Abstract
In the recent decade, the role of imaging in local staging of rectal cancer has evolved. Whereas in the past its role has been restricted mainly to endorectal ultrasound, it has recently extended to modern imaging such as CT and MRI. This chapter “imaging and staging” will address the two most frequently used imaging methods in rectal cancer management: endorectal ultrasound (ERUS) and magnetic resonance imaging (MRI). For each, experts in the field will elaborate on how these methods can identify the relevant risk factors for local recurrence and which protocol should be used to ensure a high-quality performance. In this introduction section, a helicopter view is given on the role of each method, ERUS and MRI, in the context of clinical decision making and its role put in perspective of one another. The introduction finalizes with recommendations for use in clinical practice.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Rectal Cancer
- Total Mesorectal Excision
- Local Excision
- Circumferential Resection Margin
- Lymph Vessel Invasion
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The local recurrence rate after rectal cancer surgery has significantly decreased in the past two decades. This is mainly due to the introduction of a total mesorectal excision (TME) surgery. In addition preoperative radiotherapy is now given instead of a postoperative course because trials have shown that preoperative radiotherapy is more effective in reducing the local recurrence rate than postoperative. Therefore, the role of imaging in the staging of these tumors has changed. Whereas previously most decisions on whether or not to give adjuvant treatment were based on the risk assessment for recurrence through histological evaluation of the tumor and the lymph nodes, the decisions on neoadjuvant treatment are now based on risk assessment through imaging. Although modern CT techniques are improving and to some extent able to provide information for locoregional staging, endorectal ultrasonography (ERUS) and MRI are considered as the two best locoregional staging methods for rectal cancer. When comparing ERUS with MRI, there are several issues that require consideration. In addition to the accuracy in predicting certain risk factors for local recurrence, there is the treatment strategy that dictates what information will have a clinical consequence. Besides, issues of cost, availability, and expertise may influence the local treatment strategy and thus the choice of the imaging method.
The risk factors associated with local recurrence are the T stage, N stage, distance of the tumor to the mesorectal fascia, extramural vascular invasion, perineural invasion, lymph vessel invasion, and histological grade [1, 2]. Of these risk factors, the T and N stages are commonly used for (neo)adjuvant treatment decisions (NCCN guidelines) [3] and recently the distance of the tumor to the mesorectal fascia [4]. The TNM classification system has reproducible and straightforward histological cutoff values, such as the distinction between a T2 and T3 tumor. It does however not always easily transfer to staging through imaging. All imaging methods are good in showing the bulk of the tumor but will have difficulty in predicting the exact microscopical tumor extension to a histological interface. It is therefore unrealistic to expect a 100 % accuracy from imaging technology in predicting a histological classification.
The accuracy of the T stage assessment with ERUS in the smaller series is generally higher than in larger and more recent data [5–8]. ERUS is reliable to stage rectal cancer for the degree of invasion in the rectal wall, but high accuracies are only obtained in expert centers. The agreement between the uT stage and pT stage in larger studies is 65–70 %, with 10–15 % understaging and 20 % overstaging [9–11]. In uT1 there is understaging in 15–20 % and in uT2 stage 15–30 %. Overstaging in uT3 occurred in 25–30 %. Some series address the specific question of distinguishing mucosal T0 lesions from T1 tumors, showing a risk of understaging with uT0 of only 5–15 % [12–15]. It is therefore generally considered that ERUS is good in imaging the smaller tumors and in selecting the eligible patients for a local excision. An overview of the ERUS technique and its role including the drawbacks is provided by Nonner and coauthors in Chap. 19 of this section. For the larger T3 and T4 lesions, ERUS can perfectly identify ingrowth in surrounding structures that are within the field of view such as the vagina, prostate, and seminal vesicles. The difficulties arise when tumors are located high in the rectum. It then provides insufficient anatomical information in specific on the extent to the dorsal and lateral pelvic wall.
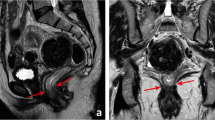
The importance of the involvement of the mesorectal fascia as a prognostic factor and as a parameter of surgical quality has been recognized and confirmed in the last 20 years [2]. The ideal plane of resection in a total mesorectal excision is just outside the mesorectal fascia, and a positive circumferential resection margin can be the result of inadequate TME surgery. An involved mesorectal fascia is defined as a closest distance of ≤1 mm between the tumor and the mesorectal fascia, as this represents the optimal prognostic cutoff point. Preoperative assessment of the mesorectal fascia involvement is important whenever a short preoperative course of 5 × 5 Gy is considered in patients without a threatened or involved margin. Although it has been shown that 5 × 5 Gy is a very efficient and cost-effective way to prevent local recurrences in many patients, it is much less effective when the tumor comes close to or invades the mesorectal fascia [16]. These tumors should be identified and treated with a preoperative long course of chemoradiation to provide downsizing. For centers that only use a long course of chemoradiation as a neoadjuvant treatment, the distance of the tumor to the mesorectal fascia is usually not very important in the preoperative decision process, as all tumors that extend beyond the muscular wall are considered candidates for a long course of chemoradiation, providing an opportunity for downsizing. Regardless of the neoadjuvant treatment strategies, it is however important for the surgeon to know the exact anatomical relation of the tumor to the mesorectal fascia and the surrounding structures in order to obtain a complete resection. Therefore, when it comes to staging the large rectal tumors, MRI is recommended as the preferred staging method [17–20]. For MRI of rectal cancer, it is important to obtain good standard high-resolution images. In Chap. 18 of this section, Hunter et al. elaborate on the state-of-the-art imaging protocol, on the strength but also the weaknesses for staging rectal tumors with modern planar imaging techniques, MRI and CT.
Nodal disease is one of the most important risk factors for both local and distant recurrence and is generally considered an indication for neoadjuvant therapy. Identifying nodal disease with imaging remains difficult because size criteria used on its own result in only a moderate accuracy. Lymph nodes with a diameter of ≥10 mm are invariably malignant, but the majority of involved nodes are smaller than 5 mm [21, 22]. In addition to size, morphological criteria such as shape, texture, and border of the nodes can be assessed in the larger nodes and improve the identification of the true node positives. But overall, the assessment of the smaller nodes remains difficult also because these criteria cannot always be applied. The difficulties in nodal staging with the standard imaging methods are illustrated by a recent multicentre report in which T3N0 tumors, staged with ERUS and/or MRI, were found to be node positive at histology in 22 %, despite preoperative chemoradiation [23].
How does one work in practice with a suboptimal accuracy of preoperative lymph node imaging? One approach is only to rely on imaging information on nodal status when the tumor is associated with round large nodes (>5 mm) that are irregular in border and/or heterogeneous in signal or echogenecity. Whenever these criteria for node positivity are absent on ERUS or MRI for any of the visualized nodes, information on nodal status is not reliable. An extreme approach is to disregard the imaging data on nodal status and to give neoadjuvant treatment in most patients, accepting overtreatment rather then undertreatment. This strategy exposes all patients to the side effects while only a few patients benefit of the improved local control. A third approach is to take into account the prevalence of nodal metastases according to the T stage and to give neoadjuvant therapy for T3 lesions, regardless of nodal imaging results, but not for T2N0 lesions [23]. This strategy of selective use of neoadjuvant radiotherapy only for patients most at risk for local recurrence is further supported by evidence from two large European trials of the lack of survival benefit of radiotherapy when good TME surgery is performed [18, 24].
Future Perspectives
Currently, there is also a trend to study alternative treatment options after a good response to treatment, such as a local excision or even a nonoperative wait and see approach. Given the increasing use of preoperative (chemo)radiation in rectal cancer, selection of the candidates for these alternative treatments by imaging should be a topic for further studies, because imaging technologies such as ERUS, CT, MRI, and PET are continuously improving. With modern more powerful machines, functional data can be generated and combined with morphological data. 3D-ERUS, diffusion MR imaging, perfusion MRI, perfusion CT, or perfusion PET/CT could all be of help in monitoring treatment response. New lymph node-specific MR contrast agents are on the way that may finally move us one step forward in our search for better identification of patients with nodal metastases. This new role of imaging to detect small volumes of residual disease in fibrotic scar tissue in the rectal wall and in the lymph nodes is now still work in progress, but it is clear that imaging in future will play an important role in the selection and follow-up of patients after neoadjuvant treatment.
Recommendations
ERUS and MRI should be seen more as complementary rather than competitive techniques. Each has its own strengths and weaknesses. ERUS has the advantage over MRI that the equipment is less costly and that it can be readily used in the office, immediately providing information that is important for further treatment planning. MRI on the other hand has the advantage over ERUS that the images can be more easily interpreted and read by other radiologists and clinicians. The images can also be used by radiotherapists for planning the radiotherapy fields and by surgeons to guide the resection in advanced cases. ERUS is without doubt the best imaging method for the selection of the candidates for local excision, whereas MRI is recommended for the larger more advanced tumors. MRI is accurate in identifying the different risk groups and in stratifying these patients into their treatment according to their risk. In the absence of easy access to MRI, MDCT is a good alternative for the high tumors, but it lacks accuracy in the low tumors. For lymph node imaging, all techniques are at present only moderately accurate. The most practical strategy seems to use the information on lymph node staging in the preoperative decision making, keeping in mind the suboptimal accuracy. In addition to the standard treatment with TME, there is a small group of patients with a superficial tumor where the surgeon is considering a local excision with a small risk of leaving behind involved lymph nodes in the mesorectum. Accurate selection of node-negative disease would be of help in the selection for this procedure, and future research should focus on developing imaging techniques that can better identify nodal disease.
References
Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N substage on survival and disease relapse in adjuvant rectal cancer: a pooled analysis. Int J Radiat Oncol Biol Phys. 2002;54(2):386–96.
Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303–12.
NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264(11):1444–50.
Marijnen CA, Nagtegaal ID, Kapiteijn E, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003;55(5):1311–20.
Edelman BR, Weiser MR. Endorectal ultrasound: its role in the diagnosis and treatment of rectal cancer. Clin Colon Rectal Surg. 2008;21(3):167–77.
Schaffzin DM, Wong WD. Endorectal ultrasound in the preoperative evaluation of rectal cancer. Clin Colorectal Cancer. 2004;4(2):124–32.
Harewood GC. Assessment of publication bias in the reporting of EUS performance in staging rectal cancer. Am J Gastroenterol. 2005;100(4):808–16.
Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging–a meta-analysis. Radiology. 2004;232(3):773–83.
Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385–90.
Ptok H, Marusch F, Meyer F, et al. Feasibility and accuracy of TRUS in the pre-treatment staging for rectal carcinoma in general practice. Eur J Surg Oncol. 2006;32(4):420–5.
Garcia-Aguilar J, Pollack J, Lee SH, et al. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45(1):10–5.
Adams WJ, Wong WD. Endorectal ultrasonic detection of malignancy within rectal villous lesions. Dis Colon Rectum. 1995;38(10):1093–6.
Kim JC, Yu CS, Jung HY, et al. Source of errors in the evaluation of early rectal cancer by endoluminal ultrasonography. Dis Colon Rectum. 2001;44(9):1302–9.
Staib L, Schirrmeister H, Reske SN, Beger HG. Is (18)F-fluorodeoxyglucose positron emission tomography in recurrent colorectal cancer a contribution to surgical decision making? Am J Surg. 2000;180(1):1–5.
Starck M, Bohe M, Simanaitis M, Valentin L. Rectal endosonography can distinguish benign rectal lesions from invasive early rectal cancers. Colorectal Dis. 2003;5(3):246–50.
Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701.
Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357(9255):497–504.
MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243(1):132–9.
Bissett IP, Fernando CC, Hough DM, et al. Identification of the fascia propria by magnetic resonance imaging and its relevance to preoperative assessment of rectal cancer. Dis Colon Rectum. 2001;44(2):259–65.
Blomqvist L, Machado M, Rubio C, et al. Rectal tumour staging: MR imaging using pelvic phased-array and endorectal coils vs endoscopic ultrasonography. Eur Radiol. 2000;10(4):653–60.
Lahaye MJ, Engelen SM, Nelemans PJ, et al. Imaging for predicting the risk factors–the circumferential resection margin and nodal disease–of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR. 2005;26(4):259–68.
Wang C, Zhou Z, Wang Z, et al. Patterns of neoplastic foci and lymph node micrometastasis within the mesorectum. Langenbecks Arch Surg. 2005;390(4):312–8.
Guillem JG, Diaz-Gonzalez JA, Minsky BD, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol. 2008;26(3):368–73.
Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811–20.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Beets-Tan, R.G.H. (2015). Introduction: Preoperative Staging by Imaging. In: Baatrup, G. (eds) Multidisciplinary Treatment of Colorectal Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-06142-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-06142-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-06141-2
Online ISBN: 978-3-319-06142-9
eBook Packages: MedicineMedicine (R0)