Abstract
Ethnic differences in the incidence and prevalence of certain obesity-related cancers are well established. African Americans have increased risk of prostate, breast (premenopausal), and colorectal cancer and myeloma, compared to Caucasians with the lowest rates in Latinos, Asians, and Native Americans. Prior work in this area suggests that there are distinct ethnic differences in obesity-related metabolic risk factors for cancer, insulin resistance in particular, that are evident early in life, and may help explain ethnic differences in the incidence and prevalence of obesity-related cancers. The focus of this chapter is to review and discuss ethnic differences in insulin resistance and its link with other cancer-related metabolic risk factors including hyperinsulinemia, insulin-like growth factors, body fat distribution, adipose tissue biology, low-grade inflammation, non-esterified fatty acids, and oxidative stress. This chapter places a particular emphasis on ethnic differences between African Americans and Latinos for two reasons: (1) African Americans and Latinos are the two largest ethnic minority groups in the USA, and (2) these populations share a similar propensity for obesity and insulin resistance but markedly different profiles for obesity-related cancers, creating an informative comparative contrast. Although the literature is limited by an inconsistency in the terminology used for various ethnicities, in most cases we refer to Caucasian for any study using the terms Caucasian, White, or non-Hispanic White; Latino to describe people of Hispanic, Latino, or Mexican-American descent; African American to describe people of African, African American, or Black-Caribbean descent; Asian to describe people of Asian, South Asian, East Asian, and Southeast Asian descent or any other specific Asian ethnicity; and Native American to describe people of American Indian, Pima Indian, Aboriginal, First Nation, or Alaska Native ethnicity. We also recognize that there may be variation within these subgroups; however, comprehensive review of this literature is beyond the scope of this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Insulin
- Insulin resistance
- Hyperinsulinemia
- Insulin-like growth factor
- Non-esterified fatty acids
- Oxidative stress
- Psychological stress
- Cortisol-induced obesity
- Body fat distribution
- Intramyocellular lipid
- Hepatic fat
- Pancreatic fat
- Ectopic fat
- Adipose tissue biology
The Scope of the Problem: Obesity and Cancer Disparities
According to the 2010 US Current Population Survey, there are 53 million people of Latino origin and 41 million African Americans in the USA, comprising 17 % and 13 % of the total population, respectively. Latinos are the fastest growing ethnic group in this country adding almost 13 million people to the population and increasing in size by 41 % in the last decade. Obesity is a significant problem in both African Americans and Latinos with the most recent National Health and Nutrition Examination Survey (NHANES) estimates from 2009 to 2010 suggesting higher rates of overweight and obesity in African American and Latino adults compared to Caucasians [1]. In adults, 20 years of age and older, African Americans had the highest age-adjusted rates of obesity (49.5 %), followed by Mexican Americans (40.4 %), all Latinos (39.1 %), and Caucasians (34.3 %). Of note, the prevalence of grade 2 [body mass index (BMI) of at least 35 kg/m2] and grade 3 obesity (BMI greater than or equal to 40 kg/m2) were highest among African Americans (26 % for grade 2, and 13.1 % for grade 3), compared to Caucasians (14.4 % for grade 2, and 5.7 % for grade 3) and Latinos (14.9 % for grade 2, and 5.4 % for grade 3). Although American Indians comprise a smaller proportion of the total US population (1.2 %), obesity is also a significant problem in this ethnic group with 39.4 % of American Indian men and women categorized as obese [2]. Among Asians, this ethnic group is 60 % less likely to be obese compared to Caucasians; however, there is substantial variation in the prevalence of overweight and obesity within this ethnic group [3]. Filipino Americans are 70 % more likely to be obese as compared to the overall Asian population. Interestingly, Southeast Asians have one of the highest prevalences of type 2 diabetes in the USA, yet the prevalence of obesity in this group is 6 % with 30–35 % of Southeast Asians classified as overweight [4]. In contrast, Chinese, Korean, and Vietnamese Americans have the lowest rates of overweight (BMI, 25 to <30 kg/m2) and one in ten Korean and Vietnamese Americans are classified as underweight [3].
In 2010, pediatric obesity rates in the USA also showed a well-defined disparity by ethnicity, where 42 % of Latinos, 41 % of African Americans, and 30 % of Caucasians between the ages of 12 and 19 years were classified as overweight or obese [5]. Of note, Native American adolescents had the highest prevalence of obesity than those in all other ethnic groups combined [6]. As a result, obesity-related complications such as prediabetes and type 2 diabetes are more common in ethnic minority children and adults compared to Caucasians [7–12]. Specifically, the risk of diagnosed diabetes is 1.8 times higher among African Americans, and 1.7 times higher among Hispanics compared to Caucasians [13]. Moreover, 16.1 % of the total adult American Indian population has diagnosed diabetes [13]. A similar trend is noted in children, with African American, Latino, and Native American children reporting the highest rates of type 2 diabetes compared to other ethnicities [11, 12, 14]. The higher risk and prevalence of type 2 diabetes among these ethnic minority groups have been attributed to more severe insulin resistance and hyperinsulinemia (relative to Caucasians [8, 15–18]).
There is convincing evidence that overweight and obesity are also associated with cancers of the kidney, breast, colon, esophagus, endometrium, prostate, and colorectum, whereas studies on the relation between obesity and other forms of cancers are less consistent [19–23].
Despite a similar predisposition towards obesity, insulin resistance, and type 2 diabetes among African Americans, Latinos and Native Americans, there are marked differences in cancer incidence across different ethnic groups [24]. African Americans have increased risk of certain forms of obesity-related cancers, whereas for these same outcomes, Latinos and Native Americans appear to be somewhat “protected.” In support of this hypothesis, data from the Surveillance Epidemiology and End Results (SEER) Database suggest that African American men have the highest incidence of cancer (all cancers combined) followed by Caucasians, with lower cancer rates among Latino, Native American and Asian men [25]. More specifically, African American men in the USA have the highest rates of prostate cancer worldwide. The prevalence rate is almost two times higher compared to Caucasians and Latinos and almost three times higher compared to Native Americans and Asians [25]. Breast cancer—the most common cancer among women—is highest among African Americans and Caucasian women compared to Latinas, Native Americans, and Asians. Interestingly, African American women have the highest rates of breast cancer before age 40 whereas Caucasians have the highest rates at older ages [26]. For both men and women, rates of colorectal cancer and myeloma are highest among African Americans followed by Caucasians with the lowest rates among Latinos, Native Americans, and Asians [25, 27]. Similar trends are observed for most other types of cancer, with rates among African Americans or Caucasians higher than those for other ethnic minority groups including Latinos [25]. Taken together, distinct differences in obesity-related cancer outcomes persist between African Americans, Latinos, and Native Americans despite all three groups having an increased propensity for obesity and similar risk for type 2 diabetes. This chapter reviews ethnic differences in cancer-related metabolic risk factors, insulin resistance, and hyperinsulinemia in particular and their potential contributions to ethnic differences in obesity-related cancer outcomes.
Obesity and Cancer Risk: Potential Mechanisms
Insulin Resistance
Obesity is the strongest contributing factor to insulin resistance and hyperinsulinemia, and this is evident early in life [8, 15, 28–30]. Many studies have shown that body fatness is positively associated with circulating fasting insulin levels in both animals and humans [31]. Insulin is a critical hormone for regulating metabolism, and its concentration in circulation is carefully coordinated, varying acutely in response to glucose and meal consumption. Insulin resistance is a condition in which muscle, fat, and liver cells are less sensitive to the metabolic effect of insulin. As a result, physiologic actions of insulin are inhibited but can be compensated for by increased insulin levels in circulation (i.e., hyperinsulinemia) to clear glucose from circulation [32, 33]. In addition, elevated insulin may stimulate cellular proliferation in pancreatic beta cells and fat cells, ensuring additional insulin production and fat storage, respectively [34]. This mechanism may have substantial advantages because it provides fat cells that can hold on to ingested fat and prevent its ectopic distribution elsewhere in the body [35, 36]. Thus, obesity results in continuous exposure of body tissues to elevated background and glucose-stimulated levels of insulin.
One of the leading hypotheses explaining why “fat is bad” relates to the role of insulin resistance and hyperinsulinemia as the mediating link between obesity and cancer risk. As mentioned above, besides its metabolic effects, insulin has promitotic and anti-apoptotic effects that may be tumorigenic [23, 37, 38]. Moreover, increased insulin resistance and hyperinsulinemia have been associated with increased risk of breast, endometrial, and colon cancer [20, 39–45]. Hence, detailed studies comparing ethnic differences in insulin resistance and hyperinsulinemia have been helpful in understanding why certain subgroups of the population are at increased cancer risk.
Research has consistently demonstrated that African Americans are more insulin resistant compared to Caucasians, which is only partially explained by greater overall adiposity in this ethnic group [8, 18, 46–56]. The Insulin Resistance Atherosclerosis Study (IRAS), a large-scale multicenter epidemiological study, was the first to provide compelling evidence in support of a metabolic predisposition towards insulin resistance in African American adults [57]. Compared to Caucasians, African Americans had significantly higher fasting and 2-h postprandial insulin concentrations, higher acute insulin responses to glucose, and greater insulin resistance [57]. These ethnic differences persisted after adjusting for differences in age, obesity, body fat distribution, self-reported physical activity, and percent calories from fat and fiber. Data from the NHANES III subsequently confirmed ethnic differences in mean fasting insulin concentrations between African American and Caucasian men and women at each BMI category [55].
Similar to African Americans, large-scale studies of obesity, insulin resistance, insulin secretion, and beta-cell response in Latino and Native American populations have consistently reported an increased insulin response to glucose [8, 50, 58–61]. Glucose-tolerant Native Americans and Latinos were found to have greater insulin resistance and fasting hyperinsulinemia compared to Caucasians [62–65]. In addition, both groups were found to have exaggerated early insulin secretory responses to both intravenous and oral glucose challenges [50, 58, 59, 66, 67]. Others have confirmed that Latino adults have greater fasting and post-challenge insulin and greater insulin resistance than Caucasians [8, 62].
Studies in children are of increased significance because they allow examination of potentially underlying biological differences across subgroups of the population to be performed in the absence of potential confounding factors such as smoking, alcohol, aging, and menopausal status. Data from the Bogalusa Heart Study were the first to report increased insulin resistance in African American compared to Caucasian children based on measures of fasting insulin [68]. Subsequently, other studies have demonstrated greater insulin resistance and greater acute insulin response to glucose in African American compared to Caucasian children [30, 69]; these differences were independent of body fat, visceral fat, dietary factors, and physical activity. A recent study, using a hyperglycemic clamp technique, supported these observations where overweight African American compared to Caucasian youth had up to a 75 % higher insulin secretion relative to their insulin sensitivity [15], an indicator of increased or up-regulated pancreatic beta-cell responsiveness.
Ethnic differences in insulin resistance have been well documented in Latino, Asian, and Native American youth, where, independent of overall adiposity, these ethnic minority groups exhibit more severe insulin resistance but an enhanced insulin secretory response when compared to Caucasian children [8, 11]. Studies comparing multiple ethnic groups confirmed greater insulin resistance during an intravenous glucose tolerance test in Native Americans compared to African Americans and Caucasians [70]. Another study reported equally greater insulin resistance assessed via hyperglycemic clamp among African Americans, Latinos, and Asians than in Caucasians [62]. In addition, Asians were the most insulin resistant followed by Latinos, African Americans, and Caucasians [62]. In prepubertal children, African American and Latino children were found to be equally more insulin resistant than Caucasian children [8]. However, in peripubertal adolescents, obese African Americans were more insulin resistant than Latinos, independent of body composition and fat distribution [60]. Pancreatic beta-cell function and the acute insulin response to a glucose challenge were also higher in African American than in Latino adolescents, suggesting that ethnic differences in pubertal induced insulin resistance may be an important contributor to ethnic differences in insulin resistance [71]. Of interest, the compensatory responses to insulin resistance were different in African American compared to Latino children and adolescents [8]. African American children tend to compensate with a higher acute insulin response to glucose, and this effect was in part due to a reduction in hepatic insulin extraction [8]. Following the ingestion of oral glucose, lower extraction rates have also been reported in African American adults [54]. In contrast, Latino children and adolescents compensate to the same degree of insulin resistance with greater second-phase insulin secretion [8]. Both beta-cell secretion and/or insulin clearance by the liver determine peripheral insulin levels and help to maintain normal glucose levels in circulation [72]. The mechanisms by which Native American and Asian populations compensate for insulin resistance is understudied; nevertheless, increased insulin resistance and secretion as well as hyperinsulinemia are present among ethnic minority children, adolescents, and adults compared to Caucasians, and these findings have been confirmed using a variety of methodologies.
The well-documented ethnic differences in insulin resistance and secretion in children and adults have been explained in part by genetic, behavioral, and/or environmental factors. Previous research has reported a positive association between African genetic admixture and insulin resistance [73]. In contrast, recent work has demonstrated that socio-behavioral factors including physical activity and self-reported racial discrimination, but not African genetic admixture, were associated with increased cardiometabolic risk (i.e., blood pressure) among African Americans [74]. Moreover, research in the area of molecular epigenetic mechanisms of gene expression has also suggested that the genome is subject to environmental regulation [75], suggesting that ethnic differences in insulin resistance may have a gene-environmental origin. Consequently, in addition to nutrition and physical activity (which is further discussed in the next chapter), research has begun to investigate the role of the social environment, particularly psychosocial stress, and its implications for obesity and insulin resistance. The physiological stress response originates from the hypothalamic-pituitary-adrenal axis and undergoes a cascade of reactions including the release of corticotrophin-releasing hormone from the hypothalamus, causing the release of adrenocorticotrophic hormone by the adrenal pituitary, and ultimately the release of cortisol by the adrenal cortex into circulation [76]. Cortisol levels increase in response to both stressors in the laboratory [77] and naturalistic social environments [78]. Designed to increase energy availability in the short term, cortisol acutely impairs insulin secretion and increases hepatic glucose output [79]. An environment of prolonged glucocorticoid exposure (i.e., chronic stress) exerts diabetogenic effects by interfering with insulin action on several different levels [80–82], including a direct inhibition of insulin secretion from pancreatic beta cells [83], impaired insulin-mediated glucose uptake [84], and disruption of the insulin signaling cascade in skeletal muscle [85]. Under chronic conditions, healthy lean individuals appear able to compensate for glucocorticoid-induced insulin resistance with increased beta-cell function or increased insulin release [86–88]. However, in the obese or the insulin-resistant state, those compensatory mechanisms fail to counteract glucocorticoid-induced insulin resistance, resulting in hyperglycemia [87, 88]. Hence, prolonged glucocorticoid exposure may further compromise the already lower insulin sensitivity in obese African Americans by exacerbating the progression towards insulin resistance in these populations. Previous research has demonstrated the negative association between cortisol and obesity in adults [89, 90], and a recent study showed that cortisol contributes to the reduction in insulin sensitivity over a 1-year period in overweight Latino children and adolescents [91], underlining the relevance of reducing glucocorticoid-induced insulin resistance in ethnic minority populations.
Prolonged glucocorticoid exposure also leads to weight gain and visceral fat accumulation [92–94], not only through behavioral pathways such as increased food consumption [92, 95, 96] and sedentariness [97–100] but also directly via the release of neuropeptide Y [93, 96]. Several longitudinal studies have reported a positive association between psychological stress and BMI in adults [101, 102]. Another study reported that higher levels of psychological stress over a 10-year period predicted significantly greater increases in BMI over time compared to lower levels of stress, and this relationship was significantly stronger for African American compared to Caucasian girls [103]. In Latino youth, a significant association between cortisol, total fat mass, and visceral fat accumulation has not found [91], suggesting that the mechanisms by which cortisol induced obesity and insulin resistance may differ by ethnicity.
In addition to responding to stressful events, the HPA axis also follows a strong circadian rhythm [78, 104]. Typically, cortisol levels are high upon waking; reach a peak about 30–40 min after waking; and then decline throughout the remainder of the day, reaching a nadir around midnight [104, 105]. The scientific literature examining ethnic differences in cortisol is not extensive but demonstrates divergent diurnal cortisol patterns for African Americans compared to Caucasians [106–110]. African Americans tend to have flatter diurnal cortisol slopes, with lower morning levels and higher evening levels, than Caucasians [106–110]. These findings have been replicated across studies of adolescents [107], pregnant women [110], adults [108, 111], and elderly populations [109]. Two studies examining ethnic differences in cortisol diurnal patterns in normal-weight African American, Latino, and Caucasian children and adolescents also reported flatter morning-to-evening cortisol slopes among African Americans and lower evening cortisol levels for Latinos relative to Caucasians [107, 112]. Deviations from the typical diurnal patterns have important implications for insulin resistance [113]. Specifically, flattened diurnal patterns previously reported in chronically stressed individuals are associated with insulin resistance and cancer-related metabolic risk factors (i.e., inflammation) [113]. Hence, greater exposure to psychosocial and environmental stressors (e.g., socioeconomic burden and racial discrimination) in African American populations may contribute to the increased obesity and insulin resistance, hyperinsulinemia, and subsequent cancer risk in this population.
Hyperinsulinemia and the IGF-1 Pathway
The direct effects of insulin resistance on cancer risk are unclear and likely do not solely explain the increased cancer risk among African Americans compared to Latinos and Native Americans since all three ethnic minority groups appear to be similar in degree of insulin resistance. Accordingly, the effect of insulin resistance is postulated to be mediated by the effects of chronic hyperinsulinemia on insulin-like growth factor (IGF)-1 bioactivity [23]. IGF-1 is a growth factor that is regulated by growth hormone levels [114, 115], present in circulation, and has insulin-like properties and functions [116]. The bioactivity of IGF-1 is determined by the circulating IGF-1 and IGF-binding protein (BPs) produced by the liver as well as paracrine effects of IGF-1, IGFBPs, and IGFBP proteases [23]. Insulin can also affect IGF-1 bioactivity via increasing IGF-1 secretion, IGF-1/IGFBP-3, IGFBP-3 proteolysis, and secretion of IGFBP-1 and IGFBP-2 and increased responsiveness of cells to IGF-1 and other growth factors. Numerous studies suggest that high level of IGF-1 is a risk factor for several cancers including breast, prostate, colon, and lung cancer [117–122].
IGF-1 bioactivity has been implicated in carcinogenesis as a function of its ability to stimulate the differentiation and proliferation of myoblasts as well as inhibit apoptosis [38]. Moreover, increasing evidence suggests that chronic hyperinsulinemia increases the risk of colon and endometrial cancer [20]. Thus, chronic exposure to high levels of insulin and IGF-1 is hypothesized to mediate many cancer risk factors [23], and as a result the IGF/insulin system has been suggested as a potential target for cancer therapy [37].
While obesity status is known to correlate with serum IGF-1 levels [123, 124], studies have reported an independent effect of ethnicity on IGF-1 bioactivity in children and adults, potentially explaining ethnic specific differences in cancer risk. Previous research has reported higher levels of IGF-1 and IGFBP-3 in African Americans compared to Caucasian and Latino adults, independent of adiposity [125]. Another study reported race by gender differences where African American females had higher IGF-1 levels compared to Caucasians with similar IGF-1 levels in males in both ethnic groups [126]. The lower IGF-1 levels in Latinos relative to African American have also been shown in prepubertal females [127].
It is important to note that previous studies have been inconsistent with respect to the relationships between obesity and circulating levels of IGF-1 [128]. Studies among healthy adults have reported a null association [129–131], a positive association [132], an inverse association [128, 133–135], and a nonlinear association [136, 137] between BMI and IGF-1 levels. However, data from studies examining ethnic differences in the relationship between obesity and circulating IGF-1 have shown more consistent trends and may help to explain the abovementioned inconsistencies in obesity–IGF relationships. In a multiethnic cohort study of 200,000 adults in Los Angeles and Hawaii, researchers reported a decline in plasma IGF-1 levels with increasing BMI in Latinos and Asians; this decline was attenuated in Caucasians and absent in African Americans [138]. After adjustment for age and BMI, African Americans had the highest IGF-1 bioactivity compared to other ethnic groups. Taken together, there appears to be a progressive increase in IGF-1 levels with increasing obesity status in African Americans compared to a decline in IGF-1 with increasing obesity in other ethnic minority groups, particularly Latinos.
Ethnic differences in IGF-1 bioactivity among children are generally similar to those observed in adults. It has been shown that African American prepubertal females have higher IGF-1 levels compared to Caucasian and Latino females [125, 127]. An inverse relationship between IGF-1 and IGFBP-3 with total fat mass and body fat distribution has been reported in overweight Latino children, whereas others have demonstrated a positive association between total body fat and IGF-1 levels in both African American and Caucasian children [139, 140]. These findings were not explained by diet, physical activity, socioeconomic status, or adiposity but were related to the degree of African admixture [141], suggesting a potential genetic basis for this difference. Taken together, these results demonstrate that African American children and adults have the highest levels of IGF-1 and exhibit a positive relationship between IGF-1 and obesity, likely contributing to the increased risk of obesity-related cancers in this population.
A possible biological mechanism mediating the association between obesity and IGF-1 may be through the effect of growth hormone. Typically, obesity results in lower circulating IGFBP-1 and IGFBP-2 levels, leading to an increased negative feedback by free IGF-1 on pituitary growth hormone secretion and a decreased IGF-1 synthesis [142]. Given the positive association between obesity and IGF-1 levels in African Americans, it is possible that the growth hormone–IGF axis may be regulated differently in this population compared to other ethnic groups. Another possible mechanism may be through the effects of cortisol on IGF-1 and growth hormone levels. IGF-1 is mainly derived from the liver, which also is the sole site of splanchnic cortisol production, which suggests a close interaction between cortisol and IGF-1 [143]. Previous research has reported a negative association between cortisol and IGF-1 in obese Latino children and adolescents [80]. Hence, high cortisol and low IGF-1 may act in concert to reduce cancer risk in Latino children and adolescents. A final mechanism centers on the relationship between IGF-1, IGFBP-1, and body fat distribution. A recent study identified a modifying effect of ethnicity on the relationship between IGF-1 and subcutaneous fat as well as IGFBP-1 and hepatic fat in overweight African American and Latino adolescents, respectively [144]. IGF-1 and IGFBP-1 were inversely correlated with BMI, total fat mass, visceral fat, and hepatic fat, while IGFBP-1 was inversely correlated with subcutaneous fat. These relationships did not differ by ethnicity; however, the relationship between IGF-1 and subcutaneous fat, as well as IGFBP-1 and hepatic fat, was stronger in African Americans compared to Latinos [144]. These results suggest that the relationship between IGF-1, IGFBP-1, and body fat distribution differs among African American and Latino adolescents, which may contribute to the higher IGF-1 levels and subsequent cancer risk in African Americans. Hence, a more in-depth discussion regarding the role of body fat distribution and its association with cancer risk is given in the section below.
Body Fat Distribution
Visceral Fat
The location of body fat is important, especially with regard to how it might affect insulin resistance. Visceral fat (adipose tissue inside the abdominal cavity) in particular has been hypothesized to be one of the major factors linking increased obesity to increased insulin resistance and subsequent cancer risk mainly due to the effects of free fatty acids released from visceral fat into the hepatic portal vein with direct exposure to the liver [145]. In addition, several studies have found that insulin sensitivity is negatively associated with adipose stores in the abdominal region [146–151], particularly visceral fat, and this is consistent across age and ethnicity [152, 153], with one notable exception [154]. Increases in visceral adipose tissue in Native American adults do not explain the greater insulin resistance and hyperinsulinemia in this population when compared to equally obese Caucasians [154].
Emerging evidence however suggests that there are ethnic differences in the relationships between BMI, waist circumference, percent body fat, and visceral fat. Much research has focused on comparisons between Caucasians and Asians, with greater visceral fat in Southeast Asian women compared with their Caucasian counterparts even at the same BMI [155–158]. In addition, Latino children and adults also have greater visceral fat compared to similarly obese Caucasians [146, 159]. In contrast, several studies have reported lower amounts of visceral fat for a given waist circumference, BMI, or waist-to-hip ratio in African American compared to Caucasian women [152, 160–163]. One study confirmed similar BMIs and waist circumference measurements in middle-aged and older African American men and women compared with Caucasians and Latinos but lower visceral fat (total visceral fat and measured at the L4L5 spinal level) in African Americans. Other studies confirmed these findings and consistently reported ethnic differences in fat distribution between African Americans and Caucasians even after significant weight gain [279] and weight loss [164, 165]. Moreover, these differences are evident before puberty, both cross-sectionally and longitudinally, with a lower growth-related increase in visceral adipose tissue in African Americans compared to Caucasians [166, 167]. Taken together, these data suggest that visceral fat is associated with insulin resistance; however, the lower volumes of visceral fat previously reported in African Americans do not appear to explain the greater insulin resistance and subsequent cancer risk in this population. On the other hand, African Americans tend to have more subcutaneous fat, which may provide a better explanation for ethnic differences in cancer-related outcomes.
Subcutaneous Fat
Although some studies suggest that visceral fat plays a larger role in the development of insulin resistance [146, 147], other studies in adults suggest that subcutaneous fat has a significant impact on metabolic disease risk given its larger volume and functional characteristics, making it more susceptible to inflammation and subsequent deposition of ectopic fat [149, 168]. More specifically, subcutaneous fat has two distinct compartments, the deep and superficial depots, which differ in their contribution to metabolic disease risk [169, 170]. For example, a study in lean and obese adults found that deep subcutaneous fat and visceral fat, but not superficial subcutaneous fat, were inversely correlated with insulin sensitivity as measured by euglycemic clamp [169]. At the same time, recent studies have identified ethnic differences in the distribution of deep and superficial subcutaneous fat with Asians reporting the lowest BMI, but the largest accumulation of visceral fat and deep subcutaneous fat when compared to Caucasian, African American, and Latino adults [171–174]. In another study, higher amounts of deep subcutaneous fat were reported in Native American and Asian adults compared to Caucasians [172]. With respect to African Americans, higher levels of subcutaneous fat have been consistently reported across populations of African descent including residents in the USA, the Caribbean, South America, or Europe [175]. Taken together, these findings suggest that ethnic differences in deep and superficial subcutaneous fat could partially explain ethnic differences in insulin sensitivity and secretion. More importantly, the greater volumes of subcutaneous fat and the previously reported stronger relationship between this fat depot and IGF-1 in African Americans offer another potential explanation for the greater insulin resistance and cancer risk previously reported in this ethnic group.
Intramyocellular Lipid
More recently evidence suggests that fat deposition outside of adipose tissue (e.g., in muscle, liver, or pancreas) contributes to increased insulin resistance [176–183]. Intramyocellular lipid, for example, has been shown to be a major determinant of insulin resistance in adults [179], obese individuals [176, 178], and obese adolescents [183]. Several studies have also reported an inverse relationship between intramyocellular lipid and insulin sensitivity in inactive individuals, independent of total body fat in both animal [184] and human models [185]. Reductions in intramyocellular lipid content have also been implicated in the improvements of insulin sensitivity in response to a short-term hypocaloric diet in both normoglycemic and type 2 diabetic patients [186]. Similar improvements in insulin sensitivity have also been observed in parallel with intramyocellular lipid depletion in morbidly obese subjects after surgical treatment of obesity [187]. These findings highlight the importance of intramyocellular lipid as a metabolically active fat depot that influences insulin resistance independent of total body fat.
Few studies have examined ethnic differences in intramyocellular lipid in adults. One study in Asian and Caucasian men reported higher intramyocellular lipid content in Asians compared to age- and BMI-matched Caucasians [178]. Interestingly, intramyocellular lipid in Asians was not related to insulin sensitivity or adiposity; this relationship was present in Caucasians [178]. Similar differences by ethnicity were reported between African Americans and Caucasians, with intramyocellular lipid content related to insulin sensitivity and adiposity in Caucasians, but not African Americans [188]. Another study in Native Americans also noted that intramyocellular lipid did not predict a reduction in peripheral or hepatic insulin sensitivity [189]. Hence, intramyocellular lipid content does not appear to explain or contribute to the increased insulin resistance in ethnic minority adults. To date, the relationship between intramyocellular lipid content and insulin sensitivity in Latino adults has not been studied.
Many more ethnic comparison studies of intramyocellular lipid content have been conducted in overweight and obese youth. One recent report demonstrated that African Americans and Latinos have more intramyocellular lipid than Caucasians, even after controlling for BMI and visceral fat [181]. Another study in African American, Latino, and Caucasian children observed an inverse relationship between intramyocellular lipid and markers of inflammation; however, the majority of these relationships were eliminated after controlling for BMI and subcutaneous and visceral fat [181], suggesting that other fat depots may be more strongly associated with low-grade inflammation and insulin resistance in ethnic minority groups. To our knowledge there are no studies examining intramyocellular lipid in Native American or Asian children. Taken together, these studies suggest that increases in intramyocellular lipid may contribute to insulin resistance in an ethnic specific manner; however, the documented correlation between intramyocellular lipid, subcutaneous, visceral, and hepatic fat makes it difficult to tease apart the exact influence of each fat depot [177, 181, 190, 191]. Hence, additional studies comparing the contribution of intramyocellular, subcutaneous, and visceral fat are warranted to better understand the relationship between body fat distribution and observed ethnic differences in insulin resistance and subsequent cancer risk in ethnic minority populations.
Hepatic Fat
Numerous studies have documented inverse associations between hepatic fat, insulin sensitivity, and pancreatic beta-cell function [171, 192–197]. In a previous study of normal-weight, overweight, and obese Caucasian adolescents, those with hepatic steatosis had lower insulin sensitivity and a twofold greater prevalence of metabolic syndrome compared to those without hepatic steatosis [196]. In another study in both Canadian Caucasian and Native American adolescents, those with type 2 diabetes had higher hepatic fat compared to those without type 2 diabetes; moreover, hepatic fat was negatively associated with insulin sensitivity [197]. A US study that included Caucasian, African American, and Asian adolescents found that obese adolescents with nonalcoholic fatty liver disease (NAFLD) had a lower pancreatic beta-cell function compared to those who were obese and without NAFLD [193]. Others have confirmed these relationships in obese Latino adolescents where those with elevated hepatic fat (>5.5 %) had a significantly lower insulin sensitivity and higher acute insulin response to intravenous glucose compared to those with lower hepatic fat [192]. These results suggest that hepatic fat is associated with metabolic abnormalities including insulin resistance and the deleterious effects of hepatic fat on insulin resistance appear consistent across different ethnic groups [171, 194, 195, 198, 199].
When making ethnic comparisons of hepatic fat content, similar to visceral fat, both African American adolescents and adults have lower amounts of hepatic fat compared to Latinos and Caucasians [200–202]. Nevertheless, the relationship between hepatic fat and insulin resistance appears to be stronger in this ethnic group. In one study, hepatic fat, not visceral fat, was inversely associated with insulin sensitivity and the effect of high hepatic fat (>5.5 %) compared to low hepatic fat was more pronounced in African American compared to Latino children [192]. In Latinos, high hepatic fat was associated with a 24 % lower insulin sensitivity, whereas in African Americans, high hepatic fat was associated with a 49 % lower insulin sensitivity [195]. These results suggest a stronger relationship between hepatic fat and insulin resistance in African Americans. Similar studies have not been performed in children belonging to other ethnic groups. Taken together, these findings suggest that for African Americans who have greater volumes of hepatic fat, this depot may contribute to increased insulin resistance. However, for the majority of African Americans who tend to have extremely low volumes of hepatic fat, this depot is not likely to be a major contributor to the increased insulin resistance and subsequent cancer risk in this population.
Pancreatic Fat
Accumulation of fat in the pancreas has also been associated with insulin resistance and hyperinsulinemia in both normal-weight and obese/type 2 diabetic individuals; this relationship appears to be independent of total body fat [195, 199, 203]. Moreover, pancreatic fat has been used as a marker of pancreatic beta-cell dysfunction, especially in Latinos [199]. A recent study examining ethnic differences in pancreatic fat determined that when comparing Caucasian, African American, and Latino adults at similar levels of adiposity, Latinos had a twofold greater volume of pancreatic fat compared to African Americans; Latinos and Caucasians had similar levels of pancreatic fat [199].
Studies in children and adolescents are limited, and no studies to date have been conducted in Asians or Native Americans. In African American and Latino overweight and obese adolescents and young adults [195, 198], one study reported greater hepatic and pancreatic fat volumes in those with prediabetes compared to those with normal glucose tolerance [195]. However, pancreatic fat predicted prediabetes in African Americans whereas hepatic fat predicted prediabetes in Latinos [195]. These results suggest that ethnic differences in the relationship between ectopic fat depots and metabolic disease risk are present with pancreatic fat playing a larger role in the metabolic abnormalities previously reported in African Americans. Of note, visceral, hepatic, and pancreatic fat are highly correlated; hence, future studies should aim to examine fat depots in an effort to elucidate the exact contributions of each fat depot, particularly pancreatic fat, to the increases in insulin resistance and subsequent cancer risk in African American populations.
Adipose Tissue Biology
There is increasing evidence to suggest that differences in body fat accumulation and patterning may result from fundamental differences in adipose tissue biology [145, 204]. The increase in body fat content with obesity can occur by either an increase in adipocyte cell size or number or the spillover of triglycerides to ectopic tissues [145, 204]. When adipocyte cell size increases with progressing obesity, it is an indication of the inability of adipocytes to expand in number to accommodate the extra triglyceride accumulation [204]. Increased adipocyte cell size is also related to greater insulin resistance independent of total body fat [67]. Larger adipocytes have also been shown to be associated with more lipid deposition in visceral and hepatic fat depots (but not muscle), and this may also contribute to insulin resistance [205]. Furthermore, it is now evident that adipose tissue is infiltrated with macrophages [206]. One animal study has shown that accumulation of excess body fat in response to excess caloric intake leads to increasing fat cell size and then to adipocyte death, with the excess fat deposited in the liver [207].
Despite the important role that adipose tissue biology appears to play in the link between obesity, insulin resistance, and related cancer risk, there are no studies to date examining potential ethnic differences in the metabolic risk factor. Some studies have compared adipocyte cell size in African Americans and Caucasians but have not shown any difference in subcutaneous abdominal or gluteal adipocytes from obese women [208]. There are no data in the literature comparing ethnic differences in adipose tissue biology in Latinos and the potential relationship between adipocyte cell size and spillover of triglycerides to other ectopic storage depots like liver and pancreas. It is plausible that Latinos may have larger fat cells than African Americans that are more likely to die due to greater macrophage infiltration, thus leading to the greater likelihood of ectopic fat accumulation in Latinos. On the other hand, the higher circulating IGF-1 present in African Americans may contribute to a greater likelihood for adipocyte proliferation during obesity [209], leading to less likelihood for spillover of fat into ectopic depots; the opposite scenario is present in Latinos (lower obesity-related IGF-1 profile). Thus, differences in the obesity–IGF pathway and adipocyte differentiation/growth factor pathways may also elucidate mechanisms explaining ethnic differences in body fat accumulation, body fat patterning, and subsequent cancer risk; additional research is warranted.
Adipose Tissue Inflammation
In conjunction with the accumulation and distribution of fat throughout the body, another potential explanation for ethnic differences in insulin resistance and subsequent cancer risk involves inflammation. Studies have shown that obesity is associated with a state of chronic low-grade inflammation, which is correlated with increased insulin resistance, and impaired glucose metabolism [210–213]. Although it was once believed that adipose tissue was only involved in the storage of free fatty acids as triglycerides, researchers now recognized that this tissue also acts as a dynamic endocrine organ, contributing to the chronic low-grade inflammation seen during obesity. For instance, during excess weight gain there is a marked increase in adipose tissue inflammation, which has been shown to be associated with insulin resistance seen during obesity [214]. Obesity is characterized by elevated circulating levels of acute-phase proteins, for example leptin, tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, and decreased adiponectin [215]. Although the cause and effect nature of these proteins on insulin action is not clear, it has been suggested that these inflammatory markers affect disease processes in part by causing or exacerbating insulin resistance. Epidemiologic studies have demonstrated a positive association between acute-phase proteins and insulin resistance [216]. For example, leptin serves as part of an “adipostat” mechanism, whereby increased fat mass sets in motion responses that will eventually reduce adiposity. Hence, the reduced responsiveness to leptin that accompanies obesity may play a role in causing obesity and also contribute to insulin resistance [217, 218]. Another example is TNF-alpha, which has been shown to impair insulin signaling by activating serine/threonine kinases in skeletal muscle and downregulate glucose transporter type 4 (GLUT 4) in adipose tissue [216]. Circulating levels of IL-6 increase hepatic glucose production and stimulate the release of free fatty acids; however IL-6 also appears to have anti-inflammatory actions since it decreases TNF-alpha [219]. Adiponectin is exclusively produced in adipose tissue, and in humans its production is slightly higher in subcutaneous fat than visceral fat [220]. Adiponectin levels are negatively correlated with BMI and body fat, and this protein has been shown to play a role in hepatic insulin sensitivity and whole-body metabolism [221]. Both experiments in humans [222] and in animals [223] have demonstrated that low-grade inflammation predicts the development of insulin resistance.
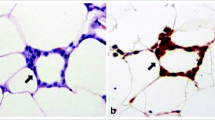
Recent studies have also examined low-grade inflammation from adipose tissue biopsies in young adults. Specifically, subcutaneous adipose tissue biopsies performed in Caucasian, African American, Latino, and Native American adults have shown that in addition to elevations in plasma markers of inflammation, increases in pro-inflammatory immune cells in adipose tissue are associated with systemic and local inflammation [224–227]. In another study, subcutaneous adipose tissue inflammation was assessed by the presence of crown-like structures in obese African American and Latino young adults. Individuals with subcutaneous adipose tissue inflammation had greater levels of visceral fat, hepatic fat, TNF-alpha, and fasting insulin and glucose and a lower beta-cell function compared to those without subcutaneous inflammation [226].
Although there are no studies in children involving adipose tissue biopsies, one study in obese youth observed macrophages and lymphocytes in perivascular positions in the adipose tissue [228] while another study in children found macrophages in the subcutaneous adipose tissue of normal-weight, overweight, and obese children as young as 5 years of age [229]. Studies using plasma markers of inflammation have also found strong associations with insulin resistance in overweight and obese youth from various ethnic groups. For example, a study in boys found that those who were overweight had higher serum levels of IL-6, IL-8, interferon-γ, monocyte chemoattractant protein (MCP)-1, and C-reactive protein (CRP) compared to those of normal weight [230]. Compared to normal-weight Latino children, higher levels of CRP and IL-1beta were reported in obese Latino children [210]. Another study in African American and Latino peripubertal females demonstrated that CRP was positively related to BMI, percent body fat, fasting insulin, and acute insulin response to glucose as well as negatively correlated with insulin sensitivity [211]. One of the few recent studies including Asian children found that, after controlling for adiposity, Asians had higher levels of CRP, A1C, and insulin levels compared to Caucasian and African American children [213]. To our knowledge, there is only one study examining inflammation in Native American children. This study found elevated levels of CRP that were associated with increased adiposity, insulin resistance, worsening lipid profile, and decreased adiponectin levels [231]. Findings from these studies in children suggest that obesity is accompanied by chronic levels of low-grade inflammation starting at an early age into adulthood, possibly contributing to increased insulin resistance in these populations.
There are only sparse data on inflammatory profiles in multiethnic cohorts in the USA. These studies suggested that inflammation may be higher in African Americans [232–234], although not all studies showed this trend [235]. Specifically, CRP concentrations were higher in African Americans than in Caucasians in several large studies [232, 234, 236]. The Women’s Health Study reported higher levels of CRP in African Americans than in Caucasians [232]. In contrast, NHANES data did not show this trend and instead observed higher CRP in Latina women compared with Caucasians [237]. In another study that measured visceral fat, the negative association between visceral adipose tissue and adiponectin was stronger in African Americans [237]. However, overall body fatness may still have played a role in inflammation because subcutaneous fat also had significant independent association with CRP in this ethnic group. Of note, African American women consistently exhibited greater markers of inflammation even after controlling for both L4L5 visceral and subcutaneous fat [159]. More importantly, the greater inflammation among these African American women was present despite similar or lower self-reported rates of smoking and similar or higher self-reported rates of taking lipid-lowering medications and nonsteroidal anti-inflammatory drugs [159]. The mechanisms contributing to greater low-grade inflammation in African Americans are unclear, but possibilities include higher intrinsic activity of cytokine pathways and/or different behavioral influences (i.e., high-fat diet and physical inactivity) on inflammation.
Aside from intrinsic cytokine production pathways, lifestyle factors such as diet or exercise may play a role in the altered visceral fat/body fat–inflammatory biomarker relationship. An observational study found that diets high in glycemic load were associated with increased concentrations of inflammation and that the dose–response gradient between glycemic load and inflammation was more exaggerated in overweight women [238]. Other dietary factors that have been shown to increase low-grade inflammation include sucrose, artificial sweeteners, fats, and processed meats [239]. In contrast, fiber, fruits, and vegetables have been associated with reduced inflammation [240]. Previous research has reported eating patterns reflecting higher consumption of fat and calories and lower consumption of fruits and vegetables in African Americans [241], which may contribute to the greater inflammation in this ethnic group. Moreover, African American women in particular have been shown to have lower rates of physical activity participation compared to Caucasians [242–245], which may independently contribute to inflammation. Hence, studies examining whether ethnic differences in exercise or dietary patterns account for the altered visceral fat–inflammation relationships among African Americans are warranted to better understand the increased cancer risk in this population.
Non-esterified Fatty Acids
Studies in obese adults have documented a relationship between adipose tissue insulin resistance and non-esterified fatty acids (NEFA) [246]. Given that increased hepatic fat, intramyocellular lipid [247, 248], and inflamed adipose tissue [249] are associated with increased whole-body insulin resistance, it is possible that NEFA play a mediating role in the link between ethnic differences in ectopic fat, inflammation, and insulin resistance. However, most of the research in this area has been conducted in children. Studies in overweight and obese youth have observed elevations in fasting NEFA and NEFA levels after an oral glucose or intravenous lipid challenge. Longitudinal data has confirmed an inverse relationship between fasting NEFA and insulin secretion following a 30-min oral glucose challenge in children with normal glucose tolerance [250]. Other researchers have shown that when compared to normoglycemic Latino children, those with prediabetes had higher fasting NEFA that were also inversely related to insulin secretion [195].
The earliest work in this field with regard to ethnicity first showed that after an intravenous lipid infusion, elevations in NEFA were associated with increased insulin resistance in African American and Caucasian adolescents [251]. Of note, ethnicity did not modify the relationship between NEFA and insulin resistance despite lower insulin sensitivity in African Americans compared to Caucasians [251]. Another study reported ethnic differences in NEFA during an intravenous glucose tolerance test [181, 252]. Independent of insulin secretion, African American women and girls had lower NEFA than Caucasian women and girls [181, 252]. To our knowledge, there are no studies examining these relationships in Asian or NA children, warranting their inclusion in future studies. Hence, NEFA contributes to insulin resistance and ethnicity does not appear to modify this relationship. However, African Americans tend to have lower NEFA suggesting that this mechanism does not explain the increased insulin resistance and subsequent cancer risk in this population.
Oxidative Stress
The potential role of oxidative stress in carcinogenesis is rapidly evolving, which may also link obesity and insulin resistance to increased cancer risk. Oxidative stress occurs when there is excessive production of reactive oxygen species (ROS) or insufficient in vivo antioxidant defense mechanisms [253]. This results in damage to DNA as well as lipid peroxidation, protein modification, membrane disruption, and mitochondrial damage [218, 254]. Data support the notion that increased formation of ROS may play an important role in carcinogenesis as well as atherosclerosis, diabetes, and neurodegenerative diseases [255]. Although ROS-induced lipid peroxides are usually described as harmful to cellular systems, they are also critical mediators of apoptosis [256] and have been shown to inhibit cancer growth in a number of experimental studies [257]. More specifically, factors that increase lipid peroxidation could also increase cancer and other degenerative diseases in people with innate or acquired high levels of ROS. However, factors that increase lipid peroxidation can increase apoptosis of precancerous and cancerous cells and thus protect against cancer, particularly in people with a low innate baseline level of ROS [256]. Thus, antioxidants may protect against certain cancers if background levels of ROS are higher in “at-risk” populations, but not if background ROS levels are lower because this may place a greater importance on the suppression of oxidation-induced apoptosis [256].
The relationship between obesity, insulin resistance, and oxidative stress has not been widely explored, but some supporting evidence suggests a link. Obese adults have elevated levels of lipid peroxidation that is reversible with weight reduction [255]. Metabolic conditions associated with insulin resistance are associated with elevated lipid peroxidation, including hypertension [255], impaired glucose tolerance [258, 259], and type 2 diabetes [258, 260–268]. In addition, increased oxidized low-density lipoprotein or susceptibility to oxidation has been reported in patients with type 2 diabetes [261, 262, 265, 268, 269]. Small dense low-density lipoprotein particles, which are also a component of the metabolic syndrome, are more susceptible than larger ones to oxidative modification [270, 271]. Finally, lipid peroxidation and oxidative stress, induced by elevations in glucose and possibly free fatty acid levels, may play a key role in causing insulin resistance by their ability to activate stress-sensitive signaling pathways [272].
Relatively few studies have compared lipid peroxidation and oxidative stress in different ethnic groups. In adults with type 2 diabetes, increased levels of lipid peroxidation were found in African Caribbeans compared to Caucasians [273]. Previous work showed greater lipid peroxidation in Latinos compared to Caucasians with [274] and without type 2 diabetes [275]. In another study, lipid peroxidation was higher in African Americans than in Caucasians during hyperlipidemia induced by lipid infusion [276]. Of note, recent data from the multiethnic IRAS cohort reported lower urinary F2-isoprostane levels, a marker of lipid peroxidation, among African American compared with Caucasians and Latinos [277, 278]. When stratified by BMI, ethnic differences in F2-isoprostance levels were not observed among participants with normal BMI but appeared among overweight participants and increased among obese participants [278]. Hence, additional studies comparing the markers of oxidative stress are warranted to better understand its potential contributions to ethnic differences in cancer risk.
Summary and Conclusions
Obesity is a predisposing risk factor for certain forms of cancer, and the link between obesity and cancer appears to be particularly complex. Obesity is associated with increased insulin resistance, and hyperinsulinemia may play a critical role in influencing cancer risk. It is notable that obesity-related cancer risk differs dramatically by ethnicity. African Americans appear particularly prone to obesity-related cancers including prostate, breast, and colorectal and myeloma, whereas Latinos appear relatively protected. Based on previous literature, it is plausible that ethnic differences in the insulin response to obesity may contribute to ethnic differences in obesity-related cancer profiles. Obese Latinos seem more prone to an ectopic fat pattern (increased visceral, hepatic, and pancreatic fat), and this might be driven by greater fat cell size, greater likelihood of adipocyte macrophage infiltration and cell death, and decreased capacity for fat cells to differentiate, possibly due to a lower obesity-related IGF-1 profile. On the other hand, obese African Americans seem more prone to some forms of obesity (subcutaneous fat pattern) and insulin-related cancers compared to Latinos and have less likelihood of ectopic fat. These differences could be driven by the much higher obesity-related hyperinsulinemia (especially in response to glucose) and IGF-1 profile in African Americans. This is important because it suggests that reducing levels of insulin in obesity in this population as a strategy to prevent obesity-related cancers may have the unwanted side effect of reducing fat cell proliferation and promotion of hepatic fat, and other ectopic fat deposition, unless it is combined with behavioral interventions to influence energy balance (reduce energy intake and increase physical activity) and subsequent weight status. Additional factors that contribute to increased insulin resistance and cancer risk in African Americans include chronic glucocorticoid exposure, chronic inflammation, and possibly greater oxidative stress. Hence, additional therapies that reduce multiple cancer-related metabolic risk factors in African American children and adults are warranted.
In summary, the causes and consequences of obesity and insulin resistance differ by ethnicity of people and much more work is needed to establish the specific mechanisms linking obesity and insulin to various cancer outcomes. These mechanistic issues are fundamental to understanding the basic pathophysiology of why increased body fat and hyperinsulinemia are related to cancer outcomes in some ethnic groups but not others and will ultimately have widespread implications for the application of more individualized prevention and treatment approaches to reduce the disparity in obesity-related cancers.
References
Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307:491–497
Barnes PM, Adams PF, Powell-Griner E (2010) Health characteristics of the American Indian or Alaska Native adult population: United States, 2004–2008 National health statistics reports, no. 20. National Center for Health Statistics, Hyattsville, MD
Barnes PM, Adams PF, Powell-Griner E (2008) Health characteristics of the Asian adult population: United States, 2004–2006. Advance data from vital and health statistics; no 394. National Center for Health Statistics, Hyattsville, MD
Narayan KM, Aviles-Santa L, Oza-Frank R, Pandey M, Curb JD, McNeely M, Araneta MR, Palaniappan L, Rajpathak S, Barrett-Connor E, Cardiovascular Disease in A, Pacific Islander Populations NWG (2010) Report of a National Heart, Lung, And Blood Institute Workshop: heterogeneity in cardiometabolic risk in Asian Americans in the U.S. opportunities for research. J Am Coll Cardiol 55:966–973
Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 307:483–490
Broussard BA, Johnson A, Himes JH, Story M, Fichtner R, Hauck F, Bachman-Carter K, Hayes J, Frohlich K, Gray N et al (1991) Prevalence of obesity in American Indians and Alaska Natives. Am J Clin Nutr 53:1535S–1542S
Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS (2009) Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 32:287–294
Goran MI, Bergman RN, Cruz ML, Watanabe R (2002) Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 25:2184–2190
Moore K (2010) Youth-onset type 2 diabetes among American Indians and Alaska Natives. J Public Health Manag Pract 16:388–393
Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF (2003) Lifetime risk for diabetes mellitus in the United States. JAMA 290:1884–1890
Nsiah-Kumi PA, Lasley S, Whiting M, Brushbreaker C, Erickson JM, Qiu F, Yu F, Larsen JL (2013) Diabetes, pre-diabetes and insulin resistance screening in Native American children and youth. Int J Obes (Lond) 37:540–545
Writing Group for the SfDiYSG, Dabelea D, Bell RA, D’Agostino RB Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B (2007) Incidence of diabetes in youth in the United States. JAMA 297:2716–2724
Schiller JS, Lucas JW, Ward BW, Peregoy JA (2012) Summary health statistics for U.S. adults: National Health Interview Survey, 2010. National Center for Health Statistics. Vital Health Stat 10(252)
American Diabetes Association (2000) Type 2 diabetes in children and adolescents. Pediatrics 105:671–680
Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J (2002) Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51:3014–3019
Hannon TS, Bacha F, Lin Y, Arslanian SA (2008) Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes Care 31:1445–1447
Ku CY, Gower BA, Hunter GR, Goran MI (2000) Racial differences in insulin secretion and sensitivity in prepubertal children: role of physical fitness and physical activity. Obes Res 8:506–515
Lindquist CH, Gower BA, Goran MI (2000) Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. Am J Clin Nutr 71:725–732
IARC Working Group (2005) IARC working group on the evaluation of cancer-preventive strategies. Breast cancer screening. IARC Press, Lyon
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591
Prentice RL, Willett WC, Greenwald P, Alberts D, Bernstein L, Boyd NF, Byers T, Clinton SK, Fraser G, Freedman L, Hunter D, Kipnis V, Kolonel LN, Kristal BS, Kristal A, Lampe JW, McTiernan A, Milner J, Patterson RE, Potter JD, Riboli E, Schatzkin A, Yates A, Yetley E (2004) Nutrition and physical activity and chronic disease prevention: research strategies and recommendations. J Natl Cancer Inst 96:1276–1287
Fuemmeler BF, Pendzich MK, Tercyak KP (2009) Weight, dietary behavior, and physical activity in childhood and adolescence: implications for adult cancer risk. Obes Facts 2:179–186
Giovannucci E (2003) Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res 35:694–704
Miller B, Kolonel LN, Bernstein L, Young JJ, Swanson G (1996) Racial/ethnic patterns of cancer in the United States 1988-1992. National Cancer Institute, Bethesda, MD
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2014) SEER cancer statistics review, 1975–2011. National Cancer Institute, Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014
Boyle P (2012) Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 23 Suppl 6:vi7–vi12
Kolonel LN, Altshuler D, Henderson BE (2004) The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer 4:519–527
Caprio S, Hyman LD, Limb C, McCarthy S, Lange R, Sherwin RS, Shulman G, Tamborlane WV (1995) Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol 269:E118–E126
Goran MI, Bergman RN, Gower BA (2001) Influence of total vs. visceral fat on insulin action and secretion in African American and white children. Obes Res 9:423–431
Gower BA, Nagy TR, Goran MI (1999) Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes 48:1515–1521
Goran MI, Ball GD, Cruz ML (2003) Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab 88:1417–1427
Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D (1984) Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest 74:1238–1246
Cutfield WS, Bergman RN, Menon RK, Sperling MA (1990) The modified minimal model: application to measurement of insulin sensitivity in children. J Clin Endocrinol Metab 70:1644–1650
Hershko DD (2008) Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer 112:1415–1424
Auld CA, Caccia CD, Morrison RF (2007) Hormonal induction of adipogenesis induces Skp2 expression through PI3K and MAPK pathways. J Cell Biochem 100:204–216
Cooke PS, Holsberger DR, Cimafranca MA, Meling DD, Beals CM, Nakayama K, Nakayama KI, Kiyokawa H (2007) The F box protein S phase kinase-associated protein 2 regulates adipose mass and adipocyte number in vivo. Obesity (Silver Spring) 15:1400–1408
Gray SG, Stenfeldt Mathiasen I, De Meyts P (2003) The insulin-like growth factors and insulin-signalling systems: an appealing target for breast cancer therapy? Horm Metab Res 35:857–871
Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4:505–518
Cheney KE, Liu RK, Smith GS, Leung RE, Mickey MR, Walford RL (1980) Survival and disease patterns in C57BL/6J mice subjected to undernutrition. Exp Gerontol 15:237–258
Grasl-Kraupp B, Bursch W, Ruttkay-Nedecky B, Wagner A, Lauer B, Schulte-Hermann R (1994) Food restriction eliminates preneoplastic cells through apoptosis and antagonizes carcinogenesis in rat liver. Proc Natl Acad Sci U S A 91:9995–9999
Ross MH, Bras G (1965) Tumor incidence patterns and nutrition in the rat. J Nutr 87:245–260
Spindler SR (2005) Rapid and reversible induction of the longevity, anticancer and genomic effects of caloric restriction. Mech Ageing Dev 126:960–966
Tannenbaum A, Silverstone H (1953) The genesis and growth of tumors. VI. Effects of varying the level of minerals in the diet. Cancer Res 13:460–463
Volk MJ, Pugh TD, Kim M, Frith CH, Daynes RA, Ershler WB, Weindruch R (1994) Dietary restriction from middle age attenuates age-associated lymphoma development and interleukin 6 dysregulation in C57BL/6 mice. Cancer Res 54:3054–3061
Weindruch R, Walford RL (1982) Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215:1415–1418
Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D (2005) Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 82:1210–1217
Cossrow N, Falkner B (2004) Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 89:2590–2594
Donahue RP, Bean JA, Donahue RA, Goldberg RB, Prineas RJ (1997) Insulin response in a triethnic population: effects of sex, ethnic origin, and body fat. Miami Community Health Study. Diabetes Care 20:1670–1676
Haffner SM, D’Agostino R Jr, Goff D, Howard B, Festa A, Saad MF, Mykkanen L (1999) LDL size in African Americans, Hispanics, and non-Hispanic whites: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol 19:2234–2240
Haffner SM, D’Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE et al (1996) Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 45:742–748
Howard BV, Mayer-Davis EJ, Goff D, Zaccaro DJ, Laws A, Robbins DC, Saad MF, Selby J, Hamman RF, Krauss RM, Haffner SM (1998) Relationships between insulin resistance and lipoproteins in nondiabetic African Americans, Hispanics, and non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Metabolism 47:1174–1179
Kasim-Karakas SE (2000) Ethnic differences in the insulin resistance syndrome. Am J Clin Nutr 71:670–671
Osei K, Cottrell DA, Harris B (1992) Differences in basal and poststimulation glucose homeostasis in nondiabetic first degree relatives of black and white patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 75:82–86
Osei K, Schuster DP (1994) Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med 11:755–762
Palaniappan LP, Carnethon MR, Fortmann SP (2002) Heterogeneity in the relationship between ethnicity, BMI, and fasting insulin. Diabetes Care 25:1351–1357
Sirikul B, Gower BA, Hunter GR, Larson-Meyer DE, Newcomer BR (2006) Relationship between insulin sensitivity and in vivo mitochondrial function in skeletal muscle. Am J Physiol Endocrinol Metab 291:E724–E728
Bogardus C, Thuillez P, Ravussin E, Vasquez B, Narimiga M, Azhar S (1983) Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Invest 72:1605–1610
Haffner SM, Miettinen H, Gaskill SP, Stern MP (1995) Decreased insulin secretion and increased insulin resistance are independently related to the 7-year risk of NIDDM in Mexican-Americans. Diabetes 44:1386–1391
Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK (1990) Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body-fat distribution. Diabetes 39:283–288
Hasson RE, Adam TC, Davis JN, Weigensberg MJ, Ventura EE, Lane CJ, Roberts CK, Goran MI (2010) Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 95:4048–4051
Stefan N, Stumvoll M, Weyer C, Bogardus C, Tataranni PA, Pratley RE (2004) Exaggerated insulin secretion in Pima Indians and African-Americans but higher insulin resistance in Pima Indians compared to African-Americans and Caucasians. Diabet Med 21:1090–1095
Chiu KC, Cohan P, Lee NP, Chuang LM (2000) Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diabetes Care 23:1353–1358
Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C (1993) Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 329:1988–1992
Lillioja S, Mott DM, Zawadzki JK, Young AA, Abbott WG, Knowler WC, Bennett PH, Moll P, Bogardus C (1987) In vivo insulin action is familial characteristic in nondiabetic Pima Indians. Diabetes 36:1329–1335
Pratley RE, Weyer C, Bogardus C (1999) Metabolic abnormalities in the development of type 2 diabetes mellitus. In: LeRoith D, Taylor SI, Olefsky JM (eds) Diabetes mellitus: a fundamental and clinical text. Lippincott, Williams & Wilkins, Philadelphia, pp 548–557
Lillioja S (1996) Impaired glucose tolerance in Pima Indians. Diabet Med 13:S127–S132
Weyer C, Bogardus C, Mott DM, Pratley RE (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794
Freedman DS, Srinivasan SR, Burke GL, Shear CL, Smoak CG, Harsha DW, Webber LS, Berenson GS (1987) Relation of body fat distribution to hyperinsulinemia in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 46:403–410
Arslanian S, Suprasongsin C, Janosky JE (1997) Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab 82:1923–1927
Moore E, Copeland KC, Parker D, Burgin C, Blackett PR (2006) Ethnic differences in fasting glucose, insulin resistance and lipid profiles in obese adolescents. J Okla State Med Assoc 99:439–443
Goran MI, Gower BA (2001) Longitudinal study on pubertal insulin resistance. Diabetes 50:2444–2450
Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R (2007) Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 293:E1–E15
Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI (2003) Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes 52:1047–1051
Klimentidis YC, Dulin-Keita A, Casazza K, Willig AL, Allison DB, Fernandez JR (2012) Genetic admixture, social-behavioural factors and body composition are associated with blood pressure differently by racial-ethnic group among children. J Hum Hypertens 26:98–107
Francis DD (2009) Conceptualizing child health disparities: a role for developmental neurogenomics. Pediatrics 124 Suppl 3:S196–S202
Pervanidou P, Chrousos GP (2012) Metabolic consequences of stress during childhood and adolescence. Metabolism 61:611–619
Dickerson SS, Kemeny ME (2004) Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130:355–391
Adam EK (2006) Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology 31:664–679
De Vriendt T, Moreno LA, De Henauw S (2009) Chronic stress and obesity in adolescents: scientific evidence and methodological issues for epidemiological research. Nutr Metab Cardiovasc Dis 19:511–519
Adam TC, Epel ES (2007) Stress, eating and the reward system. Physiol Behav 91:449–458
Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M (1993) Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 14:303–347
Rosmond R (2003) Stress induced disturbances of the HPA axis: a pathway to Type 2 diabetes? Med Sci Monit 9:RA35–RA39
Lambillotte C, Gilon P, Henquin JC (1997) Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 99:414–423
Coderre L, Vallega GA, Pilch PF, Chipkin SR (1996) In vivo effects of dexamethasone and sucrose on glucose transport (GLUT-4) protein tissue distribution. Am J Physiol 271:E643–E648
van Raalte DH, Ouwens DM, Diamant M (2009) Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest 39:81–93
Beard JC, Halter JB, Best JD, Pfeifer MA, Porte D Jr (1984) Dexamethasone-induced insulin resistance enhances B cell responsiveness to glucose level in normal men. Am J Physiol 247:E592–E596
Grill V, Pigon J, Hartling SG, Binder C, Efendic S (1990) Effects of dexamethasone on glucose-induced insulin and proinsulin release in low and high insulin responders. Metabolism 39:251–258
Larsson H, Ahren B (1999) Insulin resistant subjects lack islet adaptation to short-term dexamethasone-induced reduction in insulin sensitivity. Diabetologia 42:936–943
Bjorntorp P (1997) Neuroendocrine factors in obesity. J Endocrinol 155:193–195
Bjorntorp P, Rosmond R (2000) Obesity and cortisol. Nutrition 16:924–936
Adam TC, Hasson RE, Ventura EE, Toledo-Corral C, Le KA, Mahurkar S, Lane CJ, Weigensberg MJ, Goran MI (2010) Cortisol is negatively associated with insulin sensitivity in overweight Latino youth. J Clin Endocrinol Metab 95:4729–4735
Dallman MF (2010) Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21:159–165
Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z (2007) Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13:803–811
Weigensberg MJ, Toledo-Corral CM, Goran MI (2008) Association between the metabolic syndrome and serum cortisol in overweight Latino youth. J Clin Endocrinol Metab 93:1372–1378
Epel E, Lapidus R, McEwen B, Brownell K (2001) Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26:37–49
Newman E, O’Connor DB, Conner M (2007) Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology 32:125–132
Boutelle KN, Murray DM, Jeffery RW, Hennrikus DJ, Lando HA (2000) Associations between exercise and health behaviors in a community sample of working adults. Prev Med 30:217–224
Ng DM, Jeffery RW (2003) Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychol 22:638–642
Steptoe A, Wardle J, Pollard TM, Canaan L, Davies GJ (1996) Stress, social support and health-related behavior: a study of smoking, alcohol consumption and physical exercise. J Psychosom Res 41:171–180
Stetson BA, Rahn JM, Dubbert PM, Wilner BI, Mercury MG (1997) Prospective evaluation of the effects of stress on exercise adherence in community-residing women. Health Psychol 16:515–520
Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ (2009) Psychosocial stress and change in weight among US adults. Am J Epidemiol 170:181–192
Brunner EJ, Chandola T, Marmot MG (2007) Prospective effect of job strain on general and central obesity in the Whitehall II Study. Am J Epidemiol 165:828–837
Tomiyama AJ, Puterman E, Epel ES, Rehkopf DH, Laraia BA (2013) Chronic psychological stress and racial disparities in body mass index change between Black and White girls aged 10-19. Ann Behav Med 45:3–12
Kirschbaum C, Hellhammer DH (1989) Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22:150–169
Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C (1997) Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61:2539–2549
Cohen S, Doyle WJ, Baum A (2006) Socioeconomic status is associated with stress hormones. Psychosom Med 68:414–420
DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG (2007) Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health 41:3–13
Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C (2010) Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 35:932–943
McCallum TJ, Sorocco KH, Fritsch T (2006) Mental health and diurnal salivary cortisol patterns among African American and European American female dementia family caregivers. Am J Geriatr Psychiatry 14:684–693
Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ (2010) Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an Urban Cohort. Psychol Trauma 2:326–334
Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T (2006) Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med 68:41–50
Martin CG, Bruce J, Fisher PA (2012) Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: the role of parental psychosocial risk and monitoring. Horm Behav 61:661–668
Rosmond R, Bjorntorp P (2000) The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med 247:188–197
LeRoith D, Roberts CT Jr (2003) The insulin-like growth factor system and cancer. Cancer Lett 195:127–137
Vottero A, Guzzetti C, Loche S (2013) New aspects of the physiology of the GH-IGF-1 axis. Endocr Dev 24:96–105
Clemmons DR (2012) Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am 41:425–443, vii-viii
Cohen P (1998) Serum insulin-like growth factor-I levels and prostate cancer risk–interpreting the evidence. J Natl Cancer Inst 90:876–879
Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M (1998) Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 351:1393–1396
Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ (1999) Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 91:620–625
Vadgama JV, Wu Y, Datta G, Khan H, Chillar R (1999) Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology 57:330–340
Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, Adami HO, Trichopoulos D (1998) Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst 90:911–915
Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X (1999) Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst 91:151–156
Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H (1999) Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev 15:314–322
Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB (1997) Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord 21:355–359
Yanovski JA, Sovik KN, Nguyen TT, Sebring NG (2000) Insulin-like growth factors and bone mineral density in African American and White girls. J Pediatr 137:826–832
DeLellis K, Rinaldi S, Kaaks RJ, Kolonel LN, Henderson B, Le Marchand L (2004) Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 13:1444–1451
Girgis R, Abrams SA, Castracane VD, Gunn SK, Ellis KJ, Copeland KC (2000) Ethnic differences in androgens, IGF-I and body fat in healthy prepubertal girls. J Pediatr Endocrinol Metab 13:497–503
Copeland KC, Colletti RB, Devlin JT, McAuliffe TL (1990) The relationship between insulin-like growth factor-I, adiposity, and aging. Metabolism 39:584–587
Jernstrom H, Deal C, Wilkin F, Chu W, Tao Y, Majeed N, Hudson T, Narod SA, Pollak M (2001) Genetic and nongenetic factors associated with variation of plasma levels of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev 10:377–384
Lukanova A, Toniolo P, Akhmedkhanov A, Hunt K, Rinaldi S, Zeleniuch-Jacquotte A, Haley NJ, Riboli E, Stattin P, Lundin E, Kaaks R (2001) A cross-sectional study of IGF-I determinants in women. Eur J Cancer Prev 10:443–452
Schoen RE, Schragin J, Weissfeld JL, Thaete FL, Evans RW, Rosen CJ, Kuller LH (2002) Lack of association between adipose tissue distribution and IGF-1 and IGFBP-3 in men and women. Cancer Epidemiol Biomarkers Prev 11:581–586
Teramukai S, Rohan T, Eguchi H, Oda T, Shinchi K, Kono S (2002) Anthropometric and behavioral correlates of insulin-like growth factor I and insulin-like growth factor binding protein 3 in middle-aged Japanese men. Am J Epidemiol 156:344–348
Chang S, Wu X, Yu H, Spitz MR (2002) Plasma concentrations of insulin-like growth factors among healthy adult men and postmenopausal women: associations with body composition, lifestyle, and reproductive factors. Cancer Epidemiol Biomarkers Prev 11:758–766
Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, Rosen T, Lindstedt G, Lundberg PA, Bengtsson BA (1994) Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) 41:351–357
Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD (2005) Variation in plasma insulin-like growth factor-1 and insulin-like growth factor binding protein-3: personal and lifestyle factors (United States). Cancer Causes Control 16:917–927
Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R (2004) Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol 150:161–171
Lukanova A, Soderberg S, Stattin P, Palmqvist R, Lundin E, Biessy C, Rinaldi S, Riboli E, Hallmans G, Kaaks R (2002) Nonlinear relationship of insulin-like growth factor (IGF)-I and IGF-I/IGF-binding protein-3 ratio with indices of adiposity and plasma insulin concentrations (Sweden). Cancer Causes Control 13:509–516
Henderson KD, Goran MI, Kolonel LN, Henderson BE, Le Marchand L (2006) Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 15:2298–2302
Garnett SP, Hogler W, Blades B, Baur LA, Peat J, Lee J, Cowell CT (2004) Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr 80:966–972
Ong K, Kratzsch J, Kiess W, Dunger D, Team AS (2002) Circulating IGF-I levels in childhood are related to both current body composition and early postnatal growth rate. J Clin Endocrinol Metab 87:1041–1044
Higgins PB, Fernandez JR, Goran MI, Gower BA (2005) Early ethnic difference in insulin-like growth factor-1 is associated with African genetic admixture. Pediatr Res 58:850–854
Rosmond R, Dallman MF, Bjorntorp P (1998) Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab 83:1853–1859
Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A (2008) Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab 295:E385–E392
Alderete TL, Byrd-Williams CE, Toledo-Corral CM, Conti DV, Weigensberg MJ, Goran MI (2011) Relationships between IGF-1 and IGFBP-1 and adiposity in obese African-American and Latino adolescents. Obesity (Silver Spring) 19:933–938
Frayn KN (2000) Visceral fat and insulin resistance—causative or correlative? Br J Nutr 83 Suppl 1:S71–S77
Cruz ML, Bergman RN, Goran MI (2002) Unique effect of visceral fat on insulin sensitivity in obese Hispanic children with a family history of type 2 diabetes. Diabetes Care 25:1631–1636
Going SB, Lohman TG, Cussler EC, Williams DP, Morrison JA, Horn PS (2011) Percent body fat and chronic disease risk factors in U.S. children and youth. Am J Prev Med 41:S77–S86
Indulekha K, Anjana RM, Surendar J, Mohan V (2011) Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians (CURES-113). Clin Biochem 44:281–287
McLaughlin T, Lamendola C, Liu A, Abbasi F (2011) Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 96:E1756–E1760
Patel P, Abate N (2013) Body fat distribution and insulin resistance. Nutrients 5:2019–2027
Rosenbaum M, Fennoy I, Accacha S, Altshuler L, Carey DE, Holleran S, Rapaport R, Shelov SP, Speiser PW, Ten S, Bhangoo A, Boucher-Berry C, Espinal Y, Gupta R, Hassoun AA, Iazetti L, Jean-Jacques F, Jean AM, Klein ML, Levine R, Lowell B, Michel L, Rosenfeld W (2013) Racial/Ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 21:2081–2090
Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX (1997) Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes 46:456–462
Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN (2003) Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 52:2490–2496
Gautier JF, Milner MR, Elam E, Chen K, Ravussin E, Pratley RE (1999) Visceral adipose tissue is not increased in Pima Indians compared with equally obese Caucasians and is not related to insulin action or secretion. Diabetologia 42:28–34
Lear SA, Humphries KH, Kohli S, Birmingham CL (2007) The use of BMI and waist circumference as surrogates of body fat differs by ethnicity. Obesity (Silver Spring) 15:2817–2824
Misra A (2003) Revisions of cutoffs of body mass index to define overweight and obesity are needed for the Asian-ethnic groups. Int J Obes Relat Metab Disord 27:1294–1296
Razak F, Anand SS, Shannon H, Vuksan V, Davis B, Jacobs R, Teo KK, McQueen M, Yusuf S (2007) Defining obesity cut points in a multiethnic population. Circulation 115:2111–2118
Stevens J (2003) Ethnic-specific revisions of body mass index cutoffs to define overweight and obesity in Asians are not warranted. Int J Obes Relat Metab Disord 27:1297–1299
Carroll JF, Fulda KG, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Cardarelli R (2009) Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring) 17:1420–1427
Conway JM, Yanovski SZ, Avila NA, Hubbard VS (1995) Visceral adipose tissue differences in black and white women. Am J Clin Nutr 61:765–771
Kanaley JA, Giannopoulou I, Tillapaugh-Fay G, Nappi JS, Ploutz-Snyder LL (2003) Racial differences in subcutaneous and visceral fat distribution in postmenopausal black and white women. Metabolism 52:186–191
Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R (1996) Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism 45:1119–1124
Perry AC, Applegate EB, Jackson ML, Deprima S, Goldberg RB, Ross R, Kempner L, Feldman BB (2000) Racial differences in visceral adipose tissue but not anthropometric markers of health-related variables. J Appl Physiol (1985) 89:636–643
Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA (2012) Markers of inflammation and fat distribution following weight loss in African-American and white women. Obesity (Silver Spring) 20:715–720
Weinsier RL, Hunter GR, Gower BA, Schutz Y, Darnell BE, Zuckerman PA (2001) Body fat distribution in white and black women: different patterns of intraabdominal and subcutaneous abdominal adipose tissue utilization with weight loss. Am J Clin Nutr 74:631–636
Goran MI, Nagy TR, Treuth MS, Trowbridge C, Dezenberg C, McGloin A, Gower BA (1997) Visceral fat in white and African American prepubertal children. Am J Clin Nutr 65:1703–1708
Huang TT, Johnson MS, Figueroa-Colon R, Dwyer JH, Goran MI (2001) Growth of visceral fat, subcutaneous abdominal fat, and total body fat in children. Obes Res 9:283–289
Abate N, Chandalia M (2012) Role of subcutaneous adipose tissue in metabolic complications of obesity. Metab Syndr Relat Disord 10:319–320
Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH (2000) Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 278:E941–E948
Tordjman J, Divoux A, Prifti E, Poitou C, Pelloux V, Hugol D, Basdevant A, Bouillot JL, Chevallier JM, Bedossa P, Guerre-Millo M, Clement K (2012) Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J Hepatol 56:1152–1158
Anand SS, Tarnopolsky MA, Rashid S, Schulze KM, Desai D, Mente A, Rao S, Yusuf S, Gerstein HC, Sharma AM (2011) Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS One 6:e22112
Kohli S, Lear SA (2013) Differences in subcutaneous abdominal adiposity regions in four ethnic groups. Obesity (Silver Springs) 21(11):2288–95
Kohli S, Sniderman AD, Tchernof A, Lear SA (2010) Ethnic-specific differences in abdominal subcutaneous adipose tissue compartments. Obesity (Silver Spring) 18:2177–2183
Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Almeras N, Despres JP (2012) Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr 96:714–726
Araneta MR, Barrett-Connor E (2005) Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res 13:1458–1465
Ashley MA, Buckley AJ, Criss AL, Ward JA, Kemp A, Garnett S, Cowell CT, Baur LA, Thompson CH (2002) Familial, anthropometric, and metabolic associations of intramyocellular lipid levels in prepubertal males. Pediatr Res 51:81–86
Bennett B, Larson-Meyer DE, Ravussin E, Volaufova J, Soros A, Cefalu WT, Chalew S, Gordon S, Smith SR, Newcomer BR, Goran M, Sothern M (2012) Impaired insulin sensitivity and elevated ectopic fat in healthy obese vs. nonobese prepubertal children. Obesity (Silver Spring) 20:371–375
Forouhi NG, Jenkinson G, Thomas EL, Mullick S, Mierisova S, Bhonsle U, McKeigue PM, Bell JD (1999) Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia 42:932–935
Kelley DE, Goodpaster BH (2001) Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24:933–941
Lee S, Kim Y, White DA, Kuk JL, Arslanian S (2012) Relationships between insulin sensitivity, skeletal muscle mass and muscle quality in obese adolescent boys. Eur J Clin Nutr 66:1366–1368
Maligie M, Crume T, Scherzinger A, Stamm E, Dabelea D (2012) Adiposity, fat patterning, and the metabolic syndrome among diverse youth: the EPOCH study. J Pediatr 161:875–880
Saukkonen T, Heikkinen S, Hakkarainen A, Hakkinen AM, van Leemput K, Lipsanen-Nyman M, Lundbom N (2010) Association of intramyocellular, intraperitoneal and liver fat with glucose tolerance in severely obese adolescents. Eur J Endocrinol 163:413–419
Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S (2002) Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51:1022–1027
Kuhlmann J, Neumann-Haefelin C, Belz U, Kalisch J, Juretschke HP, Stein M, Kleinschmidt E, Kramer W, Herling AW (2003) Intramyocellular lipid and insulin resistance: a longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes 52:138–144
Thamer C, Machann J, Bachmann O, Haap M, Dahl D, Wietek B, Tschritter O, Niess A, Brechtel K, Fritsche A, Claussen C, Jacob S, Schick F, Haring HU, Stumvoll M (2003) Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 88:1785–1791
Lara-Castro C, Newcomer BR, Rowell J, Wallace P, Shaughnessy SM, Munoz AJ, Shiflett AM, Rigsby DY, Lawrence JC, Bohning DE, Buchthal S, Garvey WT (2008) Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metabolism 57:1–8
Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E (2002) Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 51:144–151
Ingram KH, Lara-Castro C, Gower BA, Makowsky R, Allison DB, Newcomer BR, Munoz AJ, Beasley TM, Lawrence JC, Lopez-Ben R, Rigsby DY, Garvey WT (2011) Intramyocellular lipid and insulin resistance: differential relationships in European and African Americans. Obesity (Silver Spring) 19:1469–1475
Koska J, Stefan N, Permana PA, Weyer C, Sonoda M, Bogardus C, Smith SR, Joanisse DR, Funahashi T, Krakoff J, Bunt JC (2008) Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr 87:295–302
Brumbaugh DE, Crume TL, Nadeau K, Scherzinger A, Dabelea D (2012) Intramyocellular lipid is associated with visceral adiposity, markers of insulin resistance, and cardiovascular risk in prepubertal children: the EPOCH study. J Clin Endocrinol Metab 97:E1099–E1105
Ou HY, Wang CY, Yang YC, Chen MF, Chang CJ (2013) The association between nonalcoholic fatty pancreas disease and diabetes. PLoS One 8:e62561
Kim JS, Le KA, Mahurkar S, Davis JN, Goran MI (2012) Influence of elevated liver fat on circulating adipocytokines and insulin resistance in obese Hispanic adolescents. Pediatr Obes 7:158–164
Singh GK, Vitola BE, Holland MR, Sekarski T, Patterson BW, Magkos F, Klein S (2013) Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr 162:1160–1168, 1168.e1161
Targher G, Rossi AP, Zamboni GA, Fantin F, Antonioli A, Corzato F, Bambace C, Pozzi Mucelli R, Zamboni M (2012) Pancreatic fat accumulation and its relationship with liver fat content and other fat depots in obese individuals. J Endocrinol Invest 35:748–753
Toledo-Corral CM, Alderete TL, Hu HH, Nayak K, Esplana S, Liu T, Goran MI, Weigensberg MJ (2013) Ectopic fat deposition in prediabetic overweight and obese minority adolescents. J Clin Endocrinol Metab 98:1115–1121
Wicklow BA, Wittmeier KD, MacIntosh AC, Sellers EA, Ryner L, Serrai H, Dean HJ, McGavock JM (2012) Metabolic consequences of hepatic steatosis in overweight and obese adolescents. Diabetes Care 35:905–910
Wittmeier KD, Wicklow BA, MacIntosh AC, Sellers EA, Ryner LN, Serrai H, Gardiner PF, Dean HJ, McGavock JM (2012) Hepatic steatosis and low cardiorespiratory fitness in youth with type 2 diabetes. Obesity (Silver Spring) 20:1034–1040
Le KA, Ventura EE, Fisher JQ, Davis JN, Weigensberg MJ, Punyanitya M, Hu HH, Nayak KS, Goran MI (2011) Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care 34:485–490
Szczepaniak LS, Victor RG, Mathur R, Nelson MD, Szczepaniak EW, Tyer N, Chen I, Unger RH, Bergman RN, Lingvay I (2012) Pancreatic steatosis and its relationship to beta-cell dysfunction in humans: racial and ethnic variations. Diabetes Care 35:2377–2383
Guerrero R, Vega GL, Grundy SM, Browning JD (2009) Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology 49:791–801
Hasson RE, Adam TC, Davis JN, Kelly LA, Ventura EE, Byrd-Williams CE, Toledo-Corral CM, Roberts CK, Lane CJ, Azen SP, Chou CP, Spruijt-Metz D, Weigensberg MJ, Berhane K, Goran MI (2012) Randomized controlled trial to improve adiposity, inflammation, and insulin resistance in obese African-American and Latino youth. Obesity (Silver Spring) 20:811–818
Liska D, Dufour S, Zern TL, Taksali S, Cali AM, Dziura J, Shulman GI, Pierpont BM, Caprio S (2007) Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One 2:e569
Maggio AB, Mueller P, Wacker J, Viallon M, Belli DC, Beghetti M, Farpour-Lambert NJ, McLin VA (2012) Increased pancreatic fat fraction is present in obese adolescents with metabolic syndrome. J Pediatr Gastroenterol Nutr 54:720–726
Ravussin E, Smith SR (2002) Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci 967:363–378
Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E (2006) Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29:1337–1344
Greenberg AS, Obin MS (2006) Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 83:461S–465S
Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS (2007) Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56:2910–2918
Dowling HJ, Fried SK, Pi-Sunyer FX (1995) Insulin resistance in adipocytes of obese women: effects of body fat distribution and race. Metabolism 44:987–995
Holly J, Sabin M, Perks C, Shield J (2006) Adipogenesis and IGF-1. Metab Syndr Relat Disord 4:43–50
Balas-Nakash M, Perichart-Perera O, Benitez-Arciniega A, Tolentino-Dolores M, Mier-Cabrera J, Vadillo-Ortega F (2013) Association between adiposity, inflammation and cardiovascular risk factors in school-aged Mexican children. Gac Med Mex 149:196–203
Spruijt-Metz D, Adar Emken B, Spruijt MR, Richey JM, Berman LJ, Belcher BR, Hsu YW, McClain AD, Lane CJ, Weigensberg MJ (2012) CRP is related to higher leptin levels in minority peripubertal females regardless of adiposity levels. Obesity (Silver Spring) 20:512–516
Utsal L, Tillmann V, Zilmer M, Maestu J, Purge P, Jurimae J, Saar M, Latt E, Maasalu K, Jurimae T (2012) Elevated serum IL-6, IL-8, MCP-1, CRP, and IFN-gamma levels in 10- to 11-year-old boys with increased BMI. Horm Res Paediatr 78:31–39
Whincup PH, Nightingale CM, Owen CG, Rudnicka AR, Gibb I, McKay CM, Donin AS, Sattar N, Alberti KG, Cook DG (2010) Early emergence of ethnic differences in type 2 diabetes precursors in the UK: the Child Heart and Health Study in England (CHASE Study). PLoS Med 7:e1000263
McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM (2013) Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne) 4:52
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Borst SE (2007) Nutrition and health: adipose tissue and adipokines in health and disease. Humana Press, Totowa
Lazar MA (2005) How obesity causes diabetes: not a tall tale. Science 307:373–375
Schwartz JL, Antoniades DZ, Zhao S (1993) Molecular and biochemical reprogramming of oncogenesis through the activity of prooxidants and antioxidants. Ann N Y Acad Sci 686:262–278, discussion 278-269
Boden G, Shulman GI (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32 Suppl 3:14–23
Fisher FM, McTernan PG, Valsamakis G, Chetty R, Harte AL, Anwar AJ, Starcynski J, Crocker J, Barnett AH, McTernan CL, Kumar S (2002) Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm Metab Res 34:650–654
Trujillo ME, Scherer PE (2005) Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 257:167–175
Festa A, D’Agostino R Jr, Tracy RP, Haffner SM, Insulin Resistance Atherosclerosis S (2002) Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 51:1131–1137
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11:183–190
Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, Chen Z, Finck BN, Han DH, Magkos F, Conte C, Bradley D, Fraterrigo G, Eagon JC, Patterson BW, Colonna M, Klein S (2013) Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 145(366–374):366.e1–374.e3
He J, Le DS, Xu X, Scalise M, Ferrante AW, Krakoff J (2010) Circulating white blood cell count and measures of adipose tissue inflammation predict higher 24-h energy expenditure. Eur J Endocrinol 162:275–280
Le KA, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, Beale E, Xie C, Greenberg AS, Allayee H, Goran MI (2011) Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes 60:2802–2809
Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE Jr, Peterson CA, Kern PA (2011) Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 96:E1990–E1998
Sbarbati A, Osculati F, Silvagni D, Benati D, Galie M, Camoglio FS, Rigotti G, Maffeis C (2006) Obesity and inflammation: evidence for an elementary lesion. Pediatrics 117:220–223
Tam CS, Tordjman J, Divoux A, Baur LA, Clement K (2012) Adipose tissue remodeling in children: the link between collagen deposition and age-related adipocyte growth. J Clin Endocrinol Metab 97:1320–1327
Kyrgios I, Galli-Tsinopoulou A, Stylianou C, Papakonstantinou E, Arvanitidou M, Haidich AB (2012) Elevated circulating levels of the serum acute-phase protein YKL-40 (chitinase 3-like protein 1) are a marker of obesity and insulin resistance in prepubertal children. Metabolism 61:562–568
Retnakaran R, Hanley AJ, Connelly PW, Harris SB, Zinman B (2006) Elevated C-reactive protein in Native Canadian children: an ominous early complication of childhood obesity. Diabetes Obes Metab 8:483–491
Albert MA, Glynn RJ, Buring J, Ridker PM (2004) C-reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study). Am J Cardiol 93:1238–1242
Albert MA, Ridker PM (2004) Inflammatory biomarkers in African Americans: a potential link to accelerated atherosclerosis. Rev Cardiovasc Med 5 Suppl 3:S22–S27
Lin SX, Pi-Sunyer EX (2007) Prevalence of the metabolic syndrome among US middle-aged and older adults with and without diabetes—a preliminary analysis of the NHANES 1999–2002 data. Ethn Dis 17:35–39
Duncan BB, Schmidt MI, Chambless LE, Folsom AR, Carpenter M, Heiss G (2000) Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults—the ARIC study. Atherosclerosis Risk in Communities. Obes Res 8:279–286
Patel DA, Srinivasan SR, Xu JH, Li S, Chen W, Berenson GS (2006) Distribution and metabolic syndrome correlates of plasma C-reactive protein in biracial (black-white) younger adults: the Bogalusa Heart Study. Metabolism 55:699–705
Ford ES, Giles WH, Mokdad AH, Myers GL (2004) Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem 50:574–581
Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM (2002) Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr 75:492–498
Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR Jr (2006) Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 83:1369–1379
King DE, Egan BM, Woolson RF, Mainous AG 3rd, Al-Solaiman Y, Jesri A (2007) Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med 167:502–506
Madan AK, Barden CB, Beech B, Fay K, Sintich M, Beech DJ (2002) Self-reported differences in daily raw vegetable intake by ethnicity in a breast screening program. J Natl Med Assoc 94:894–900
Despres JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, Theriault G, Pinault S, Bouchard C (1989) Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes 38:304–309
Kushner RF, Racette SB, Neil K, Schoeller DA (1995) Measurement of physical activity among black and white obese women. Obes Res 3 Suppl 2:261s–265s
Lovejoy JC, Smith SR, Rood JC (2001) Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: The Healthy Transitions Study. Obes Res 9:10–16
Tuten C, Petosa R, Sargent R, Weston A (1995) Biracial differences in physical activity and body composition among women. Obes Res 3:313–318
Heilbronn L, Smith SR, Ravussin E (2004) Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord 28 Suppl 4:S12–S21
Bays H, Mandarino L, DeFronzo RA (2004) Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 89:463–478
Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A (2013) Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 5:1544–1560
Manteiga S, Choi K, Jayaraman A, Lee K (2013) Systems biology of adipose tissue metabolism: regulation of growth, signaling and inflammation. Wiley Interdiscip Rev Syst Biol Med 5:425–447
Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB (2012) Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab 97:3302–3309
Burns SF, Kelsey SF, Arslanian SA (2009) Effects of an intravenous lipid challenge and free fatty acid elevation on in vivo insulin sensitivity in African American versus Caucasian adolescents. Diabetes Care 32:355–360
Goree LL, Darnell BE, Oster RA, Brown MA, Gower BA (2010) Associations of free fatty acids with insulin secretion and action among African-American and European-American girls and women. Obesity (Silver Spring) 18:247–253
Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A (2009) Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 89:27–71
Emerit I (1994) Reactive oxygen species, chromosome mutation, and cancer: possible role of clastogenic factors in carcinogenesis. Free Radic Biol Med 16:99–109
Gago-Dominguez M, Castelao JE, Yuan JM, Ross RK, Yu MC (2002) Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States). Cancer Causes Control 13:287–293
Salganik RI (2001) The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr 20:464S–472S, discussion 473S-475S
Welsch CW (1995) Review of the effects of dietary fat on experimental mammary gland tumorigenesis: role of lipid peroxidation. Free Radic Biol Med 18:757–773
Niskanen LK, Salonen JT, Nyyssonen K, Uusitupa MI (1995) Plasma lipid peroxidation and hyperglycaemia: a connection through hyperinsulinaemia? Diabet Med 12:802–808
Vijayalingam S, Parthiban A, Shanmugasundaram KR, Mohan V (1996) Abnormal antioxidant status in impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabet Med 13:715–719
Cederberg J, Basu S, Eriksson UJ (2001) Increased rate of lipid peroxidation and protein carbonylation in experimental diabetic pregnancy. Diabetologia 44:766–774
Collier A, Rumley A, Rumley AG, Paterson JR, Leach JP, Lowe GD, Small M (1992) Free radical activity and hemostatic factors in NIDDM patients with and without microalbuminuria. Diabetes 41:909–913
Griesmacher A, Kindhauser M, Andert SE, Schreiner W, Toma C, Knoebl P, Pietschmann P, Prager R, Schnack C, Schernthaner G et al (1995) Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am J Med 98:469–475
Jain SK, McVie R, Jaramillo JJ, Palmer M, Smith T, Meachum ZD, Little RL (1996) The effect of modest vitamin E supplementation on lipid peroxidation products and other cardiovascular risk factors in diabetic patients. Lipids 31 Suppl:S87–S90
MacRury SM, Gordon D, Wilson R, Bradley H, Gemmell CG, Paterson JR, Rumley AG, MacCuish AC (1993) A comparison of different methods of assessing free radical activity in type 2 diabetes and peripheral vascular disease. Diabet Med 10:331–335
Neri S, Bruno CM, Raciti C, D’Angelo G, D’Amico R, Cristaldi R (1994) Alteration of oxide reductive and haemostatic factors in type 2 diabetics. J Intern Med 236:495–500
Sato Y, Hotta N, Sakamoto N, Matsuoka S, Ohishi N, Yagi K (1979) Lipid peroxide level in plasma of diabetic patients. Biochem Med 21:104–107
Velazquez E, Winocour PH, Kesteven P, Alberti KG, Laker MF (1991) Relation of lipid peroxides to macrovascular disease in type 2 diabetes. Diabet Med 8:752–758
Yaqoob M, McClelland P, Patrick AW, Stevenson A, Mason H, White MC, Bell GM (1994) Evidence of oxidant injury and tubular damage in early diabetic nephropathy. QJM 87:601–607
Santini SA, Marra G, Giardina B, Cotroneo P, Mordente A, Martorana GE, Manto A, Ghirlanda G (1997) Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes 46:1853–1858
Wakatsuki A, Ikenoue N, Okatani Y, Fukaya T (2001) Estrogen-induced small low density lipoprotein particles may be atherogenic in postmenopausal women. J Am Coll Cardiol 37:425–430
Wakatsuki A, Okatani Y, Ikenoue N, Shinohara K, Watanabe K, Fukaya T (2003) Effect of lower dose of oral conjugated equine estrogen on size and oxidative susceptibility of low-density lipoprotein particles in postmenopausal women. Circulation 108:808–813
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2003) Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 52:1–8
Mehrotra S, Ling KL, Bekele Y, Gerbino E, Earle KA (2001) Lipid hydroperoxide and markers of renal disease susceptibility in African-Caribbean and Caucasian patients with Type 2 diabetes mellitus. Diabet Med 18:109–115
Haffner SM, Agil A, Mykkanen L, Stern MP, Jialal I (1995) Plasma oxidizability in subjects with normal glucose tolerance, impaired glucose tolerance, and NIDDM. Diabetes Care 18:646–653
Haffner SM, Miettinen H, Stern MP, Agil A, Jialal I (1996) Plasma oxidizability in Mexican-Americans and non-Hispanic whites. Metabolism 45:876–881
Lopes HF, Morrow JD, Stojiljkovic MP, Goodfriend TL, Egan BM (2003) Acute hyperlipidemia increases oxidative stress more in African Americans than in white Americans. Am J Hypertens 16:331–336
Il’yasova D, Spasojevic I, Base K, Zhang H, Wang F, Young SP, Millington DS, D’Agostino RB Jr, Wagenknecht LE (2012) Urinary F2-isoprostanes as a biomarker of reduced risk of type 2 diabetes. Diabetes Care 35:173–174
Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB Jr, Wagenknecht LE (2012) Racial differences in urinary F2-isoprostane levels and the cross-sectional association with BMI. Obesity (Silver Spring) 20:2147–2150
Lara-Castro C, Weinsier RL, Hunter GR, Desmond RE (2002) Visceral adipose tissue in women: longitudinal study of the effects of fat gain, time, and race. Obes Res 10:868–874
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hasson, R.E., Goran, M.I. (2014). Ethnic Differences in Insulin Resistance as a Mediator of Cancer Disparities. In: Bowen, D., Denis, G., Berger, N. (eds) Impact of Energy Balance on Cancer Disparities. Energy Balance and Cancer, vol 9. Springer, Cham. https://doi.org/10.1007/978-3-319-06103-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-06103-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-06102-3
Online ISBN: 978-3-319-06103-0
eBook Packages: MedicineMedicine (R0)