Abstract
A new technique, based on the combination of time-resolved X-ray diffraction (TRXRD) and high-temperature laser scanning confocal microscopy (LSCM), was developed for direct observation of morphological evolution and simultaneous identification of the phases. TRXRD data and LSCM images under the desired thermal cycles were measured simultaneously. As several observation examples, the microstructural evolutions in the steel materials were observed to investigate the phase transformation kinetics under the thermal cycle including the rapid heating and cooling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Martensitic Transformation
- Thermal Cycle
- Duplex Stainless Steel
- Mild Steel
- Laser Scanning Confocal Microscope Image
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Over the past decade, two synchrotron based techniques have been developed at Lawrence Livermore National Laboratory for direct observation of phase transformations induced by welding. These techniques are spatially resolved X-ray diffraction (SRXRD), which was developed to map the phases that exist in the HAZ [1–6], and time-resolved X-ray diffraction (TRXRD).
Elmer et al. [7–12] showed that TRXRD could track phase transformation during welding in real time. Synchrotron radiation makes time-resolved diffraction measurements possible in local areas; phases that exist in the HAZ and fusion zone (FZ) of metal can be identified in real time. This technique was used to analyse the phase transformation during solidification of carbon–manganese (C–Mn) steel, and Babu et al. [10] verified the existence of non-equilibrium phases directly in the rapid cooling cycle of spot welds. In addition, TRXRD can be applied in tracking the phase evolution in the HAZ. The formation of the microstructures of duplex stainless steel (DSS) [11] and C–Mn [8] steel were observed in the HAZ during the thermal cycle using TRXRD system. In experiments with DSS, the phase balance between ferrite and austenite was estimated, and the precipitation of the sigma phase in the thermal cycle of the HAZ was assessed. In TRXRD experiments with C–Mn steel, the effect of transformation strain on the diffraction pattern profile during martensitic transformation was discussed.
Our research group began TRXRD experiments for welding by developing a new technology for the system [13–26]. We focussed on the details of the weld solidification phenomena in the directional solidification process under rapid cooling because the influence of a preferred orientation was important for observing directional solidification along the 〈100〉 direction towards the moving heat source. First, the solidification process was confirmed by SRXRD as a function of the distance from the weld pool, which was melted by an arc of the quenched metals after welding [13, 14]. However, the crystallisation at a lateral resolution corresponding to a time resolution of 0.1 s was impossible to observe. That is, because the microstructure was ultimately dynamic, understanding the crystallography during heating and cooling was not possible. For instance, the eutectic microstructure is formed in the liquid phase during solidification in a short period; the displacement of interplanar spacing by thermal expansion and shrinkage could not be observed. Next, the phase transformation was dynamically observed along a certain direction on the reciprocal space using an imaging plate [15–17]. A crystallinity change was observed with a temperature drop, and the growth of dendrites was captured. We assumed the rotation of dendrites from the discontinuous diffraction pattern recorded by the imaging plates along one direction of reciprocal space. However, eutectic growth in the remaining liquid phase was confirmed, though peritectic growth of the hetero phase on the primary phase was expected. Therefore, it was difficult to simultaneously observe the primary phase and the hetero phase along a certain direction because interfaces have coherency and preferred crystal orientation.
With the availability of intense X-ray beams from synchrotron storage rings, it is now possible to directly observe phase transformation and microstructural evolution in situ and in real time as a function of welding time. Therefore, we developed a two-dimensional time-resolved X-ray Diffraction (2D-TRXRD) system for real welding [18–28]. Weld metal rapid solidification was then dynamically observed at a time resolution of 0.01–0.1 s.
The monochromatic X-ray is used as a probe with the incident beam from one direction in the study described above. Detecting a wider area of the Debye circle is very important. For analysing the solidification process, the weak and broad halo pattern is clear sign of the existence of liquid. Thus, detecting halo patterns with a high S/N ratio detector indicates the beginning and the end of solidification [22].
Further, a combination of analyses methods (the in situ phase identification system, morphological observation by high-temperature laser scanning confocal microscopy (LSCM) and observation of the microstructure at room temperature with an optical microscope, scanning electron microscope and micro diffraction system) was suggested for analysing the phase transformation during welding [20–22, 24–30].
The TRXRD data obtained during welding needs to be combined with the appropriate temperature history to obtain the phase transformation kinetic data. The LSCM technique can give us information such as the morphological development of microstructures and precise temperature [31, 32].
Then a new technique based on the TRXRD and LSCM system was developed in the present study. As some observation examples, the microstructural evolutions in the steel materials were observed to investigate the phase transformation kinetics under the thermal cycle including the rapid heating and cooling.
2 Hybrid System for In Situ Observation in Real and Reciprocal Space
2.1 Overview of System
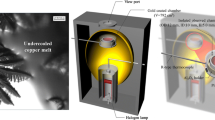
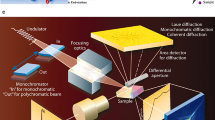
Figure 1 shows a photograph of the experimental setup on the 46XU beam line at SPring-8 in Hyogo, Japan. The infrared furnace was set on the theta-axis of a goniometer situated within the hatch of the beam line. In this system, the head of the laser scanning confocal microscope (LSCM) was also set by fitting it to the theta-axis, as shown in the photograph. The focus point of the LSCM is on the surface of the observed sample which is set in the furnace. A two-dimensional pixel detector was placed on the two-theta axis. The incident beam, i.e., an ultra bright X-ray, was introduced to the furnace and the diffractions were recorded by the pixel detector with high time resolution. Simultaneously, the microstructural changes were observed through the LSCM in situ.
2.2 Detailed Experimental Procedures
Figure 2 shows a schematic illustration of the control flow for the experiment of in situ observations in real and reciprocal space [31]. The specimens, 5 mm in diameter and 1 mm thick, were place in the boron-nitride (BN) crucible in which the X-ray absorption is quite small, and held in a platinum holder, which was inserted in the furnace. The temperature was measured by a thermocouple incorporated into the crucible holder. The specimens were placed at the focal point of halogen lamp. The temperature controller, which was connected to a personal computer (PC), the thermocouple and the halogen lamp in the furnace were placed inside of the beam line hatch. When the thermal cycles that simulate welding were developed on the PC, the profiles were sent to the temperature controller, which reproduced the desired thermal cycles by switching the halogen lamp on and off, based on the measured temperature. The LSCM head makes it possible to carry out in situ observations of microstructural changes at a rate of 30 frames/s, at high temperature [21, 22, 27]. A CCD camera was connected to the PC located outside the hatch through the monitor, and the images were stored at a rate of 30 frames/s. The control program could trigger the temperature controller, the X-ray shutter, the x-y-z stages on θ-axis, goniometer-axis control and the exposure of the pixel detector.
Hybrid in situ observation system in real and reciprocal lattice space [31]
Before the measurements, the specimen position was adjusted in the manner explained in the following section. Then, the θ-axis was tilted to a fixed angle (10° in the present study). The temperature controller was then triggered at a set timing and, the exposure of the detector was activated with the time resolution of 0.2 s. TRXRD data and LSCM images under the desired thermal cycles were measured simultaneously.
2.3 Scattering Geometry of X-rays in the Experimental Setup
Figure 3 shows the scattering geometry of the TRXRD part of system. The undulator beam was monochromatized by the double Si-crystal, and 30 keV of X-ray energy was used. The X-ray was introduced into the hatch through a mirror—the incident beam shown in Figs. 1 and 2. The X-ray was shaped by the slit. In the present study, the beam is shaped into dimensions of 0.5 mm height and width. The X-ray beam was introduced into the furnace through an X-ray window made with polyimide film. Before the measurements, the position of the sample surface was adjusted. By controlling the z-stage, the sample surface is forced to be at the half position of the beam height as indicated by the dashed line in Fig. 3 the θ-axis was then rotated to the angle of 10° in the present study. The resulting irradiated area was 1.4397 mm2. The penetration depth is calculated using Eq. (1) [33]:
where \( I_{0} \) is the intensity of the incident beam, γ is the angle between the incident beam and the sample surface and β is the angle between the diffracted beam and sample surface.
3 In Situ Investigation of the Allotropic Transformation in Iron
3.1 In Situ Observations of the α → γ and γ → δ Transformations Using LSCM
Two types of metals, iron and steel whose chemical compositions were shown in Table 1 were studied to provide more kinetics information about the phase transformation [34]. The aim is to differentiate between the two types of transformation because there are no any composition changes between parent and product phases during the allotropic and martensitic transformations. Figure 4 displays the microstructural evolution of the iron sample during the heating process (indicated by Fig. 7). The snapshots of the LSCM images for α → γ transformation are shown in Fig. 4a–e. At the temperature of 1,202.2 K (Fig. 4a), a microstructural change was observed on the ferrite grain boundary as indicated by the arrow. It can be supposed that this is austenite phase. Austenite phases then spread to the surrounding areas (as designated by the arrows) with increasing temperature to 1,205.8 K (Fig. 4b). Subsequently (Fig. 4c), the austenite phases quickly spread throughout the region by half the sample. In the temperature of 1,218.4 K (Fig. 4d), the phase transformation in the other half sample can be also observed clearly. The transformation spreads to the center from all directions, and eventually is completed at the point, as presented in the Fig. 4e. By calculation, the α → γ transformation is completed within 2.5 s (see Fig. 7). It is obviously seen the microstructural evolution during the α → γ transformation even the transformation is rapid.
The microstructural evolution of iron during the heating process, a–e the snapshots of the LSCM mages for α → γ transformation, and f–j the snapshots for γ → δ transformation [34]
Figure 4f–j show the snapshots of the LSCM images for the γ → δ transformation. The iron is further heated to 1,689.2 K (Fig. 4f). No changes are observed in the surface morphology of the austenite since 1,222.9 K, which can be seen from the Fig. 4e, f. When the temperature is increased to 1,689.6 K (Fig. 4g), the transformation of austenite to δ-ferrite begins, as indicated by the arrow. Within 1 s (Fig. 4h–j), this γ → δ transformation (indicated by the arrows) can be completed. However, this contrast is concealed by the product generated during the previous heating transformation. Thus the image contrast of the δ product is weak. On the other hand, one cannot observe the austenite grain boundary during austenitizing process due to the short holding time at high temperature. So, it is hard to distinguish the nucleation sites before the onset of the allotropic δ-ferrite formation.
3.2 In Situ Observations of the δ → γ and γ → α Transformations Using LSCM
Figure 5 presents the morphological evolution in iron sample at the micron scale under the cooling cycle (indicated by Fig. 7) [34]. The snapshots of the LSCM images for the δ → γ transformation are shown in Fig. 5a–d. When the temperature drops to 1,646.1 K (Fig. 5a), no changes are observed in the surface morphology of the δ-ferrite compared to the Fig. 4j. In the case of the δ → γ transformation (Fig. 5b–d), it can be completed within 1 s during the temperature span of 4.8 K. The arrows in Fig. 5b–d indicate the austenite growth process. Similarly, the contrast is also concealed by the product generated during the previous heating transformation. In addition, the austenite grain boundary during the δ → γ transformation cannot be observed due to the short holding time at high temperature.
The microstructural evolution of iron during the cooling process, a–d the snapshots of the LSCM mages for δ → γ transformation, and e–h the snapshots for γ → α transformation [34]
Figure 5e–h show the snapshots of the LSCM images for the γ → α transformation. When the temperature is cooled to 1,083.8 K, two austenite grain boundaries can be observed in Fig. 5e. The thick arrows indicate the locations of the grain boundaries. Subsequently, the nucleated ferrite spreads across the austenite grain boundary A, as indicated by the small arrow (Fig. 5f, g). When encountering the grain boundary B, the ferrite crosses it (Fig. 5h) and continues to spread, as designated by the arrow. In other words, the ferrite can cross the austenite grain boundaries and grow rapidly into the adjacent grains. Similarly, the γ → α transformation is completed within 1 s based on the in situ observations.
3.3 Ex-situ Observations of the Crystallographic Relationships During the Allotropic Transformation Using EBSD
To examine the crystallography and the orientation relationship during the allotropic transformation, the iron samples before and after the transformation are selected for further EBSD analysis [34]. The results of the EBSD analysis are shown in Fig. 6. Figure 6a exhibits the original distribution of the α-ferrite before the transformation, while the distribution of the α-ferrite after the thermal cycle of heating to 1,700 K and cooling is displayed in Fig. 6c. It seems the transformation product phase (α-ferrite) that possessed no crystallographic relationship with respect to the parent phase (austenite). As a good example to determine the orientation relationship, Zhang et al. [35] have observed the ferrite/cementite orientation relationships in near-eutectoid steel using SEM-FEG/EBSD.
The results of the EBSD analysis of iron before and after transformation; a and c the original distribution of α-ferrite before and after transformation, respectively; b and d the ferrite grain sizes before and after transformation, respectively [34]
It is also to be noted that the α-ferrite grain sizes before and after the transformation have created a great difference, as displayed in Fig. 6b, d. The grain size of the α-ferrite after the transformation is beyond 115 μm (Fig. 6d), while that of the α-ferrite before the transformation is about 51 μm (Fig. 6b). It suggests that the grain size of the re-transformed α-ferrite from the γ-austenite becomes much larger than that before α to γ transformation. Takasaki et al. [36] also observed the similar experimental results during the allotropic transformation.
3.4 In Situ Observation of the Allotropic Transformation in Iron Using TRXRD
To further probe the characteristics of this rapid transformation, synchrotron radiation technology has been used in this study. This investigation is interesting from a fundamental as well as a technological viewpoint. The dynamics of phase transformations can be well monitored using this method. Figure 7 presents the d-spacing, time, temperature, and intensity diagram of the iron during heating and cooling. The diffraction data are analyzed using the method and programming described in the Ref. [37]. A series of thousands of diffraction rings from ferrite (110), (200), (211), and austenite (111), (200), (220), (311) are integrated into an image format, as summarized in Fig. 7. Before the α → γ transformation, the clear diffraction peaks, marked as α(110), α(200), and α(211), can be observed. When the temperature reaches 1,202.2 K, the transformation from ferrite to austenite occurs. After fully austenitizing, the diffraction peaks, marked as γ(111), γ(200), γ(220), and γ(311), can be observed clearly. However, only δ(200) diffraction peak can be captured after γ → δ transformation. The start transformation temperature from austenite to δ-ferrite is about 1,689.6 K. Under this condition, the signal/noise ratio decreases with the increase of temperature because the larger grain size in the δ-phase implies a smaller number of crystals to satisfy Bragg’s conditions. Thus, the diffraction peaks, such as δ(110) and δ(211), cannot collected by the pixel detector.
The d-spacing, time, temperature, and intensity diagram of the iron during phase transformation. The peak temperature is 1,698 K [34]
For the cooling process, the δ → γ transformation occurs at 1,644.9 K, and only γ(311) can be detected due to the increased austenite grain size. It can be found that the austenite grain size is huge after the δ → γ transformation, as shown in Fig. 5e. For the γ → α transformation (occurs at 1,081.9 K), the α(110) and α(200) can be observed, but the α(211) disappears. This indicates that the grain size of α-ferrite gets large after the transformation, and then a smaller number of crystals are to satisfy Bragg’s conditions. In fact, EBSD results of Fig. 6 have indicated that the grain size of the α-ferrite becomes extremely large after the transformation. Since the final microstructure is mainly determined by the γ → α transformation, direct tracking of austenite lattice parameter changes before onset of the transformation can provide more kinetics information about the transformation, especially for the elemental partitioning behaviors during the transformation [32, 34, 37]. As mentioned above, the austenite grain size gets large after the δ → γ transformation, and it continues to grow with the increasing of annealing time. Accordingly, the signal/noise ratio decreases with the increase of austenite grain size because the larger grain size in the austenite phase implies a smaller number of crystals to satisfy Bragg’s conditions. Under these situations, alternative thermal cycle for tracking lattice expansion during the γ → α transformation should be performed. Figure 8a displays the d-spacing, time, temperature, and intensity diagram of the iron by lowering the peak temperature to 1,473 K. Based on the TRXRD analysis, the start transformation temperature from austenite to α-ferrite is 1,087.7 K. Figure 8b presents the progression of the measured γ(200) d-spacing value as a function of temperature. The d-spacing value displays a steady decrease, which generally corresponds to lattice contraction due to decreasing temperature. In other words, the lattice expansion shows a linear change along thermal cycle during the γ → α transformation. The result of the linear change is verified by other repeated experiments. In general, the LSCM images are compatible with the diffraction pattern during the allotropic transformation. Hence, the developed system could follow the phase transformation in both real and reciprocal space.
a The d-spacing, time, temperature, and intensity diagram of the iron by lowering the peak temperature to 1,473 K; b the progression of the measured γ(200) d-spacing value as a function of temperature during the γ → α transformation [34]
3.5 Characteristic of the Morphology in the Allotropic Transformation
Figure 9a displays the morphology of the martensitic product phase in the studied steel based on EBSD observations. When the temperature is decreased to 651 K, the martensitic laths generates quickly with the needle-like appearance. The martensitic transformation is completed within 4 s in a cooling rate of 8.3 K s−1. As shown in Fig. 9a, a lenticular or needle-like appearance is the most typical characteristic of a martensitic phase produced in a polycrystalline material. However, the phases in the allotropic transformation display irregular appearances, as displayed in Figs. 4, 5 and 6. Accordingly, the morphological differences in the allotropic and the martensitic transformations can be distinguished by in situ observations and ex-situ EBSD analysis. Furthermore, for the γ → α transformation, the α-ferrite phase grows rapidly into the adjacent grains (Fig. 5f–h), that is, the allotropic product can cross the grain boundaries. The heterogeneous nucleation of ferrite may take place at austenite grain boundary by forming a low energy interface with at least one of the two grains, while the ferrite nuclei meet difficulty in growing in the same grain due to a highly coherent character of the interface and tend to purse their growth into the opposite grain.
a An inverse pole figure (IPF) color map of the lath martensite structure showing the K–S orientation relationship, and the white lines indicate the prior austenite grain boundaries; b a linear change of d-spacing value with temperature during martensitic transformation [34]
3.6 Characteristic of the Crystallographic Relationships in the Allotropic Transformation
Figure 9a shows an inverse pole figure (IPF) color map of the lath martensite structure for a limited area. The white lines in Fig. 9a indicate the prior austenite grain boundaries. Based on the report of Morito et al. [38] and Kitahara et al. [39], variant analysis of the lath martensite in the present study is conducted. By analysis, the martensite under investigation maintains the Kurdjumov–Sachs (K–S) orientation relationship with respect to retained austenite. For example, the variants of V1, V2, V3, V7, and V8 appear in the selected region (the white arrow). In other words, austenite grains appear by the martensitic transformation as cooling proceeds leading to an interconnected austenite-ferrite structure. Both phases are related by near K–S crystallographic relationships. However, the allotropic transformation possesses no strict crystallographic relationship with respect to the parent phase based on the analysis of Fig. 6. On the other hand, the phase appearance of the allotropic transformation is irregular compared to the needle-like lath during the martensitic transformation. Therefore, the K–S relationship between parent phase and the product cannot be observed due to its irregular transformation.
3.7 Characteristic of Short Range Diffusion in the Allotropic Transformation
In light of the present observations, it is useful to study the kinetics of phase transformations by this hybrid in situ observations system. Figure 9b exhibits a linear change of d-spacing value with temperature during the martensitic transformation. It can therefore be concluded that there is no partitioning of carbon before onset of austenite to α-ferrite transformation. The shear-type diffusionless mechanism during the martensitic transformation can be drawn. This is consistent with the previous reports [32].
Similar result has also been observed during the allotropic transformation (Fig. 8b). According to the early reports [40–42], the growth of the allotropic product may be achieved by thermally activated short range jumps of atoms across an incoherent interface. This mechanism leads to the curved interfaces between the two phases. This has been confirmed as mentioned above, that is, the curved interfaces between the two phases have been observed during the transformation (Figs. 4, 5, 6). On the other hand, Banerjee [43] argues that only a few atomic jumps are required for an atom to cross-over the transformation front during the allotropic transformation. The transformation front can be treated as a thin film of certain thickness. The diffusivity within this thin film is much higher than that in the bulk of parent and product crystals. Accordingly, only a few Fe atoms might gather on the transformation front under the atom-vacancy interchange process. However, this does not lead to the lattice expansion in the parent phase during allotropic transformation, which is consistent with the linear change of d-spacing in Fig. 8b.
As described in the Introduction, the tracking of lattice parameter changes can provide information about the element partitioning behaviors during the phase transformation. But for the case of the allotropic transformation, knowledge of lattice parameter change is still lacking. In this study, the d-spacing value of the austenite displays a steady decrease along thermal cycle during the γ → α transformation. This information will be helpful to understand the kinetic information about the phase transformation.
4 Thermal Stability of Carbides and Carbonitrides During Thermal Cycle
Precipitation reactions of heat-resistant steel occurring during welding, both growth and coarsening of existing phases and the formation of new phases, can be detrimental for creep strength. However, understanding of the thermal stability of precipitates during the heat cycle of welding is insufficient. In this study, an attempt has been made to investigate the possibility of analyzing carbides/carbonitrides stability occurring in the heat affected zone based on in situ time-resolved X-ray diffraction using a synchrotron.
In this study, two types of heat-resistant steels were selected for study, ferritic steel and austenitic steel. The chemical compositions of the steels ware listed in Table 2. Then the in situ observation was carried out using the hybrid observation system.
Figure 10a [44] displays both the TRXRD and the corresponding temperature data obtained during the continuous heating and cooling of the investigated austenitic heat-resistant steel at heating and cooling rates of 8.3 °C s−1. The temperatures and times at which certain phases are present during the heat treatment can be obtained from these plots. Figure 10b shows selected X-ray diffractograms of experimental steel corresponding to Fig. 10a. In the case of the heating process, the diffraction patterns do not exhibit significant changes in the range of temperate from 24 to 1,365.5 °C. However, the diffraction intensity of the ε-Nb(C,N) decreased sharply at 1,365.5 °C, indicating that dissolution in the precipitates is occurring under the thermodynamic driving force. The diffraction intensity of the ε-Nb(C,N) continued to decrease until it had completely disappeared at 1,372.5 °C. At the same temperature, the δ-Nb(C,N) just begins to dissolve, corresponding to the reduction of its diffraction intensity. This indicates that the dissolution of the δ-Nb(C,N) begins at a higher temperature (1,372.5 °C) than the 1,365.5 °C of the ε-Nb(C,N). Finally, only weak δ-Nb(C,N) diffraction peaks could be detected when the temperature dropped to 1,148.3 °C. This indicates that the δ-Nb(C,N) is just starting to re-precipitate in the austenite. The re-precipitation was completed at 1,115.5 °C. Moreover, according to the weak diffraction intensity, there should only be a small amount of reprecipitation in the austenite.
a Changes in diffraction patterns and lattice parameter of austenitic heat-resistance steel during the heating and cooling process. b Selected X-ray diffractograms of experimental steel corresponding to (a) [44]
Figure 11 [44] shows the progression of the measured δ-Nb(C,N) (111) and ε-Nb(C,N) (102) d-spacing values of austenitic steel as a function of temperature. In the initial stages of the experiment, the d-spacing values of δ-Nb(C,N) and ε-Nb(C,N) display the steady increase which generally corresponds to lattice expansion due to increasing temperature. After the temperature of 1,274 °C, which are indicated on the plot by the arrows, the d-spacing of δ-Nb(C,N) exhibits a sharp decrease during continuous heating when an expansion is expected, indicating that changes in the δ-Nb(C,N) precipitates are occurring before the dissolution. The contraction of δ-Nb(C,N) carbonitrides might be related with covalent bonding energy. According to electronic structure of niobium carbonitrides, the covalent bonds Nb–N and Nb–C are formed by p–d hybridization, which determines its thermodynamic stability. Once the Nb–N or Nb–C covalent bonds have been destroyed by heat prior to the dissolution, N and C atoms dissolve from the interstitial sites of niobium carbonitrides. Thus, the contraction of δ-Nb(C,N) will be manifested in the form of a decrease in d-spacing. With the further increase of temperature, more and more Nb–N and Nb–C covalent bonds will be broken, which leads to a further contraction of the lattice. This seems to indicate that δ-Nb(C,N) particles are not thermodynamically stable during the coarsening process. Until the Nb–N and Nb–C covalent bonds are totally destroyed, δ-phase particles will be completely dissolved into austenite. But the d-spacing of the ε-Nb(C,N) exhibits the continued increase along with the increasing temperature. It is likely that the hexagonal ε-phases possess a larger covalent contribution to the chemical bonding than the cubic one, which may give rise to higher thermodynamic stability as discussed above.
Changes of d-spacing of ε-NbN (102) and δ-NbN (111) in austenitic heat-resistance steel for heating rate 8.3 °C s−1 as function of temperature. Arrows on plot indicate the initiation of the niobium carbonitrides coarsening and dissolution, respectively [44]
Figure 12 [45] exhibits the progression of the measured M23C6 (420) d-spacings of ferritic steel as a function of temperature. Below 800 °C, the crystal lattice expands as a result of the thermal expansion during heating. The thermal expansion coefficient (CTE) for M23C6 is reported to be 7.9 × 10−6/°C near room temperature. This value corresponds very well with the experimentally measured CTE value (8.76 × 10−6/°C). The result displays a rapid increase in CTE to a value of 16.4 × 10−6/°C above 800 °C, which is approximately 1.8 times that observed at lower temperatures. Similarly, the measured CTE value of the M23C6 (422) is approximately 1.7 times that observed at lower temperatures.
Tracing the d-spacing changes of 420 and 422 diffraction peaks of M23C6 carbides in ferritic heat-resistance steel [45]
To understand this difference in lattice expansion, the mean crystallite size of M23C6 carbides is considered as a function of temperature. Figure 13 [45] shows the calculated mean particle size using the integral breadth method. Above 800 °C, coarsening of M23C6 carbide particles occurred. The different behaviors before and after 800 °C are most likely explained by the diffusion of alloying elements. M23C6 carbides, though generally Cr rich, can accommodate varying amounts of Fe, Mo, and W, depending on the temperature and time of formation [46, 47]. It is clear that dislocations are energetically stable for the M23C6 precipitates, since Cr-rich M23C6 particles mainly precipitate on prior austenite grain boundaries, ferrite subgrain boundaries and on dislocations inside subgrains. The coarsening of M23C6 precipitates could possibly be caused by a solute drag effect where substitutional atoms are carried by migrating dislocations. Calculations made by Hald on 9–12 % Cr steels show that solubility and diffusion of Mo control the coarsening of M23C6 carbides. Therefore an increased mobility of Mo and W atoms could contribute to the coarsening of carbides. Although it is generally accepted that Cr and Fe are the main elements in M23C6 carbides, Mo and W will also play a role in the coarsening of M23C6 precipitates. The atomic radii of Mo and W are 1.90 Å and 1.93 Å, which are larger than the radii of 1.66 Å and 1.56 Å for Cr and Fe respectively. Thus, lattice expansion increases markedly during the coarsening of M23C6 carbides.
Calculated crystallite size of M23C6 versus temperature [45]
5 Diffusional and Displacive Transformation Behavior in Low Carbon Low Alloy Steels
As an observational example for hybrid system, the phase transformation behaviour of diffusional (mild steel (MS)) and displacive (low-temperature transformation steel (LTT)) transformations were compared with this system. The origin and morphology of the microstructural change were analysed in real space. The d-spacing change was also analysed to assess the carbon partitioning behaviour in the reciprocal lattice space. An LTT specimen was prepared in order to observe the displacive transformation. The LTT material should reduce the residual stress by using transformation strain in the welding field [48]. The chemical composition of the LTT was 0.03 C, 0.07 Si, 0.09 Mn and 9.88 Ni (wt.%). The chemical composition of the MS was 0.06 C, 0.4 Si, 1.01 Mn, 0.01 P and 0.008 S (wt.%). The specimen was 5.0 mm in diameter and 2.0 mm in height, and it was inserted into a boron nitride crucible. Both specimens were subjected to the same thermal cycle, heated up to 1,000 °C and cooled down to room temperature. The time resolution of XRD detection was 0.2 s. The diffraction data were analysed using the method and programming described in Ref. [37]. On the other hand, the LSCM images were recorded in the time resolution of 0.03 s throughout the thermal cycle. Figure 14 [32] shows snapshots of the LSCM images for the LTT and MS specimens during the cooling cycle. In the case of the LTT, austenite was supercooled to 300 °C, as shown in Fig. 14 LTT-(i). Further cooling caused a martensitic transformation, as shown in Fig. 14 LTT-(ii) and (iii). The image contrast of the precipitate was very clear because of the surface relief of the martensite. The yield stress increased linearly as the temperature decreased. Then the elastic strain balanced the transformation strain and it was effective in reducing the residual stress in the restricted weld. In the case of the MS, at 640 °C, a very weak contrast was observed on the grain boundary of the austenite and spread throughout the images at 550 °C, as shown in Fig. 14 MS-(ii) and (iii). The nucleation site and the morphology of the precipitate were of the nature of a ferrite allotriomorph. The nucleation temperature should be higher than 640 °C because the magnification of LSCM images is 100. The image contrast of the ferrite allotriomorph was very weak because there was no relief on the surface of the specimen. The contrast in the LSCM image is affected by two factors: the formation of relief and a difference in reflectivity. In the case of relief formation, such as displacive transformation and thermal grooving at the grain boundary, the difference in the reflection direction of the laser light is responsible for the clear contrast in the LSCM image. On the other hand, in diffusional transformation, the contrast is very weak, as shown in Fig. 14. This means that there is no surface relief, and the contrast is due to the differences of reflectivity between the ferrite and austenite. The light reflectivity is governed by the electrical conductivity. The difference of electrical conductivity between the ferrite and austenite should be small in the current chemical composition, and this difference causes a very weak contrast in the case of diffusional transformation. Figure 15 [32] shows the d-spacing, time, temperature and intensity diagram [LTT-(a) and MS-(a)] during the phase transformations in the cooling cycle. The displayed reflection is that of austenite (111) and ferrite (110). The intensity was normalized. In the macro-view, the d-spacing of the austenite and ferrite decreased because of thermal shrinkage in the LTT and MS. When the phase transformation occurred, the intensity of the austenite decreased, as shown in Fig. 15. The marks (i), (ii) and (iii) in the temperature profile correspond to the number in Fig. 14 for each steel sample. There was a discrepancy of approximately 20 °C between the surface temperature of the specimen and the thermocouple set behind the platinum plate stage. This discrepancy suggests that the LSCM images were compatible with the diffraction pattern. Hence, the developed system could follow the phase transformations in both real and reciprocal space. Figure 15 LTT-(b) and MS-(b) show enlarged views of the austenite (111) diffraction peaks when the austenite decreased because of the phase transformations. The d-spacing continuously decreased along the trends in the macro-view in the LTT, whereas it increased against the trends in the MS. In order to analyse this phenomenon, a fit of Gaussian peaks [37] to the austenite reflection (111) was performed.
Snapshots of solid-state transformation during the cooling cycle in LTT [LTT-(i), (ii), (iii)] and MS [MS-(i), (ii), (iii)] [32]
The d-spacing, time and temperature—intensity diagram during the cooling cycle in LTT [LTT-(a)] and MS [MS-(a)] [32]
Figure 16 shows the result of d-spacing that was derived using a fit of Gaussian peaks in the LTT and MS (shown in the low-temperature and high temperature region, respectively, in Fig. 10). As expected, in the case of the LTT, the d-spacing continuously decreased along the cooling cycle. On the other hand, in the case of the MS, d-spacing continuously decreased until 643 °C and then suddenly increased at the point shown in Fig. 15 MS-(b). There is a possibility that the inhomogeneous carbon distribution occurred because of the insufficient austenitization and affected the d-spacing change. However, the continuous decreasing of d-spacing prior to phase transformation (as shown in Fig. 16, marked “MS”) means the discontinuous change around 643 °C was due to the phenomena during phase transformation. The d-spacing change in an austenite phase in the diffusional transformation was found to be discontinuous to than in the displacive transformation. This difference reflected the carbon partitioning behaviour. Furthermore, there was no splitting behaviour [49] in the austenite peaks. Thus, it could be concluded that the carbon partitioning expanded the lattice parameter as observed in the ferrite allotriomorph formation. In the case of the displacive transformation, there was no partitioning of carbon, and the expansion was not observed in the TRXRD data. A large-area detector, Pilatus 2M [9], was installed in the experimental system. Then the austenite reflections of (111), (200), (220) and (311) were recorded over time. All the reflections had the same tendency (i.e., increasing d-spacing because of carbon partitioning in MS), and the results were verified statistically. As is clearly observed in Fig. 16, the d-spacing change in the MS looks very smooth, whereas that in the LTT looks uneven. Note that the uneven form appeared before phase transformation and was smooth after phase transformation, as shown in the low-temperature region of Fig. 16. The major difference in the phase transformation of each austenite is the degree of supercooling. The supercooling may affect crystal stability. Further statistical research is needed to assess the effect from this perspective.
Tracing of the d-spacing change during phase transformations in LTT and MS, derived from the fitting of Gaussian peaks [32]
The phase transformation behaviour of mild steel and low-temperature-transformation steel was observed in situ with a developed hybrid TRXRD/LSCM system. When the ferrite allotriomorph was formed in the mild steel, carbon partitioning occurred, and the d-spacing of the austenite reflection increased even when thermal shrinkage occurred during the cooling thermal cycle. Our system should also help to analyse other transformation mechanisms of steel, such as bainite and massive transformations.
6 Summary
A new technique, based on the combination of time-resolved X-ray diffraction (TRXRD) and laser scanning confocal microscopy (LSCM), was developed for direct observation of morphological evolution and simultaneous identification of the phases. TRXRD data and LSCM images under the desired thermal cycles were measured simultaneously. As described above, the combination of LSCM and TRXRD is effective in investigating the phase transformation kinetics during thermal cycles of rapid heating and cooling. The system can be applied to the analysis of microstructural changes for improved control of properties in welds.
7 Future Works
Synchrotron based X-ray diffraction techniques combined with high-temperature laser scanning confocal microscopy; provide new and powerful tools for the study of phase transformations and microstructural evolution during welding.
Detecting a wider area of the Debye circle is very important. Mounting several detectors on the arm of the diffractometer increases the detection range for the part of the Debye circle. Continual improvements in synchrotron based methods can only increase the ability to monitor these transformations at higher spatial and temporal resolutions during welding. When combined with additional experiments and modelling, these techniques enable a deeper understanding of the kinetics of phase transformations.
References
Elmer JW, Wong J, Ressler T (1998) Spatially resolved X-ray diffraction phase mapping and alpha → beta → alpha transformation kinetics in the heat-affected zone of commercially pure titanium arc welds. Metall Mater Trans A 29:2761–2773
Elmer JW, Wong J, Ressler T (2001) Spatially resolved X-ray diffraction mapping of phase transformations in the heat-affected zone of carbon-manganese steel arc welds. Metall Mater Trans A 32:1175–1187
Elmer JW, Palmer TA, Wong J (2003) In situ observations of phase transitions in Ti-6Al-4V alloy welds using spatially resolved x-ray diffraction. J Appl Phys 93:1941–1947
Elmer JW, Palmer TA (2006) In-situ phase mapping and direct observations of phase transformations during arc welding of 1045 steel. Metall Mater Trans A 37A:2171–2182
Elmer JW, Palmer TA, Zhang W, Wood B, DebRoy T (2003) Kinetic modeling of phase transformations occurring in the HAZ of C-Mn steel welds based on direct observations. Acta Mater 51:3333–3349
Zhang W, Elmer JW, DebRoy T (2005) Integrated modelling of thermal cycles, austenite formation, grain growth and decomposition in the heat affected zone of carbon steel. Sci Technol Weld Joining 10:574–582
Elmer JW, Wong J, Ressler T (2000) In-situ observations of phase transformations during solidification and cooling of austenitic stainless steel welds using time-resolved X-ray diffraction. Scripta Mater 43:751–757
Elmer JW, Palmer TA, Babu SS, Zhang W, DebRoy T (2004) Direct observations of austenite, bainite, and martensite formation during arc welding of 1045 steel using time-resolved X-ray diffraction. Weld J 83:244S–253S
Elmer JW, Palmer TA, Babu SS, Zhang W, DebRoy T (2004) Phase transformation dynamics during welding of Ti-6Al-4V. J Appl Phys 95:8327–8339
Babu SS, Elmer JW, Vitek JM, David SA (2002) Time-resolved X-ray diffraction investigation of primary weld solidification in Fe-C-Al-Mn steel welds. Acta Mater 50:4763–4781
Palmer TA, Elmer JW, Babu SS (2004) Observations of ferrite/austenite transformations in the heat affected zone of 2205 duplex stainless steel spot welds using time resolved X-ray diffraction. Mater Sci Eng, A 374:307–321
Wong J, Ressler T, Elmer JW (2003) Dynamics of phase transformations and microstructure evolution in carbon-manganese steel arc welds using time-resolved synchrotron X-ray diffraction. J Synchrotron Radiat 10:154–167
Komizo Y, Osuki T, Yonemura M, Terasaki H (2004) Analysis of primary weld solidification in stainless steel using X-ray diffraction with synchrotron radiation (materials, metallurgy & weldability). Trans JWRI 33:143–146
Osuki T, Yonemura M, Ogawa K, Komizo Y, Terasaki H (2006) Verification of numerical model to predict microstructure of austenitic stainless steel weld metal using synchrotron radiation and trans varestraint testing. Sci Technol Weld Joining 11:33–42
Komizo Y, Terasaki H, Yonemura M, Osuki T (2005) In-situ observation of steel weld solidification and phase evolution using synchrotron radiation. Trans JWRI 34:51–55
Yonemura M, Osuki T, Terasaki H, Komizo Y, Sato M, Kitano A (2006) In-situ observation for weld solidification in stainless steels using time-resolved X-ray diffraction. Mater Trans 47:310–316
Terasaki H, Komizo Y, Yonemura M, Osuki T (2006) Time-resolved in-situ analysis of phase evolution for the directional solidification of carbon steel weld metal. Metall Mater Trans A 37A:1261–1266
Yonemura M, Komizo Y, Toyokawa H (2006) SPring 8 Res Front 129–130
Yonemura M, Osuki T, Terasaki H, Komizo Y, Sato M, Toyokawa H (2006) Two-dimensional time-resolved X-ray diffraction study of directional solidification in steels. Mater Trans 47:2292–2298
Komizo Y, Terasaki H (2008) In-situ observation of weld solidification and transformation using synchrotron radiation. Tetsu To Hagane—J Iron Steel Inst Jpn 94:1–5 (in Japanese)
Komizo Y (2008) In-situ microstructure observation techniques in welding. J Jpn Weld Soc 77:26–31 (in Japanese)
Komizo Y, Terasaki H, Yonemura M, Osuki T (2008) Development of in-situ microstructure observation techniques in welding. Weld world-Lond 52:56–63
Hashimoto T, Terasaki H, Komizo Y (2008) Effect of solidification velocity on weld solidification process of alloy tool steel. Sci Technol Weld Joining 13:409–414
Terasaki H, Yamada T, Komizo Y (2008) Analysis of inclusion core under the weld pool of high strength and lowalloy steel. ISIJ Int 48:1752–1757
Terasaki H, Yanagita K, Komizo Y, Sato M, Toyokawa H (2009) In-situ observation of solidification behavior of 14Cr-Ni steel weld. Q J Jpn Weld Soc 27:118s–121s
Zhang D, Terasaki H, Komizo Y (2009) In situ observation of morphological development for acicular ferrite in weld metal. J Alloy Compd 484:929–933
Terasaki H, Komizo Y (2006) In situ observation of morphological development for acicular ferrite in weld metal. Sci Technol Weld Joining 11:561–566
Yamada T, Terasaki H, Komizo Y (2008) Microscopic observation of inclusions contributing to formation of acicular ferrite in steel weld metal. Sci Technol Weld Joining 13:118–125
Komizo Y, Terasaki H (2011) Optical observation of real materials using laser scanning confocal microscopy Part 1-techniques and observed examples of microstructural changes. Sci Technol Weld Joining 16:56–60
Komizo Y, Terasaki H (2011) Optical observation of real materials using laser scanning confocal microscopy Part 2-direct observation of ferrite nucleation sites in weld metal and heat affected zone. Sci Technol Weld Joining 16:61–67
Komizo Y, Terasaki H (2011) In situ time resolved X-ray diffraction using synchrotron. Sci Technol Weld Joining 16:79–86
Terasaki H, Komizo Y (2011) Diffusional and displacive transformation behaviour in low carbon-low alloy steels studied by a hybrid in situ observation system. Scripta Mater 64:29–32
Warren BE (1990) X-ray diffraction. Courier Dover Publications
Zhang XF, Komizo Y (2013) In Situ Investigation of the Allotropic Transformation in Iron. Steel Res Int 84:751–760
Zhang YD, Esling C, Calcagnotto M, Zhao X, Zuo L (2007) New insights into crystallographic correlations between ferrite and cementite in lamellar eutectoid structures, obtained by SEM-FEG/EBSD and an indirect two-trace method. J Appl Crystallogr 40:849–856
Takasaki A, Ojima K, Taneda Y (1995) In-situ transmission electron microscopy of the α ↔ γ allotropic transformation in thin foil of iron. Phys Status Solidi A 148:159–165
Babu SS, Specht ED, David SA, Karapetrova E, Zschack P, Peet M, Bhadeshia H (2005) In-situ observations of lattice parameter fluctuations in austenite and transformation to bainite. Metall Mater Trans A 36A:3281–3289
Morito S, Tanaka H, Konishi R, Furuhara T, Maki T (2003) The morphology and crystallography of lath martensite in Fe-C alloys. Acta Mater 51:1789–1799
Kitahara H, Ueji R, Tsuji N, Minamino Y (2006) Crystallographic features of lath martensite in low-carbon steel. Acta Mater 54:1279–1288
Perepezko JH (1984) Nucleation in undercooled liquids. Metall Trans A 15:437–447
Massalski TB (1984) Distinguishing features of massive transformations. Metall Trans A 15:421–425
Kinsman KR, Richman RH, Verhoeven JD (1976) Geometric surface relief and the allotropic transformation in iron. J Mater Sci 11:1487–1493
Banerjee S (1984) Solubility of organic mixtures in water. Mater Sci Forum 1:239–255
Zhang XF, Terasaki H, Komizo Y (2012) In situ investigation of structure and stability of niobium carbonitrides in an austenitic heat-resistant steel. Scripta Mater 67:201–204
Zhang XF, Komizo Y (2013) Direct observation of thermal stability of M23C6 carbides during reheating using in situ synchrotron X-ray diffraction. Philos Mag Lett 93:9–17
Hald J (2008) Microstructure and long-term creep properties of 9–12% Cr steels. Int J Press Vessels Pip 85:30–37
Mythili R, Paul VT, Saroja S, Vijayalakshmi A, Raghunathan VS (2003) Microstructural modification due to reheating in multipass manual metal arc welds of 9Cr–1Mo steel. J Nucl Mater 312:199–206
Zenitani S, Hayakawa N, Yamamoto J, Hiraoka K, Morikage Y, Kubo T, Yasuda K, Amano K (2007) Development of new low transformation temperature welding consumable to prevent cold cracking in high strength steel welds. Sci Technol Weld Joining 12:516–522
Palmer TA, Elmer JW (2005) Direct observations of the formation and growth of austenite from pearlite and allotriomorphic ferrite in a C–Mn steel arc weld. Scripta Mater 53:535–540
Acknowledgments
The synchrotron radiation experiments were performed at the SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2009B2086 and 2011B1968). The authors are grateful to Dr. Sato and Dr. Toyokawa, JASRI, for profitable discussion.
This study was conducted as a part of research activities of ‘Fundamental Studies on Technologies for Steel Materials with Enhanced Strength and Functions’ by the Consortium of The Japan Research and Development Center of Metals (JRCM). Financial support from New Energy and Industrial Technology Development Organization (NEDO) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Komizo, Yi., Zhang, X.F., Terasaki, H. (2014). Hybrid System for In Situ Observation of Microstructure Evolution in Steel Materials. In: Kannengiesser, T., Babu, S., Komizo, Yi., Ramirez, A. (eds) In-situ Studies with Photons, Neutrons and Electrons Scattering II. Springer, Cham. https://doi.org/10.1007/978-3-319-06145-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-06145-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-06144-3
Online ISBN: 978-3-319-06145-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)