdova et al. 2004). In this type of aquaculture system, shrimp farms take advantage of natural tides for water exchange in the shrimp pond which at the same time helps to maintain proper water quality in the ponds. This system can use pumps to facilitate this process during harvest time or during extreme low water quality conditions (Páez-Osuna et al. 2003).
Semi-intensive shrimp farming have been utilized since 1980s, being the main shrimp farming systems used in Mexico today (Table 5.1, Fig. 5.12a, b). The increase of semi-intensive and intensive production systems was possible because the Mexican government allowed private investment in the shrimp aquaculture industry, although the exploitation of shrimp species had been exclusively reserved to organizations of fishermen named ‘cooperatives’ (INAPESCA 2006). This situation generated an exponential growth in shrimp farming enabling it to reach the actual levels of productivity, where farmed shrimp production has practically doubled the capture shrimp fishery production (Fig. 5.7).
Mexican farms that use these systems recognize two culture phases. The first phase or nursery (from post larva to juvenile shrimp ≤0.5 g) use natural food obtained through previous fertilization (inorganic) with triple superphosphate or TSP [Ca(H2PO4)2] + Urea [CO(NH2)2] on shrimp ponds (Treece 2001; Martínez-Córdova et al. 2004). The post larvae acclimatization is very relevant to reduce post larval stress caused by changes in temperature, salinity and pH when they are transferred from hatcheries to shrimp farms.
During this phase, shrimp ponds are filled with less than 100 % of their water capacity, which is increased as the shrimp grows. Pond fertilization is strongly monitored during the shrimp culture focusing on obtaining a proper productivity of algae, mollusks, polychaetes, organic matter, small crustaceans, and diverse benthic organisms that provide a natural feed source for shrimp (Treece 2001; Martínez-Córdova et al. 2004). However, as shrimp grow natural feeding is replaced by artificial feed (pellets). Several farms with intensive systems have specialized areas such as greenhouse or nursery areas with a high control of water quality, where shrimp are maintained until reaching a suitable body size before they are transported to the grow-up ponds.
In the second phase or growth phase (shrimp ≥0.5 g to harvest) there is a constant control of the different parameters of shrimp production, such as disease prevention and/or control, feeding and growing rates, feed conversion ratio, feed intake, shrimp density, among other parameters. Commercial feed is supplied one or two times per day. Dissolved oxygen levels, pH, and water temperature in the shrimp pond is assessed daily, whereas ammonium, nitrites, nitrates and primary productivity parameters are monitored weekly. Semi-intensive and intensive shrimp farms utilize water exchange and aeration supply to maintain water quality. Additionally, shrimp survival, their size and weight, and intestinal fullness are monitored biweekly in order to calculate the feed necessary for the next weeks (Arredondo-Figueroa 2002; Rojas et al. 2005).
Recently, the use of liners or geo-membrane of synthetic polymers (high-density polyethylene or HDPE, and ethylene-propylene-diene-monomer or EPDM) is used in shrimp farms with intensive systems (Horowitz et al. 2001; Samocha 2011a). Shrimp farms use these polymeric geosynthetic barriers principally as pond cover to prevent water filtration (Horowitz et al. 2001; Samocha 2011a); this type of material also facilitates the collection, treatment and/or removal of organic material and sediment produced during shrimp farming. Initially, these geo-membranes were used in maturation and larval rearing tanks, later they were also utilized in nursery ponds and finally in grow-out ponds. However, the use of EPDM has decreased due to its apparent toxicity to shrimp (Samocha 2011b). In most shrimp farms one to two crops a year are obtained; in tropical climates, even three crops a year are possible.
Some farms in Mexico grow shrimp in freshwater conditions. To this end, shrimp post larvae are previously adapted to freshwater in tanks before they are transferred to the grow-out ponds (Araneda et al. 2008, 2013; Miranda et al. 2010; Ortega-Salas and Rendón 2013). Some of these types of farms that culture shrimp in freshwater conditions, are located in the Mexican states of Baja California, Colima, Sinaloa, Sonora, and Tamaulipas which operate at commercial scale, using freshwater from artesian wells (2–10 ‰) (CONAPESCA 2011; Ortega-Salas and Rendón 2013). Early post larvae of L. vannamei (PL 2–7) are acclimated from seawater to freshwater at a rate of 1 ‰ per day until obtaining a salinity water level similar to that in grow-up ponds (Fig. 5.12c). The post larvae are acclimated in greenhouses at low densities for a better control of the environmental conditions, and they are feed with Artemia spp. nauplii and microparticle feed (Treece 2001; Araneda et al. 2008; Miranda et al. 2010).
When organisms show an adequate body size, they are transferred to the grow-out ponds (land and/or liners). The freshwater shrimp farm close to Reynosa, Tamaulipas (Mexico) located at 65–70 km from the coast is a good example of this type of production system (Gutiérrez-Salazar et al. 2011).
4.3 Future Considerations and Concerns
The further development of the shrimp aquaculture industry may generate a high environmental pressure (mangrove deforestation, increase of organic material and sediments in estuaries and coastal lagoons) that at the same time might diminish or stop the continuous growth of this industry. In this regard, the implementation of measures in order to develop aquaculture methods environmentally sustainable is critical (Páez-Osuna 2001; Ferreira et al. 2007).
The rapid expansion of the shrimp farming industry brought with it frequent outbreaks of diseases affecting the shrimp growth and survival (Walker and Winton 2010). Pathogens represent a critical problem to the shrimp industry, causing important declines in shrimp production with the concurrent economic losses. Viruses are known as the most important pathogens faced by the shrimp farming industry. In Mexico, the Infectious hypodermal and hematopoietic necrosis virus (IHHNV) was introduced during early 1980s into shrimp farms, producing the collapse of young farmed L. stylirostris which encouraged shrimp farmers to change the cultured shrimp species to L. vannamei (Aguirre-Guzmán and Ascencio-Valle 2000). Later, the Taura Syndrome Virus (TSV) affected the shrimp cultures of L. vannamei during the 1990s causing the return of L. stylirostris to the shrimp cultures (Lightner 1999; Aguirre-Guzmán and Ascencio-Valle 2000; Lightner 2005). Currently, L. vannamei is the most important shrimp cultured in Mexico; however, in early 2000s the White Spot Syndrome Virus (WSSV) generated important shrimp losses in shrimp farms particularly on L. vannamei (Lightner 1999, 2005; Aguirre-Guzmán and Ascencio-Valle 2000; Sánchez-Martínez et al. 2007). Recently, the Mexican government stopped all shrimp imports from Asia due to a new pathogen (Vibrio parahaemolyticus) detected in those shrimps; which has produced high shrimp mortality in the Mexican Pacific region where the vast majority of shrimp farms are located. These bacteria are transmitted orally, colonizing the shrimp gastrointestinal tract, and producing toxins that cause tissue destruction and dysfunction of the hepatopancreas. This bacterial disease is called early mortality syndrome (EMS) or acute hepatopancreatic necrosis syndrome (AHPNS) (FAO 2013).
The future of shrimp farming in Mexico is relevant for shrimp production in the country. For that reason and according to previous experience where significant losses of shrimp production were due to several pathogens, some strategies have been established with the aim to obtain a healthy shrimp aquaculture industry. The strategies implement are based on animal health and technological elements, such as the use of SPF (specific pathogen free) and SPR (specific pathogen resistant) post larvae (Walker and Winton 2010). Mexico implemented this measure of diseases control in the mid-1990s after TSV outbreaks in shrimps from Hawaii, USA, France and Tahiti. To date, this biosecurity strategy has been complemented with a reduced water exchange, hazard analysis and critical control points (HACCP) systems, and the careful monitoring and management of shrimp culture (Fig. 5.12d). The Mexican government decreed a law project (NOM-062-ZOO-1999 and Project NOM-000-ZOO-2009) on aquatic animal health showing the relevance of using SPF organisms in shrimp production (Lightner 2005), with special attention to quarantine laws to prevent the import of exotic pathogens.
Shrimp farmers associations (SFA) in Mexico are highly communicated and organized (intra and inter-associations) allowing them to achieve common objectives for the shrimp aquaculture industry. They also have a close relationship with research groups to work in common areas of interest; this is especially true in the Mexican Pacific, which accounts for 97 % of total farmed shrimp (Fig. 5.10). This organizational arrangement has also enabled them to get financial support for technology to improve shrimp production, as well as to get better national and/or international prices. Currently, the SFA have been working on the issue of animal health in shrimp farms in real time with different laboratories focused on the diagnosis of shrimp diseases in Mexico, USA, and France.
5 Trends and Advances on the Interaction of Fishing and Aquaculture for Tuna Production
5.1 Situation of the Tuna Fishery and Wild Tuna Stocks in Mexico
Tuna represents the third most important marine resource in Mexico in terms of volume, after sardine and shrimp; but it is the second most economically important fishery resource, just after shrimp, reaching about US$86 million in 2011. The Mexican tuna fishery occurs along the Pacific Ocean and Gulf of Mexico; however, the Mexican Pacific is the most important region for tuna fishing in the country. In fact, the catch of tuna in the Mexican Pacific in 2011 was 106 thousand tonnes (107 tuna vessels) whereas in the Mexican Atlantic (Gulf of Mexico and the Caribbean Sea) was 1.5 thousand tonnes (31 tuna vessels) (CONAPESCA 2011). This fishery on the Pacific side of Mexico is supported primarily by the catch of the yellowfin tuna (Thunnus albacares) accounting for about 80 % of total tuna catch, followed by skipjack tuna (Katsuwonus pelamis), bluefin tuna (T. orientalis) and bonito (Sarda spp.) among others (INAPESCA 2006), which are mainly caught with purse seine gears. In the Gulf of Mexico, the fishery is based on yellowfin tuna primarily caught with long-line fishing gears.
Tuna catch by Mexican vessels started in the 1950s in the Pacific Ocean reaching around 109 thousand tonnes; before this year the fishery was artisanal (INAPESCA 2006). However, after the following two decades tuna catches decreased to 13,825 tonnes in 1972 reaching its maximum in 2003 with 188,821 tonnes; but during the last 5 years (2007–2011) tuna catch has fluctuated around 130 thousand tonnes. Additionally, tuna farming has also contributed to tuna production in Mexico; however, its contribution in terms of volume has been much lower than from the tuna fishery. Tuna aquaculture production in Mexico was recorded in official statistics of CONAPESCA since the mid-2000s, and its production has fluctuated from 4,193 tonnes in 2004 to 3,689 tonnes in 2011 (Fig. 5.13).
There are three bluefin tuna species: Southern bluefin tuna (T. maccoyii), Atlantic and Mediterranean bluefin tuna (T. thynnus), and Northern Pacific bluefin tuna (T. orientalis); of these three species, the Northern Pacific bluefin tuna is currently farmed in Mexico. Thunnus orientalis is a highly migratory species, and its commercial value exceeds that of most other species of tunas (Kitagawa et al. 2007). This species also has the largest home range of any tuna of the genus Thunnus (Withlock et al. 2012).
Thunnus orientalis spawns in the North Pacific in the sea between Japan and the Philippines from April to June, south of Honshu Island in July, and in the Japan Sea in August (del Moral-Simanek and Vaca-Rodríguez 2009a); however, their reproductive biology is not well understood (Chen et al. 2006). Juvenile bluefin tuna remain in the Western Pacific during their first year of life (Kitagawa et al. 2007), and then, when the carrying capacity of the sea begins to saturate, part of the 2-year-old bluefin tuna population migrate to California and Baja California as a survival strategy, using the cold water of the Subarctic Frontal Zone (Kitagawa et al. 2009). These organisms constitute the tuna fishery found in the East Pacific from May to October, where they stay until they are 6 years old; no tuna larvae have been reported from the East Pacific, and it is surmised that the tuna return to Japan to spawn. Several studies have reported on the seasonal movements of pacific bluefin tuna in the Eastern North Pacific using archival tags, and they have revealed that in the summer, tuna are located primarily over the continental shelf of Baja California and migrate north to Central California in the fall, to return to Southern California by mid-winter (Kitagawa et al. 2007). In another study, electronic tagged bluefin tuna showed repeatable seasonal movements along the west coast of North America, and were found farthest south in the spring off southern Baja California, Mexico and farthest north in the fall when fish were found predominately off central and northern California (Boustany et al. 2010), concentrating in areas of high productivity, with dispersed fish in areas of low productivity.
There are commercial fisheries for Pacific bluefin tuna throughout their distribution range, from the Western to the Eastern Pacific Ocean (Withlock et al. 2012). Of the total population of Northern Pacific bluefin tuna, about 15 % is captured in the Eastern Pacific (California and Baja California), where due to its high value in the Japanese market, it is ranched and fattened for export to Japan (del Moral-Simanek et al. 2010). The Mexican fleet usually captures tuna from May to October (del Moral-Simanek and Vaca-Rodríguez 2009a).
There are other valuable tuna species that are valuable alternatives to bluefin tuna; these species are bigeye tuna (T. obesus) and yellowfin tuna (T. albacares), which have been farmed in the Cedros Island farm, located on the Mexican Pacific (Belle et al. 2003).
5.2 Tuna Culture in Mexico
Global demand for tuna has increased at a rapid rate, resulting in a downward trend in supplies of tuna, which in turn has resulted in increased catch restrictions by the Tuna Regional Fisheries Management Organization (RFMO); at the same time there has been an increase in tuna stock originating from farms (Ariji 2010).
The tuna industry in Mexico consisted principally in the capture of wild tuna for canning. However, after the 1990 US tuna embargo on Mexico, tuna captures stopped being one of the main export fisheries products, and revenue from tuna capture fell (del Moral-Simanek and Vaca-Rodríguez 2009a). Because of this, Baja California businessmen saw the opportunity to establish North Pacific bluefin tuna (T. orientalis) farms, with the purpose of capturing, farming and exporting tuna to the Japanese market (del Moral-Simanek et al. 2010).
Tuna farming operations in Mexico constitute about 3 % of the world total (Belle et al. 2003). Most tuna farming operations in Mexico are located on the Baja Peninsula, on the Pacific side. Tuna farming in Mexico started in 1996 (Belle et al. 2003) because of its temperate water, low labor costs, and abundant supply of local feed, especially sardines (Sardinops sagax) (del Moral-Simanek et al. 2010). The first bluefin tuna farm was established in Isla de Cedros, Baja California in 1997, with a total production of 64 tonnes of live tuna in 3 years (del Moral-Simanek et al. 2010) and a second farm, Maricultura del Norte, S de RL de CV in 1998. By 2003 there were already five tuna farms in operation, and by 2007 there were ten farms (del Moral-Simanek and Vaca-Rodríguez 2009a). From 1999 to 2003, the tuna production from farming increased from less than 50 to about 600 tonnes (Belle et al. 2003), and up to 4,535 tonnes in 2005 (CONAPESCA 2005) by the ten companies in operation (del Moral-Simanek and Vaca-Rodríguez 2009b). However, due to the world financial crisis, by 2009, there were only two farms in production, Baja Acuafarms and Maricultura del Norte (Anonymous 2009), which limited the number of captures. An economic reactivation in Baja California, Mexico, began in August 2010, when three more farms were opened and newly capture tuna were transported and caged (Anonymous 2010). By 2013, 79 % of farmed tuna in Tokyo was imported from Mexico, which exported from January to April 2013 about 7,000 tuna (Anonymous 2013). Tuna aquaculture in Mexico is only practiced on the Pacific side of the country; in fact, the Mexican Pacific accounts for almost 99 % of the total national production of tuna with 108 thousand tonnes (capture fishery and aquaculture) whereas catches of tuna from the Gulf of Mexico fleet accounts for 1.5 thousand tonnes (CONAPESCA 2011) (Fig. 5.14).
Tuna farming in Mexico is done by fattening wild caught tuna (del Moral-Simanek and Vaca-Rodríguez 2009a). The production cycle lasts from 3 to 8 months, depending on the fish size. When the fish are small they are usually held for longer periods of time. Water temperatures range from 18 to 22 °C and the cage systems are 40–50 m in diameter, 15–20 m deep with holding volumes of 18,000–20,000 m3, with fish densities ranging from 2 to 5 kg/m3. Fish are fed both fresh and frozen sardines, mackerel and squid. Weight gain ranges from 30–90 % of initial weight. Fish are harvested from December to April/May, using the Australian method (Belle et al. 2003) (see Fig. 5.15).
Tuna farming on the Pacific Ocean in Baja California (Mexico): (a) circular cages for capture-based aquaculture of tuna located along the coast; (b) sea lions surrounding the cages attracted by the uneaten food; (c, d) bluefin tunas (Thunnus orientalis) swimming inside the cages which are fattened for several months; (e) tuna farmer overseeing the feeding of fish; (f) harvesting of tuna from the cages to be iced and transported to sashimi markets of Japan and USA (Photographs provided by Luz Estela Rodríguez-Ibarra)
A particular problem of tuna farming in Mexico is the presence of predators, in the form of sea lions, since many areas along the coastline contain large colonies, which are attracted to the tuna cages by the excess feed that falls through the cages and that is discarded. Therefore, stress and poor performance of fish has been a problem and is common in most farms. Tuna farmed in Mexico is generally smaller in size (Belle et al. 2003) (Fig. 5.15).
The primary market for tuna farmed in Mexico is Japan. However, the rapidly expanding US market for sashimi products has increased the number of exports from Mexico to the US. It is predicted that a larger percentage of farmed tuna from Mexico will be marketed in the US. Bluefin tuna farmed in Mexico is highly prized for its meat within the sashimi market, and consequently the price per tonne of the farmed tuna is about tenfold the price per tonne of tuna caught in the fishery. For instance, during 2004–2011 the price of 1 tonne of farmed tuna in Baja California (Mexico) was 7–13 times the price per tonne of tuna caught by the Mexican fleet (Fig. 5.16).
The increase in tuna farming in Mexico has also resulted in an increased demand of live feed to fatten the tuna in the form of sardines, which has resulted in an increased price per tonne, benefiting the sardine local fishery with prices of up to US$100–120 per tonne (del Moral-Simanek et al. 2010).
Capture-based aquaculture of bluefin tuna in Mexico is similar to that occurring in other parts of the world, and has been expanding rapidly but little is known about its environmental impact. Studies on Mediterranean tuna farms, have reported high nutrient concentrations at the cage station, however, monitoring of physico-chemical parameters, nutrients, and chlorophyll in the water column together with organic carbon in sediment did not show detectable impact of fattening of Atlantic bluefin tuna, probably caused by strong currents present in the area, water depth, controlled feeding, and periodic presence of tuna farming activity in the study area (Aksu et al. 2010). However, fish farms cause wild fish to aggregate nearby, but the spatial and temporal extent of the attraction effect around farms is still poorly understood (Bacher et al. 2012).
5.3 Future Considerations and Concerns
Tuna from the genus Thunnus are high commercial value species that until very recently could only be acquired by wild captures (Munday et al. 2003) which has resulted in the decline of tuna populations worldwide. Japan continues to be the most important market for tuna products, however the fishery productivity has decreased, especially for bluefin tuna (T. orientalis, T. thynnus), because of the strict capture limits implemented, which are needed to conserve the resources (Ariji 2010). Tuna production from farms or ranches has become increasingly significant, but, since these farms rely on natural young tuna as a raw material, they are also affected by the resource management efforts (Ariji 2010).
Complete tuna domestication may be a solution to stop the tuna population decline due to overexploitation, fortunately, a full-cycle farming technology for T. orientalis has recently been developed in Japan (Sawada et al. 2005; Ariji 2010); however, in Japan, there is a negative image of farmed products; therefore it is necessary to change the consumer perception.
Particularly, the reproductive biology of Pacific bluefin tuna (T. orientalis) appear to be poorly known when compared with other tunas (Juan-Jordá et al. 2013); while larval production technology is underdeveloped (Sawada et al. 2005). There are studies that report the reproductive stock parameters associated with this species; and it seems that condition factor decreases from late May to early June, and the sex ratio might be 1:1 for spawners, with a relatively high gonadosomatic index, which is markedly increased from late May to early June. Histological examination of oocytes revealed that all specimens were sexually mature at the start of the spawning activity which starts in May and peaks in late May to early June and fecundity increased with fork length, and preliminary estimates of spawning frequency between batches range 2–4.5 days based on analysis of postovulatory follicles (Chen et al. 2006).
Completion of the life cycle of Pacific bluefin tuna may open the possibility of genetic improvement by selecting useful biological traits such as growth, feed conversion rate, meat quality, disease resistance, resistance to environmental stress, etc. (Sawada et al. 2005). However, difficulties in maturation, spawning and seedling production of Pacific bluefin tuna still exist, and the key factors initiating spawning have not been clearly identified. Furthermore, there are high levels of cannibalism in tuna larvae, which needs to be suppressed to ensure high survival rates for practical larval and juvenile production of Pacific bluefin tuna (Sawada et al. 2005).
A concern in tuna farming is mercury accumulation. Studies on southern bluefin tuna (SBT) edible tissues showed that there is a reduction in the mercury concentration over a typical farming period of 136 days, due to growth dilution from 0.51 mg/kg down to 0.33 mg/kg. Culture beyond 136 days resulted in an increase in mercury concentration due to the combined effects of mercury accumulation and seasonal lipid depletion (Balshaw et al. 2008).
Intensive aquaculture is affected by disease epizootics and the introduction of parasites as a consequence of the transport of live fish (Guo and Woo 2009). Marine fish parasites are usually considered benign in the wild, except when they may impact its host’s fecundity or induce their death (Jones 2005). However, in aquaculture settings, parasites are associated with high mortalities and reduced productivity, especially when these parasites are direct cycle (Hayward et al. 2007, 2008) and they propagate from fish to fish, reaching high infection rates due to the high density at which fish are kept.
Tuna are energetic pelagic fish with a remarkable migratory activity, with a unique physiology reflected in high metabolic rates. However, knowledge of microbial and environmental diseases of tuna is still limited (Di and Mladineo 2008). Many pathogens can be potentially dangerous for tuna of the genus Thunnus, both in natural populations, as those kept in captivity for aquaculture (Munday et al. 2003), although only a few have caused noticeable economic losses.
Among the different pathogens affecting tuna are viruses. There are two major viral diseases affecting Pacific bluefin tuna; juvenile production has often failed in Japan because of the occurrence of viral nervous necrosis (VNN) caused by betanoda viruses (Sugaya et al. 2009). The mortalities mostly occur at larval stages, and in some, but not all mortality cases, the diseased fish was characterized by vacuolation in the central nervous systems and retina. VNN is a major cause of larval mortality of Pacific bluefin tuna (Nishioka et al. 2010). Another virus that affects under 1 year old juveniles is the Red Sea Bream Iridovirus (RSIV) (Masuma et al. 2011), which causes high mortality at the grow-out phase (Munday et al. 2003).
Bacteria are another source of disease for tuna. Most bacterial diseases described for tuna, mainly Aeromonas sp. have been reported as secondary infections following skin damage caused by ectoparasites, such as Caligus elongatus (Munday et al. 2003). Infections with Photobacterium damselae subsp. piscicida have been associated with high mortalities of blue fin tuna in the Adriatic Sea (Mladineo et al. 2006). Tuna are also susceptible to Mycobacterium sp., which causes piscine tuberculosis (Munday et al. 2003).
Protozoan infections include Coccidia (Goussia auxidis) and Scuticociliates (Uronema nigricans), which cause significant mortalities in Pacific bluefin tuna larvae (Munday et al. 2003). However, most parasitic infections in tuna are due to metazoan infections, although just a few species are of economic importance. Kudoa sp. is a myxozoon that is capable of liquefying the fish muscle in T. maccoyii and T. thynnus, which results in high economic losses. There are also many monogenean species that infect tuna, affecting gills and skin. Hexostoma thynni is one of the most common monogenean species found in tuna, causing branchial hyperplasia, lamellar fusion and hemorrhages (Mele et al. 2010). Copepod crustacea are also another group of problematic parasites, with three potentially pathogenic species for tuna: Caligus elongatus, Eyryphorus brachypterus and Penella filosa, which cause external lesions, including branchial epithelia hyperplasia, lamellar fusion or hemorrhages (Mele et al. 2010). Cardicola forsteri, a digenean sanguinicolid blood fluke has been identified as a moderate risk for farmed southern bluefin tuna (Nowak 2004), although the fish are able to control de blook fluke infection on its own (Aiken et al. 2006) growth may be compromised. Furthermore, studies demonstrate the development of acquired resistance in fish against a parasite in an aquaculture environment under natural infection conditions (Aiken et al. 2008).
6 Conclusions
From the viewpoint of food security, the particular case of shrimp aquaculture in Mexico has been a very significant alternative to increase the shrimp production for human consumption, and perhaps it represents one of the few examples worldwide where aquaculture production of a native shrimp species (i.e. Litopenaeus vannamei) has practically doubled the national shrimp fishery production. According to official statistics of fishery landings in Mexico, the maximum level of shrimp caught by the fishing fleet was reached in a period of 40 years (1947–1987) with 83.8 thousand tonnes. However, it only took about 20 years for shrimp aquaculture industry to reach a similar production which was even almost doubled in the following 5 years up to 133 thousand tonnes of farmed shrimp.
Contrarily to the exponential development of shrimp aquaculture industry in the Mexican Pacific, the number of shrimp farms along the Gulf of Mexico is still incipient accounting only for 2.6 % of the total farmed shrimp production. The opportunity to increase the development of shrimp farming in the Gulf of Mexico is evident. However, any future expansion of shrimp aquaculture along the Gulf of Mexico coast should be made after a complete analysis of the potential environmental risks associated with this activity, which is based on the culture of L. vannamei, an exotic species from the Pacific Ocean. Recently, seven individuals of this exotic species were reported in a coastal lagoon on the Southern Gulf of Mexico; but, the establishment of a local population of this species has not been confirmed (Wakida-Kusunoki et al. 2011). Irrespective of this, the introduction of exotic species to the marine ecosystem of the Gulf of Mexico could have negative and unforeseen impacts to the biota. However, the lack of culture technologies for native shrimp species is also a limiting factor to avoid the use of L. vannamei in shrimp farms along the Gulf of Mexico.
Shrimp pond effluents may contribute to nutrient over-enrichment and eutrophication of coastal ecosystems (Páez-Osuna et al. 2003) and the residual waters from shrimp farms may also contain chemical products such as fertilizers, pesticides and antibiotics (Páez-Osuna 2001). In this regard, the rapid increase in the number of shrimp farms along the Pacific Ocean also increase the pollution risks to the coastal environment which have not been sufficiently documented excepting some particular cases.
Because of the capture shrimp fishery in Mexico is at its maximum level of exploitation exhibiting stagnating catches, coupled with the fact that in some regions this fishery resource is clearly depleted (Campeche Sound, Gulf of Mexico), shrimp aquaculture might be a potential alternative to maintain or increase the current shrimp production levels. However, aquaculture development should be supported by a most participative environmental research in order to avoid possible negative effects to coastal ecosystems and their resources.
On the other hand, the interaction between fishing and farming of tuna is very different to the case of the interactive contribution of the fishery and aquaculture of shrimp mentioned above. Tuna fishing is the main contributor to the total tuna production in Mexico with approximately 130 thousand tonnes per year, with a very little contribution of bluefin tuna (Thunnus orientalis) farming with around 3–4 thousand tonnes per year. Contrary to the advanced development regarding shrimp farming, capture-based aquaculture of tuna continues to prevail. This means that tuna farming depends entirely on wild captures of bluefin tuna to be transported and fattened in circular cages. Furthermore, this relatively new industry requires catching small pelagic, primarily sardines, which are utilized as feed for tunas. These two facts concerning tuna farming make it difficult to expect that tuna aquaculture may have an important contribution to national fish production, because it is directly dependent on the capture of wild tunas to be fattened and of small pelagics to be utilized as feed. Indeed, amount of bluefin tuna farmed accounts for only 3.4 % of the total tuna production in Mexico, a completely opposite situation to farmed shrimp production. However, the price of 1 tonne of farmed tuna is about 7–13 times that of tuna caught by the Mexican fleet. Therefore, capture-based aquaculture of bluefin tuna might not be a plausible option to increase the availability of fish for human consumption; but it is a good alternative as source of employment and incomes.
The occurrence of outbreaks of infectious diseases in aquatic organisms is one of the major threats to aquaculture production. Disease outbreaks have resulted in important losses in several countries around the world. In this regard, the use of SPF and SPR post larvae has been an important tool in shrimp aquaculture in Mexico as biosecurity strategy. However, knowledge of certain microbial diseases in farmed tuna is still limited. Thus, future research on this critical topic would be required.
In the case of Mexico, the combined contribution of fisheries and aquaculture to food security has allowed to reach seafood production and income levels that could not have been obtained from the fishing or aquaculture activities individually. Therefore, aquaculture should not be considered as a replacement of capture fisheries, but as a complementary productive activity to meet the growing human demand for food from the sea.
References
Aguirre-Guzmán G, Ascencio-Valle F (2000) Infectious disease in shrimp species with aquaculture potential. In: Pandalai SG (ed) Resent research developments in microbiology, vol 4. Research Signpost, Trivandrum, pp 333–348
Aiken HM, Hayward CJ, Nowak BF (2006) An epizootic and its decline of a blood fluke, Cardicola forsteri, in farmed southern bluefin tuna, Thunnus maccoyii. Aquaculture 254:40–45
Aiken HM, Hayward CJ, Crosbie P, Watts M, Nowak BF (2008) Serological evidence of an antibody response in farmed southern bluefin tuna naturally infected with the blood fluke Cardicola forsteri. Fish Shellfish Immunol 25:66–75
Aksu M, Kaymakci-Basaran A, Egemen O (2010) Long-term monitoring of the impact of a capture-based bluefin tuna aquaculture on water column nutrient levels in the Eastern Aegean Sea, Turkey. Environ Monit Assess 171:681–688
Anonymous (2009) Disminuye producción de atún en Ensenada. http://www.iaes.gob.mx/index.php?pag=m_blog&gad=detalle_entrada&entry=19
Anonymous (2010) Comienza Siembra de atún aleta azul en Baja California, México. Panorama Acuícola Noticias del día. http://www.panoramaacuicola.com/noticias/2010/08/23/comienza_siembra_de_atun_aleta_azul_en_baja_california_mexico.html
Anonymous (2013) México copó el 70% del atún de criadero llegado a Tokio. Dinero en Imagen Economia. http://www.dineroenimagen.com/2013-05-13/20107
Araneda M, Pérez EP, Gasca-Leyva E (2008) White shrimp Penaeus vannamei culture in freshwater at three densities: condition state based on length and weight. Aquaculture 283:13–18
Araneda ME, Hernández JM, Gasca-Leyva E, Vela MA (2013) Growth modelling including size heterogeneity: application to the intensive culture of white shrimp (P. vannamei) in freshwater. Aquacult Eng 56:1–12
Ariji M (2010) Conjoint analysis of consumer preference for bluefin tuna. Fish Sci 76:1023–1028
Arredondo-Figueroa JL (2002) El cultivo de camarón en México, actualidades y perspectivas. ContactoS 43:41–54
Bacher K, Gordoa A, Sague O (2012) Spatial and temporal extension of wild fish aggregations at Sparus aurata and Thunnus thynnus farms in the north-western Mediterranean. Aquacult Environ Interact 2:239–252
Balshaw S, Edwards JW, Ross KE, Ellis D, Padula DJ, Daughtry BJ (2008) Empirical models to identify mechanisms driving reductions in tissue mercury concentration during culture of farmed southern bluefin tuna Thunnnus maccoyii. Mar Pollut Bull 56:2009–2017
Bekker-Nielsen T (2005) Ancient fishing and fish processing in the Black Sea region. Aarhus University Press, Aarhus
Belle S, Smart A, Sylvia P (2003) Current status and future prospective of bluefin tuna (Thunnus thynnus orientalis) farming in Mexico and the West Coast of the United States. In: Bridges CR, García A, Gordin H (eds) Domestication of the bluefin tuna Thunnus thynnus thynnus. CIHEAM, Zaragoza
Boustany AM, Matteson R, Castleton M, Farwell C, Block BA (2010) Movements of pacific bluefin tuna (Thunnus orientalis) in the Eastern North Pacific revealed with archival tags. Prog Oceanogr 86:94–104
Brander KM (2007) Global fish production and climate change. Proc Natl Acad Sci U S A 104:19709–19714
Castilla JC, Defeo O (2001) Latin American benthic shellfisheries: emphasis on co-management and experimental practices. Rev Fish Biol Fish 11:1–30
Chen K-S, Crone P, Hsu C-C (2006) Reproductive biology of female Pacific bluefin tuna Thunnus orientalis from south-western North Pacific Ocean. Fish Sci 72:985–994
Cifuentes-Lemus JL, Torres-García MP, Frías-Mondragón M (1995) El océano y sus recursos, IX. La pesca, 2nd edn. Fondo de Cultura Económica, Mexico City
CONAPESCA (2005) Anuario estadístico de acuacultura y pesca 2005. SAGARPA, CONAPESCA, Mazatlán
CONAPESCA (2011) Anuario estadístico de acuacultura y pesca 2011. SAGARPA. CONAPESCA, Mazatlán
del Moral-Simanek RJ, Vaca-Rodríguez JG (2009a) Administración de la pesquería del atún aleta azul en Baja California: Una visión global. Front Norte 21:151–175
del Moral-Simanek RJ, Vaca-Rodríguez JG (2009b) Captura de atún aleta azul en Baja California, México: ¿pesquería regional o maquiladora marina? Región y Sociedad XXI:159–190
del Moral-Simanek RJ, Vaca-Rodríguez JG, Alcalá Alvarez MC (2010) Análisis socioeconómico e interrelación de las pesquerías de sardina y atún aleta azul en la región noroeste de México. Región y Sociedad XXII:9–29
DeWalt BR, Zavala JRR, Noriega L, González RE (2002) Shrimp aquaculture, the people and the environment in coastal Mexico. Report prepared under the World Bank, NACA, WWF and FAO Consortium program on shrimp farming and the environment. The World Bank, NACA, WWF, FAO, Washington
Di MA, Mladineo I (2008) Ultrastructure of Didymocystis semiglobularis (Didymozoidae, Digenea) cysts in the gills of Pacific bluefin tuna (Thunnus orientalis). Parasitol Res 103:641–647
FAO (2011) Aquaculture development. 6. Use of wild fishery resources for capture based aquaculture. FAO technical guidelines for responsible fisheries. No. 5, Suppl. 6. FAO, Rome
FAO (2012) The state of world fisheries and aquaculture 2012. FAO, Rome
FAO (2013) Report of the FAO/MARD technical workshop on early mortality syndrome (EMS) or acute hepatopancreatic necrosis syndrome (AHPNS) of cultured shrimp (under TCP/VIE/3304). Hanoi, Vietnam, on 25–27 June 2013. FAO Fisheries and Aquaculture Report No. 1053. FAO, Rome
Ferreira JG, Hawkins AJS, Bricker SB (2007) Management of productivity, environmental effects and profitability of shellfish aquaculture—the farm aquaculture resource management (FARM) model. Aquaculture 264:160–174
Gartside DF, Kirkegaard IR (2009) A history of fishing. In: Equires VR (ed) The role of food, agriculture, forestry, and fisheries in human nutrition, vol 2. EOLSS Publishers, Paris, pp 105–139
Guo FC, Woo PTK (2009) Selected parasitosis in cultured and wild fish. Vet Parasitol 163:207–216
Gutiérrez-Salazar GJ, Molina-Garza ZJ, Hernández-Acosta M, García-Salas JA, Mercado-Hernández R, Galaviz-Silva L (2011) Pathogens in Pacific white shrimp (Litopenaeus vannamei Boone, 1931) and their relationship with physicochemical parameters in three different culture systems in Tamaulipas, Mexico. Aquaculture 321:34–40
Hayward CJ, Aiken HM, Nowak BF (2007) Metazoan parasites on gills of Southern Bluefin Tuna (Thunnus maccoyii) do not rapidly proliferate after transfer to sea cages. Aquaculture 262:10–16
Hayward CJ, Aiken HM, Nowak BF (2008) Epizootics of metazoan gill parasites did not threaten feasibility of farming southern bluefin tuna (Thunnus maccoyii) in a trial extending over summer months. Vet Parasitol 154:122–128
Horowitz A, Samocha TM, Gandy RL, Horowitz S (2001) Toxicity tests to assess the effect of a synthetic tank liner on shrimp survival and nitrification in a recirculating superintensive production system. Aquacult Eng 24:91–105
INAPESCA (2006) Sustentabilidad y pesca responsable en México. Evaluación y manejo. SAGARPA. INAPESCA, México
Jones B (2005) Mass mortalities in the oceans. In: Rohde K (ed) Marine parasitology. CSIRO, Canberra, pp 371–374
Juan-Jordá MJ, Mosqueira I, Freire J, Dulvy NK (2013) The conservation and management of tunas and their relatives: setting life history research priorities. PLoS ONE 8:e70405
Kitagawa T, Boustany AM, Farwell CJ, Williams TD, Castleton MR, Block BA (2007) Horizontal and vertical movements of juvenile bluefin tuna (Thunnus orientalis) in relation to seasons and oceanographic conditions in the eastern Pacific Ocean. Fish Oceanogr 16:409–421
Kitagawa T, Kimura S, Nakata H, Yamada H, Nitta A, Sasai Y, Sasaki H (2009) Immature Pacific bluefin tuna, Thunnus orientalis, utilizes cold waters in the Subarctic Frontal Zone for trans-Pacific migration. Environ Biol Fish 84:193–196
Larsen L, Roney JM (2013) Farmed fish production overtakes beef. Plan B updates. June 12, 2013. Earth Policy Institute. http://www.earth-policy.org/plan_b_updates/2013/update114. Accessed 25 Nov 2013
Lightner DV (1999) The penaeid shrimp viral pandemics due to IHHNV, WSSV, TSV and YHV: current status in the Americas, available diagnostic methods, and management strategies. J Appl Aquac 9:27–52
Lightner DV (2005) Biosecurity in shrimp farming: pathogen exclusion through use of SPF stock and routine surveillance. J World Aquacult Soc 36:229–248
Ludwig D, Hilborn R, Walters C (1993) Uncertainty, resource exploitation, and conservation: lessons from history. Science 260:17–36
Martínez-Córdova LR (1999) Cultivo de camarones peneidos: principios y prácticas. AGT Editor, México City
Martínez-Córdova LR, Campaña-Torres A, Martínez-Porchas M (2004) Manejo de la productividad natural en el cultivo del camarón. In: Cruz-Suárez LE, Ricque-Marie D, Nieto-López MG, Villarreal D, Scholz U, González M (eds) Avances en nutrición acuícola VII. Memorias del VII simposium internacional de nutrición acuícola, Hermosillo, 2004. Universidad Autónoma de Nuevo León. Monterrey
Masuma S, Takebe T, Sakakura Y (2011) A review of the broodstock management and larviculture of the Pacific northern bluefin tuna in Japan. Aquaculture 315:2–8
Mele S, Merella P, Macias D, Gomez MJ, Garippa G, Alemany F (2010) Metazoan gill parasites of wild albacore Thunnus alalunga (Bonaterre, 1788) from the Balearic Sea (western Mediterranean) and their use as biological tags. Fish Res 102:305–310
Miranda I, Valles JL, Sánchez R, Álvarez Z (2010) Culture of marine shrimp Litopenaeus vannamei (BOONE, 1931) in freshwater. Rev Cien FCV-LUZ XX:339–346
Mladineo I, Miletic I, Bocina I (2006) Photobacterium damselae subsp. piscicida outbreak in cage-reared Atlantic bluefin tuna Thunnus thynnus. J Aquat Anim Health 18:51–54
Munday BL, Sawada Y, Cribb T, Hayward CJ (2003) Diseases of tunas, Thunnus spp. J Fish Dis 26:187–206
Nishioka T, Mori K, Sugaya T, Tezuka N, Takebe T, Imaizumi H, Kumon K, Masuma S, Nakai T (2010) Involvement of viral nervous necrosis in larval mortality of hatchery-reared pacific bluefin tuna Thunnus orientalis. Fish Pathol 45:69–72
Nowak BF (2004) Assessment of health risks to southern bluefin tuna under current culture conditions. Bull Eur Assoc Fish Pathol 24:45–51
Ortega SC (2012) Veterinary medical education and veterinary involvement in aquatic-animal health and aquaculture in Mexico. J Vet Med Educ 39:195–199
Ortega-Salas AA, Rendón MLA (2013) Hyper-intensive farming of white shrimp Litopenaeus vannamei in a freshwater tank under semi-controlled conditions (Decapoda: Penaeidae). Cuad Invest UNED 5:63–68
Páez-Osuna F (2001) The environmental impact of shrimp aquaculture: causes, effects, and mitigating alternatives. Environ Manag 28:131–140
Páez-Osuna F, Gracia A, Flores-Verdugo F, Lyle-Fritch LP, Alonso-Rodriguez R, Roque A, Ruiz-Fernández AC (2003) Shrimp aquaculture development and the environment in the Gulf of California ecoregion. Mar Pollut Bull 46:806–815
Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F Jr (1998) Fishing down marine food webs. Science 279:860–863
Pauly D, Christensen V, Guénette S, Pitcher TJ, Sumaila UR, Walters CJ, Watson R, Zeller D (2002) Towards sustainability in world fisheries. Nature 418:689–695
Pauly D, Watson R, Alder J (2005) Global trends in world fisheries: impacts on marine ecosystems and food security. Philos Trans R Soc B 360:5–12
Rabanal HR (1988) History of aquaculture. ASEAN/UNDP/FAO Regional small-scale coastal fisheries development project. ASEAN/SF/88/Tech. 7. ASEAN/UNDP/FAO, Manila
Ramírez-Rodríguez M, Chávez EA, Arreguín-Sánchez F (2000) Perspective of the pink shrimp (Farfantepenaeus duorarum Burkenroad) fishery of Campeche Bank, Mexico. Cienc Mar 26:97–112
Rojas AA, Haws MC, Cabanillas JA (eds) (2005) Buenas prácticas de manejo para el cultivo de camarón. Prácticas de desarrollo sostenible en ambientes costeros de prioridad de los ecosistemas del Golfo de California camaronicultura. The David and Lucile Packard Foundation. United States Agency for International Development (Cooperative Agreement No. PCE-A-00-95- 0030-05)
SAGARPA (2012) Actualización de la carta nacional acuícola. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Diario Oficial. pp 33–112, 6 June 2012
Samocha TM (2011a) The effect of different synthetic polymer liners in tank-systems stocked with Litopenaeus vannamei. Panor Acuic Mag 17:11–15
Samocha TM (2011b) The effect of different synthetic polymer liners on growth, survival and selected water quality indicators in tank-system stocked with Litopenaeus vannamei under limited water discharge and in the presence of natural productivity. Panor Acuic Mag 17:16–21
Sánchez-Martínez JG, Aguirre-Guzmán G, Mejía-Ruíz H (2007) White spot syndrome virus (WSSV) in cultured shrimp: a review. Aquacult Res 38:1339–1354
Sawada Y, Okada T, Miyashita S, Murata O, Kumai H (2005) Completion of the Pacific bluefin tuna Thunnus orientalis (Temminck et Schlegel) life cycle. Aquac Res 36:413–421
Secretaría de Pesca (1993) Anuario estadístico de pesca 1990. Secretaría de Pesca, México City
SEMARNAP (1996) Anuario estadístico de pesca 1995. SEMARNAP, México City
Squires VR (2009) The role of food, agriculture, forestry, and fisheries in human nutrition. In: Equires VR (ed) The role of food, agriculture, forestry, and fisheries in human nutrition, vol 1. EOLSS Publishers, Paris, pp 1–28
Sugaya T, Mori K, Nishioka T, Masuma S, Oka M, Mushiake K, Okinaka Y, Nakai T (2009) Genetic heterogeneity of betanodaviruses in juvenile production trials of Pacific bluefin tuna, Thunnus orientalis (Temminck & Schlegel). J Fish Dis 32:815–823
Treece GD (2000) Shrimp maturation and spawning. In: Tamaru CCT, Tamaru CS, McVey JP, Ikuta K (eds) Spawning and maturation of aquaculture species. US-Japan Natural Resources Technical Report No. 28. University of Hawaii Sea Grant College Program, Hawaii, pp 121–133
Treece GD (2001) Fertilizacion. In: Boyd CE, Haws MC (eds) Métodos para mejorar la camaronicultura en Centroamérica. UCA, Managua, pp 93–106
United Nations (2013) World population prospects. The 2012 revision. Highlights and advance tables. Department of Economic and Social Affairs, Population Division. Working Paper No. ESA/P/WP. United Nations, New York
Wakida-Kusunoki AT, Amador-del Angel LE, Carrillo P, Quiroga C (2011) Presence of Pacific white shrimp Litopenaeus vannamei (Boone, 1931) in the Southern Gulf of Mexico. Aquat Invasions 6:139–142
Walker PJ, Winton JR (2010) Emerging viral diseases of fish and shrimp. Vet Res 41:51–75
Withlock RE, McAllister MK, Block BA (2012) Estimating fishing and natural mortality rates for Pacific bluefin tuna (Thunnus orientalis) using electronic tagging data. Fish Res 119–120:115–127
Ye Y, Cochrane K (2011) Global overview of marine fishery resources. In: FAO (ed) Review of the state of world marine fishery resources. FAO fisheries and aquaculture technical paper, no. 569. FAO, Rome, pp 3–18
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pérez-Castañeda, R., Sánchez-Martínez, J.G., Aguirre-Guzmán, G., Rábago-Castro, J.L., de la Luz Vázquez-Sauceda, M. (2015). Interaction of Fisheries and Aquaculture in the Production of Marine Resources: Advances and Perspectives in Mexico. In: Finkl, C., Makowski, C. (eds) Environmental Management and Governance. Coastal Research Library, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-319-06305-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-06305-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-06304-1
Online ISBN: 978-3-319-06305-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)