Abstract
Different laser-based methods for the fabrication of nanoparticles and ordered nanoparticle structures, including possibilities for their functionalization and replication in polymeric materials, are discussed. Nanoparticles made from noble metals, supporting collective electron oscillations, and low absorbing dielectric nanoparticles, having large permittivity values, can both be resonantly excited by external electromagnetic fields which make them attractive for biophotonic and sensing applications. For applications in biomedicine especially polymeric nanoparticles, as drug delivery systems, are very important. Fabrication of all these types of nanoparticles can be realized with laser technologies, which are briefly reviewed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In this chapter, we discuss different laser-based methods for the fabrication of nanoparticles and ordered nanoparticle structures, including possibilities for their functionalization and replication in polymeric materials. Research fields involving nanoparticles and their applications in photonics, biomedicine, and sensorics are rapidly growing. At present, “nanoparticles” search in Google provides approximately 8 million references.
Nanoparticles made from noble metals support collective electron oscillations, which can be resonantly excited by external electromagnetic fields and are known as localized surface plasmon resonances. The frequency of these resonances strongly depends on the size, shape, and environment of the nanoparticles, making them very attractive for different practical applications, for example real-time sensor technologies. Alternatively, low absorbing dielectric nanoparticles having large permittivity values can also resonantly interact with light and support strong Mie resonances. Such particles represent dielectric resonators or antennas, which can trap external electromagnetic fields. It is well-known from Mie theory that the first and second lowest frequency resonances of dielectric spheres correspond to the magnetic and electric dipole contributions. Scattering diagrams of the resonant dielectric nanoparticles are determined by interference of electromagnetic waves generated by the electric and magnetic dipoles, which can result in directional back or forward light scattering.
For applications in biomedicine especially polymeric nanoparticles, as drug delivery systems, are very important. Fabrication of all these types of nanoparticles can be realized with laser technologies, which are briefly reviewed below.
2 Laser Printing of Nanoparticles and Nanoparticle Arrays
2.1 Laser Printing of Nanoparticles
Femtosecond lasers have opened new possibilities for the generation of spherical nanoparticles with predefined sizes and positions, based on laser-induced melting, fluid dynamics, and molten material transfer. In this section, a printing process for the controllable generation of noble metal and semiconductor nanoparticles by laser-induced transfer of liquid material droplets is described. The liquid droplets are captured on a receiver substrate, e.g. glass, where they obtain a near-spherical shape after solidification, due to the surface tension of molten material. Resonant optical properties of the nanoparticles generated by this method can be applied for the development of novel sensor concepts.
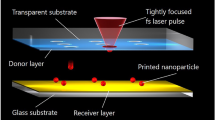
During the laser printing of metallic nanoparticles, a thin material layer deposited on a glass substrate, acting as a donor substrate, is irradiated by single femtosecond laser pulses. Due to the ultrashort pulse duration, the pulse energy is absorbed by electrons in the metallic film and is slowly transferred to the lattice. Processes which are important for structural changes of the laser-excited solid, such as carrier–phonon scattering and thermal diffusion, occur after the laser pulse absorption [1]. Therefore, only the material within the laser beam spot size on the target is affected and a highly controlled and reproducible material transformation is possible. Due to the solid-liquid phase transition, the irradiated material changes its density and its volume. This effect induces strong temperature and pressure gradients, leading to a complex fluid dynamics [2]. Noble metals, such as gold, silver, and copper expand during melting. The liquid metal then forms a protrusion with a back-jet structure in its center [3, 4]. One example of the irradiation of a thin gold film by a single tightly focused femtosecond laser pulse is shown in the SEM image in Fig. 5.1 [4, 5]. When the amount of melted material increases, the surface tension induces the material to form spherical droplets and a spherical nanodroplet appears on top of the back jet. With higher laser pulse energy the nanodroplet starts to separate from the back-jet and is ejected upwards [6]. By placing the receiver substrate on top of the thin gold layer the ejected nanoparticles can be collected [5]. A schematic illustration of this laser printing process is shown in Fig. 5.2. Each laser pulse is producing one nanoparticle, which is deposited on the receiver substrate. By focusing laser pulses on different positions on the donor substrate it is possible to generate large nanoparticle arrays with precisely arranged nanoparticles [7]. Furthermore, the printing process allows generating nanoparticles with reproducible diameters by using the same laser pulse energy for every nanodroplet ejection process [8]. The right part of Fig. 5.2 shows SEM images of gold nanoparticles which are deposited on a receiver substrate by the described process. The diameter of these nanoparticles can controllably be influenced by the experimental parameters such as the layer thickness of the material film, laser pulse energy, and focusing conditions of the laser pulse. To demonstrate the precise deposition of nanoparticles on the receiver substrate and its capabilities, an example is given by the SEM and dark field microscopic images in Fig. 5.3. Here, the nanoparticles with a diameter of 400 nm are deposited at a distance of 5 \(\upmu \)m, forming the word “NANO”. The observed strong light scattering is due to multipole plasmon resonances in the visible spectral range [8].
This process, however, cannot be used with materials (e.g. silicon) reducing their volume during melting. In this case, no back-jet is generated and the ejection of molten material is different, as will be discussed below.
2.2 Laser Fabrication of Large-Scale Nanoparticle Arrays
For sensing applications of nanoparticle structures and arrays, the main technological drawback is the high cost and low-throughput of existing fabrication methods. Most of the experimentally studied structures are fabricated by electron or ion beam lithography, which are not suitable for large-scale and low-cost production. This stimulates the development of novel high-throughput methods for the fabrication of nanoparticle structures with particular optical properties, for example, biosensors or metamaterials [9, 10]. In this section, a method for high-speed low-cost fabrication of large-scale nanoparticle arrays is introduced [11]. This method is based on a combination of nanosphere lithography and femtosecond laser-induced transfer. The metallic film, e.g. gold, on the donor substrate is pre-structured by nanosphere lithography. Both, interparticle distance and particle size can be independently controlled, which allows engineering of optical properties of such arrays. A scheme of high-speed fabrication of large-scale nanoparticle arrays based on a combination of the nanosphere lithography and laser-induced transfer is shown in Fig. 5.4. The final structure consists of hexagonal arrays of spherical gold nanoparticles partially embedded into a polymeric substrate. First, submicron sized spheres of silica are deposited onto a glass plate forming a large area closely packed monolayer. Subsequently, gold is evaporated onto the substrate and the silica spheres are removed, leaving triangular metallic islands on the donor substrate. After laser irradiation, the triangles are melted and forming spherical droplets. This process is accompanied by a raise in the center-of-mass, resulting in an upward acceleration. The particles are finally captured in a receiver substrate consisting of a thin layer of PDMS (poly-dimethoxysiloxane) [11].
In this metallic nanoparticle array, a collective plasmonic mode with diffractive coupling between the nanoparticles can be excited. The excitation of this mode leads to the appearance of a narrow (fwhm \(=\) 14 nm) Fano-type resonance dip in the optical transmission spectra. The spectral position of this dip is sensitive to the refractive index changes of the local environment, allowing the realization of novel sensing concepts [11].
In principle, this process can also be applied with other materials, for example, silicon. The magnetic Mie resonances of Si nanoparticles would allow the realization of novel type sensors for magnetic fields at optical frequencies. The optical properties of silicon nanoparticles are briefly discussed in the next section.
3 Resonant Electric and Magnetic Response of Silicon Nanoparticles
Metal nanoparticles are characterized by a strong resonant response to the electric field of light [12, 13], which appears due to excitation of collective electron oscillations known as localized surface plasmons. The main drawback of using plasmonic particles in the visible spectral range is their intrinsic Ohmic loss, which strongly affects their overall performance and limits practical applications. One of the possible ways to avoid such limitation and still to have similar resonant properties is to use high-refractive index dielectric nanoparticles [14, 15]. Nanoparticles of crystalline silicon (Si), the basic material of silicon photonics, provide a promising choice [16, 17]. Scattering properties of Si nanostructures with Mie resonances are attracting growing interest due to their potential applications for solar cells [18, 19], for tuning optical responses of nanostructured systems [20, 21], and for field-enhanced surface spectroscopy [22]. Spherical silicon nanoparticles with sizes of few hundred nanometers exhibit unique optical properties due to their strong electric and magnetic dipole responses in the visible spectral range [23, 24]. The spectral position of these resonances can be tuned throughout the whole visible spectral range from violet to red by changing the nanoparticle size in the range of 100–200 nm. Experimentally measured scattering intensities of spherical Si nanoparticles with diameters between 100 and 150 nm are shown in Fig. 5.5. For every nanoparticle in this range, two resonance peaks in the visible spectral region are present. These two resonance peaks have their origin in the excitation of magnetic and electric dipole resonances. As it can be seen in Fig. 5.5, resonances are red-shifted for increased nanoparticle radius.
a Experimentally measured scattering intensities of Si nanoparticles with different diameters. b Extinction efficiencies of Si nanoparticles with the radius R calculated by Mie theory (along vertical axis measured wavelengths of scattered light). Circles and squares correspond to magnetic and electric dipole resonances, respectively, of fabricated Si nanoparticles measured experimentally
4 Generation of Silicon Nanoparticles from Bulk Silicon
Silicon nanoparticles, as they have been used in the measurements discussed in the previous section, can be generated by focussing single femtosecond laser pulses onto the surface of a silicon wafer. However, as already pointed out, silicon material reduces its volume during melting, leading to a depression on the material surface. Melted silicon forms a smooth toroidal ring around this depression. When the amount of material increases the formerly smooth ring gets instable and the surface tension contracts liquid material into a number of small spherical droplets. These droplets are ejected from the molten zone and can be captured on a receiver glass substrate. Since the contraction of the molten material into droplets is a statistical process, the sizes and the positions of the ejected particles are centered around some average value, but cannot be controlled precisely.
A schematic illustration of this process is given in Fig. 5.6. In this case, every single femtosecond laser pulse generates a small group of silicon nanoparticles with sizes of 100–300 nm in diameter. An example of Si nanoparticles, which are generated by this method, is shown in the darkfield microscopic image in Fig. 5.7. The silicon nanoparticles deposited on the glass receiver substrate scatter white light in different colors, depending on the sizes of these particles. As already shown in the previous section, the reason for these colors is in the strong electric and magnetic Mie resonances depending on the particles size and located in the visible spectral range.
As it can be seen in Fig. 5.7, the nanoparticles are grouped around a position above the ejection point. The number of particles per group varies from 1 to 7. The contraction of the molten material into spherical nanodroplets is the driving force transferring particles onto the receiver substrate. This process might in future enable formation of Si nanodroplets from pre-structured silicon films, which will allow achieving better control over their sizes and positions.
5 Microreplication of Laser-Transferred Gold Nanoparticles/Nanomolding
Laser printed nanoparticles can be reproduced in a polymer by applying the so-called soft lithography technique (see Fig. 5.8). This procedure represents a non-photolithographic method and is based on replica molding of micro- and nanostructures. It provides a fast, effective and low-cost strategy for the manufacturing of complex nanostructures and 3-D features. In soft lithography, an elastomeric template (stamp) with patterned features on its surface is applied to fabricate replica patterns and structures on the target surface with the sizes ranging from tens of nanometers to hundreds of micrometers. Such elastomeric stamps with patterned relief structures are the basis of soft lithography [25].
Schematic illustration of soft-lithography replication of laser generated nanoparticles: 1 laser transferred gold nanoparticles on the glass substrate are 2 casted with liquid PDMS; after removal of air gaps in the vacuum chamber and curing 3 PDMS mold is detached from the master substrate; 4 Liquid photomonomer is coated onto the PDMS mold; 5 it is covered with a glass coverslip and cured with UV light
The replication procedure involves the fabrication of negative poly-dimetylsiloxane (PDMS) molds of the original (master) structure, which are then used as templates to form structures in polymer (see Fig. 5.8). The elastomeric mold is manufactured by casting liquid PDMS onto a master structure consisting from an array of metal nanoparticles. After curing PDMS at room temperature or by heating, the PDMS mold can be peeled-off of the master structure.
PDMS forms a conformal contact with all surfaces and therefore, superiorly encloses convex structures and penetrates into cavities. Its elastomeric properties allow PDMS to be easily released from a master without damaging either the master or the mold itself. PDMS’s thermal and chemical stability enables most polymers to be patterned. The material is optically transparent down to 300 nm what permits molding of photocurable polymers. The PDMS is not hygroscopic; it does not swell with humidity [26]. For replication of laser-generated gold nanoparticles we used photochemically curable resist NIL 6000.2 (Microresist Technology). The unique features of this polymer, like low viscosity, non-harmful solvents in its composition, and room temperature conditioning enable the fabrication of smallest feature sizes down to 50 nm with a very low residual layer thickness \(<\)10 nm. Moreover, NIL 6000.2 has an excellent film quality after curing. The replication results are shown in Fig. 5.9.
Even though in general PDMS is chemically inert, it readily swells in a number of nonpolar organic solvents such as toluene and hexane [26], which can limit the application of such molds for polymers containing nonpolar organic solvents. Rolland et al. applied photocurable perfluoropolyether (PFPE) for mold fabrication. PFPE-based molds are both non-wetting and non-swelling in contact with both inorganic and organic materials [27]. Such physicochemical properties of the mold material are essential for repetitive fabrication of nanoobjects using the same mold, which is essential in industrial fabrication. The same group has developed a general technique of Particle Replication In Nonwetting Templates (PRINT) for the fabrication of monodispersed particles with simultaneous control over their structure and function. Using highly fluorinated PFPE surfaces that are non-wetting to organic materials, they have fabricated isolated objects with superior shape and composition control without harsh processing steps. Although the PRINT technique is similar to the soft lithography methods, it is unique because it can produce isolated free standing particles instead of particles anchored to the film [28].
Micro- and nanospheres play important role in many applications, such as drug delivery systems [29], optical materials [30], cosmetics [31], chemical and biological diagnostics [32] and other biomedical fields [33]. Applying soft lithography technique, nanoparticles can be fabricated also from synthetic hydrogels. These hydrogel nanoparticles, usually called “nanogels”, are very promising as drug-delivery carriers [34]. The nano-replication soft lithography and PRINT methods enable strict control over the particle size, shape, composition and permit the loading of delicate units, including pharmaceutical drugs and biomacromolecules. For example, monodisperse 200 nm PEG-based swellable particles were fabricated with the PRINT method by UV-induced copolymerization of several vinyl monomers such as PEG triacrylate, PEG monomethyl ether monomethacrylate, and p-hydroxystyrene [35].
6 Laser-Based Synthesis of Nanoparticles and Surface Modified Nanoconjugates
6.1 Ultrapure Nanoparticles by Pulsed Laser Ablation in Liquids
Besides the direct deposition of single nanoparticles by laser-printing, ultrapure nanoparticles can also be produced using laser irradiation of solids in liquid media [36]. This green technology allows generation of high amounts of spherical nanoparticles by complex ablation phenomena depending on pulse duration, pulse energy, irradiation wavelength, and liquid-material combination. Compared to conventional manufacturing approaches of nanoparticles such as wet chemical synthesis, mechanical milling and grinding, laser ablation does not require usage of any additional material such as chemical precursors or reducing agents [37] and lacks any other form of contaminations arising from application of mechanical or abrasive components. Therefore, laser ablation in liquid environments attracts more and more attention, enabling the generation of nanoparticle colloids of a great variety of materials with outstanding purity, as illustrated in Fig. 5.10. Taking benefits of the simplicity and flexibility of this technique, a vast diversity of functional materials have been already prepared including metal nanoparticles [38, 39], semiconductors [40, 41], ceramics, alloys [42] and highly photosensitive bioconjugates [43]. Working in dense environments, such as liquid media, results in less efficient and more complex ablation process than laser ablation in air or under vacuum conditions. In the femtosecond and picosecond time regimes [37], nanoparticle generation is mainly limited by relatively low pulse energy of currently available laser sources. Employing nanosecond lasers reveals that the ablation efficiency can be further increased under rigorous control of laser and process parameters. Note that generation of nanoparticles based on continuous wave laser ablation has also been reported [44].
Common for all liquid-based nanoparticle generation methods applying laser ablation is that high reproducibility was only achieved using axial separation of the incoming laser beam and the generated air bubbles. Usually, this is obtained by horizontal beam guidance, by transmitting the laser beam through an entrance media that allows accurate control of the liquid layer. This way also allows elimination of the liquid meniscus which considerably reduces inaccuracy in laser focusing. Besides, ablation efficiency strongly depends on the deposited laser energy into the target material involving linear and non-linear absorption regimes [45, 46]. The thickness of applied liquid layer is another major parameter determining and limiting nanoparticle generation due to absorption and scattering of laser radiation by previously ablated nanoparticles. Flow rate of the applied liquid media has only recently been considered to impact ablation efficiency due to removal of ablated particles and generated air bubbles from the ablation zone. Without liquid circulation, the ablated nanomaterials disperse into the entire liquid volume by slow diffusion and Brownian motion, hence after each ablation sequence a dense particle cloud is ejected, having a relatively long residual living time in front of the target leading to significant absorption of the subsequent laser beam.
Therefore, considerable increase in ablation rate can be observed by increasing liquid flow rate from stationary to several hundreds of mL/min using the same laser parameters. Optimal flow rate however strongly depends on the laser fluence and pulse duration. Besides these evident parameters, recent studies demonstrated that the interpulse distance has a dramatic effect on the material removal rate, when laser ablation takes place in liquid environment [47]. Figure 5.11 (top) shows ablation rate of alumina as a function of interpulse distance for 4 mm liquid layer, 4.6 mJ pulse energy at 4 kHz repetition rate, and a focal spot size of 50 \(\upmu \)m. The term interpulse distance defines the distance between two pulses, from one pulse center to the center of the neighboring pulse. As expected, material removal rate and nanoparticle productivity strongly depends on overlapping of spatially separated laser pulses. By adjusting position of separated laser pulses using a galvanometric laser scanner, optimal interpulse distance of 125 \(\upmu \)m for 4 kHz repetition rate has been identified. Two competitive effects influence the nanoparticle generation rate. First, for strongly overlapping laser pulses, the pulse interaction with previously ablated nanoparticles and previously generated cavitation bubbles are the most important mechanisms, preventing higher material ablation. Referring to the literature, laser ablation in liquids using Nd:YAG nanosecond laser irradiation at 36 J/cm\(^{2}\) laser fluence generates cavitation gas bubbles which last around 300 \(\upmu \)s. This cavitation bubble contains primary nanoparticles of extreme high local concentration which can scatter, reflect or absorb subsequent laser pulses. This absorption and scattering effects become less important with increasing interpulse distance. On the other hand, increasing interpulse distance also results in significant temperature variations and temperature gradients in the solid target. Above the optimal pulse overlap, the regime of thermally isolated ablation areas is reached, that negatively influences the achievable material removal. This hypothesis of cavitation bubble and heat accumulation affected laser ablation has been clearly confirmed by comparison of nanoparticle generation in gas and liquid media [48]. By changing the repetition rate with equal interpulse distances at constant pulse energy of 3.3 mJ and pulse duration of 40 ns FWHM, experimental results shown in Fig. 5.11 (bottom) are obtained. Investigations revealed a strong decrease in the ablation rate when the repetition rate was increased from 0.5 to 20 kHz. An enhancement of almost two orders of magnitude in the ablation rate was identified by decreasing the laser frequency from 20 to 0.5 kHz resulting in 0.26 ng/pulse and 17 ng/pulse, respectively. The maximum ablation rate of 17 ng/pulse was achieved at 2 kHz repetition rate. Nanosecond laser ablation initiates a complex sequence of events occurring both during and after the laser pulse. Nanoparticle formation starts at the nanosecond to sub-microsecond time scales during plasma plume expansion. Due to rapid cooling, which can occur faster than condensation, exceptionally high saturation ratios can be achieved. Therefore, even at high laser fluences relatively small nanoparticles can be generated by laser ablation in liquids. For nanoparticle generation, the subsequent events are even more critical, since on the time scale of several microseconds a cavitation bubble is growing on the target surface due to the local heating of solvent in the vicinity of ablated spot. In the highly confined region of the cavitation bubble, large amount of primary nanoparticles are formed and trapped. They are ejected when the bubble collapses on the time scale of 200–300 \(\upmu \)s (or even later depending on the applied pulse energy). During the lifetime of the cavitation bubble, the target cannot be ablated efficiently due to light scattering. As shown in Fig. 5.11 (bottom), maximum nanoparticle generation rate is obtained at the laser repetition rate of 2 kHz, which corresponds to 500 \(\upmu \)s time delay between laser pulses [47]. This particular time delay is in the order of the cavitation bubble lifetime. It is likely, that when the time delay between two laser pulses is high enough, the cavitation bubble collapses between subsequent laser pulses, resulting in a saturation plateau in the ablation rate. A similar behavior has been identified for picosecond laser ablation, however with shorter cavitation bubble lifetimes.
6.2 Surface-Functionalized Nano(Bio)Conjugates
Small gold and other plasmonic nanoparticles, characterized by enhanced resonant absorption and scattering properties, are particularly useful in numerous biomedical applications, including cell-targeted drug delivery [49], high resolution bioimaging [50], biomedical diagnostics and therapeutics, when conjugated with functional molecules such as DNA, RNA, oligonucleotides, peptides, drugs, etc. Pulsed laser ablation in liquids offers an alternative single step surface functionalization, allowing size-controlled generation of stable nanoparticle colloids with outstanding purity and novel surface chemistry, not possible by conventional manufacturing methods. Due to the presence of partial oxidation into high oxidation states, such as Au\(^{+}\) and Au\(^{3+}\), laser-generated gold nanoparticles act as electron acceptors [51]; hence, they are easily coordinated by molecules bearing electron donor moieties such as thiol, amine or carboxyl groups. A (bio)molecule, having a particular functional group, added to ablation media prior (in-situ conjugation) or after (ex-situ conjugation) the laser process will be chemically or physically bound to the surface. However, in-situ conjugation leads to higher conjugation efficiencies than ex-situ functionalization. Figure 5.12 shows absorption spectra of ligand-free gold nanoparticles and gold nanoconjugates generated by 100 \(\upmu \)J, 7 ps laser pulses at 2 kHz repetition rate in aqueous media and in 5 \(\upmu \)M Cy5-tagged model peptide (TAT) derived from the human immunodeficiency virus type-1. High resolution scanning electron microscopy, in SEM and STEM modus, can reveal the presence of cohesive organic molecules around the nanoparticle core as shown in Fig. 5.13, whereas the ligand-free nanoparticles lack such organic shell.
Although ultrashort pulsed laser ablation presents a promising tool for in-situ bioconjugation, due to minimal thermal impact to ablated material and surrounding media when working at laser fluences close to the ablation threshold, it induces similar residual thermal effects as nanosecond lasers in the high fluence regime, including melting and photothermal reshaping of ablated particles. During laser ablation (especially during ultrashort-pulsed ablation), the ejected material is in a chemically activated state of growth which allows to significantly influence the nanoparticle size by the amount of conjugative agents in solution. Reciprocal dependency of the primary nanoparticle size as a function of the concentration of active molecules was reported by several groups using the term growth-quenching. As described above, ablation efficiency and nanoparticle yield increase with the laser pulse energy, although at the same time, heat impact and the risk of molecule degradation also increase. In order to investigate whether the ablation process itself, or presence of nanoparticles trigger biomolecule degradation during in-situ conjugation of biomolecules to nanoparticles by femtosecond pulsed laser ablation, we ablated gold in the presence of a photo-sensitive model biomolecule (fluorescence-tagged single stranded oligonucleotide with 1.5 \(\upmu \)M concentration) using various pulse energies in stationary solution and in biomolecule flow using the experimental setup schematically illustrated in Fig. 5.14 (top). It was clearly observed that femtosecond laser ablation in stationary biological media induces drastic degeneration of the sensitive molecular compartments by denaturing the fluorophore tag, while ablation in a biomolecule flow with the same laser parameters minimizes degradation [52]. The degree of degradation reduces considerably with higher flow rates. Since only the total residence time of ablated species in the ablation zone is varied by the liquid flow rate, it was assumed that biomolecule disintegration or laser/heat-induced DNA depurination by femtosecond laser irradiation is mainly induced by absorption and scattering of subsequent laser pulses on previously ablated, suspended nanoparticles/nanoparticle conjugates in the colloidal solution and is not triggered directly by the ablation itself.
7 Novel Laser-Based Conjugation Concepts
Both ex-situ and in-situ laser-based bioconjugation techniques have major disadvantages The ex-situ method does not allow precise nanoparticle size control as colloidal species might have already been aggregated in the moment of ligand addition. In contrast, the in-situ method generates stable and size-controlled bioconjugates due to rapid size-quenching, but has limited productivity and induces photo-degradation to biomolecules at high laser fluences. In order to overcome limitations of the existing methodologies, a combination of both techniques (referred below as fast ex-situ synthesis) represents a novel single-step method [53] for design of highly-controlled biofunctionalized nanoparticle surfaces. In this method the sensitive molecules are not irradiated but promptly added to the formed nanoparticles, presented schematically in Fig. 5.14 (bottom). Our investigations demonstrate that longer time delays for biomolecule introduction (in the range of 200 ms to 120 s) induce exponential increase in bioconjugate sizes, varied from about 20 to 50 nm, under conditions when gold nanoparticles are conjugated with fluorophore-labeled model peptides using 6 mJ, 40 ns laser pulses at 3 kHz and 1,064 nm wavelength. The central position of the plasmon resonance band follows the size of nanoparticle bioconjugates. Conjugation efficiency and bioconjugate stability also correlates with the observed size-variation. By identifying the amount of non-conjugated biomolecules, it was found that the conjugation efficiency depends exponentially on the delay time. At 200 ms delay time the smallest nanoparticle size is 20 nm, and the highest conjugation efficiency of 93 % is reached. The importance of delay time for the quality of synthesized colloids is thus obvious. Our results confirm that primary nanoparticles, synthesized by laser ablation in solution, continue to grow on the multisecond time scale, until achieving their final sizes.
8 Conclusion
We have focused here on laser printing, replication, generation, and conjugation of nanoparticles. Nanoparticles and ordered nanoparticle arrays can be fabricated from metal, semiconductor, dielectric and polymer materials. The described technologies are very simple and allow high throughput fabrication of nanomaterials for different applications.
References
S. Sundaram, E. Mazur, Inducing and probing non-thermal transitions in semiconductors using femtosecond laser pulses. Nat. Mater. 1, 217–224 (2002)
F. Korte, J. Koch, B.N. Chichkov, Formation of microbumps and nanojets on gold targets by femtosecond laser pulses. Appl. Phys. A 79, 879–881 (2004)
J. Koch, F. Korte, T. Bauer, C. Fallnich, A. Ostendorf, B.N. Chichkov, Nanotexturing of gold films by femtosecond laser-induced melt dynamics. Appl. Phys. A 81, 325–328 (2005)
A.I. Kuznetsov, J. Koch, B.N. Chichkov, Nanostructuring of thin gold films by femtosecond lasers. Appl. Phys. A 94, 221–230 (2009)
A.I. Kuznetsov, J. Koch, B.N. Chichkov, Laser-induced backward transfer of gold nanodroplets. Opt. Express 17(2), 18820–18825 (2009)
A.I. Kuznetsov, C. Unger, J. Koch, B.N. Chichkov, Laser-induced jet formation and droplet ejection from thin metal films. Appl. Phys. A 106, 479–487 (2012)
A.I. Kuznetsov, A.B. Evlyukhin, C. Reinhardt, A. Seidel, R. Kiyan, W. Cheng, A. Ovsianikov, B.N. Chichkov, Laser-induced transfer of metallic nanodroplets for plasmonics and metamaterial applications. J. Opt. Soc. Am. B 26, B130–137 (2009)
A.B. Evlyukhin, A.I. Kuznetsov, S.M. Novikov, J. Beermann, C. Reinhardt, R. Kiyan, S.I. Bozhevolnyi, B.N. Chichkov, Optical properties of spherical gold mesoparticles. Appl. Phys. B 106, 841–848 (2012)
S. Aksu, A.A. Yanik, R. Adato, A. Artar, M. Huang, H. Altug, High-throughput nanofabrication of infrared plasmonic nanoantenna arrays for vibrational nanospectroscopy. Nano Lett. 10(7), 2511–2518 (2010)
J. Henzie, M.H. Lee, T.W. Odom, Multiscale patterning of plasmonic metamaterials. Nat. Nanotechnol. 2(9), 549–554 (2007)
A.I. Kuznetsov, A.B. Evlyukhin, M.R. Gonc-alves, C. Reinhardt, A. Koroleva, M.L. Arnedillo, R. Kiyan, O. Marti, B.N. Chichkov, Laser fabrication of large-scale nanoparticle arrays for sensing applications. ACS Nano 5(6), 4843–4849 (2011)
L. Novotny, N. van Hulst, Antennas for light. Nat. Photonics 5(2), 83–90 (2011)
A. Alù, N. Engheta, Theory, modeling and features of optical nanoantennas. IEEE Trans. Antennas Propag. 6, 1508–1517 (2013)
N. Liu, H. Guo, L. Fu, S. Kaiser, H. Schweizer, H. Giessen, Three-dimensional photonic metamaterials at optical frequencies. Nat. Materi. 7(1), 31–37 (2007)
N. Liu, H. Liu, S. Zhu, H. Giessen, Stereometamaterials. Nat. Photonics 3(3), 157–162 (2009)
A.B. Evlyukhin, C. Reinhardt, A. Seidel, B.S. Luk’yanchuk, B.N. Chichkov, Optical response features of Si-nanoparticle arrays. Phys. Rev. B 82, 045404 (2010)
A.B. Evlyukhin, C. Reinhardt, B.N. Chichkov, Multipole light scattering by nonspherical nanoparticles in the discrete dipole approximation. Phys. Rev. B 84, 235429 (2011)
L. Cao, P. Fan, A.P. Vasudev, J.S. White, Z. Yu, W. Cai, J.A. Schuller, S. Fan, M.L. Brongersma, Semiconductor nanowire optical antenna solar absorbers. Nano Lett. 10(2), 439–445 (2010)
P. Spinelli, M. Verschuuren, A. Polman, Broadband omnidirectional antireflection coating based on subwavelength surface mie resonators. Nat. Commun. 3, 692 (2012)
L. Cao, P. Fan, E.S. Barnard, A.M. Brown, M.L. Brongersma, Tuning the color of silicon nanostructures. Nano Lett. 10(7), 2649–2654 (2010)
K. Seo, M. Wober, P. Steinvurzel, E. Schonbrun, Y. Dan, T. Ellenbogen, K.B. Crozier, Multicolored vertical silicon nanowires. Nano Lett. 11(4), 1851–1856 (2011)
S.M. Wells, I.A. Merkulov, I.I. Kravchenko, N.V. Lavrik, M.J. Sepaniak, Silicon nanopillars for field-enhanced surface spectroscopy. ACS Nano 6(4), 2948–2959 (2012)
A.B. Evlyukhin, S.M. Novikov, U. Zywietz, R.L. Eriksen, C. Reinhardt, S.I. Bozhevolnyi, B.N. Chichkov, Demonstration of magnetic dipole resonances of dielectric nanospheres in the visible region. Nano Lett. 12(1), 3749–3755 (2012)
A.I. Kuznetsov, A.E. Miroshnichenko, Y.H. Fu, J. Zhang, B. Luk’yanchuk, Magnetic light. Scientific reports, vol. 2 (2012)
Y. Xia, G.M. Whitesides, Soft lithography. Annu. Rev. Mater. Sci. 28, 153–84 (1998)
S.J. Clarson, J.A. Semlyen (eds.), Siloxane Polymers (Prentice Hal, Englewood Cliffs, 1993)
J.P. Rolland, R.M. Van Dam, D.A. Schorzman, S.R. Quake, J.M. DeSimone, J. Am. Chem. Soc. 126, 8349–8349 (2004)
J.P. Rolland, B.W. Maynor, L.E. Euliss, A.E. Exner, G.M. Denison, J.M. DeSimone, Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 127, 10096–10100 (2005)
K.J. Pekarek, J.S. Jacob, E. Mathiowitz, Double-walled polymer microspheres for controlled drug-release. Nature 367, 258–260 (1994)
M.C.W. van Boxtel, R.H.C. Janssen, D.J. Broer, H.T.A. Wilderbeek, C.W.M. Bastiaansen, Polymer-filled nematics: a new class of light-scattering materials for electro-optical switches. Adv. Mater. 12, 753–757 (2000)
M.N.V.R. Kumar, A review of chitin and chitosan applications. React. Funct. Polym. 46, 1–27 (2000)
Y.J. Zhao, X.W. Zhao, J. Hu, J. Li, W.Y. Xu, Z.Z. Gu, Z.Z. Multiplex, Label-free detection of biomolecules with an imprinted suspension array. Angew. Chem. Int. Ed. 48, 7350–7352 (2009)
F. Danhier, E. Ansorena, J.M. Silva, R. Coco, A. Le Breton, V. Préat, PLGA-based nanoparticles: an overview of biomedical applications. J. Controlled Release 161, 505–522 (2012)
A.V. Kabanov, S.V. Vinogradov, Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew. Chem. Int. Ed. 48, 5418–5429 (2009)
S.E. Gratton, P.D. Pohlhaus, J. Lee, J. Guo, M.J. Cho, J.M. DeSimone, Nanofabricated particles for engineered drug therapies: a preliminary biodistribution study of PRINT nanoparticles. J. Controlled Release 121, 10–18 (2007)
P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice (Oxford University Press, New York, 1998), p. 160
S. Besner, A.V. Kabashin, M. Meunier, Two-step femtosecond laser ablation-based method for the synthesis of stable and ultra-pure gold nanoparticles in water. Appl. Phys. A. 88, 269 (2007)
F. Mafuné, J. Kohno, Y. Takeda, T. Kondow, H. Sawabe, Formation of gold nanoparticles by laser ablation in aqueous solution of surfactant. J. Phys. Chem. B 105, 5114 (2001)
A.V. Kabashin, M. Meunier, Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J. Appl. Phys. 94, 7941 (2003)
L. Sajti, S. Giorgio, V. Khodorkovsky, W. Marine, Femtosecond laser synthesized nanohybrid materials for bioapplications. Appl. Surf. Sci. 253, 8111 (2007)
H. Usui, Y. Shimizu, T. Sasaki, N. Koshizaki, Photoluminescence of ZnO nanoparticles prepared by laser ablation in different surfactant solutions. J. Phys. Chem. B. 109, 120 (2005)
A. Hahn, S. Barcikowski, Production of bioactive nanomaterial using laser generated nanoparticles. J. Laser Micro/Nanoeng. 4, 51 (2009)
A. Barchanski, N. Hashimoto, S. Petersen, C.L. Sajti, S. Barcikowski, Impact of spacer and strand length on oligonucleotide conjugation to the surface of ligand-free laser-generated gold nanoparticles. Bioconj. Chem. 23(5), 908–915 (2012)
A. Abdolvand, S.Z. Khan, Y. Yuan, P.L. Crouse, M.J.J. Schmidt, M. Sharp, Z. Liu, L. Li, Generation of titanium-oxide nanoparticles in liquid using a high-power, high-brightness continuous-wave fiber laser. Appl. Phys. A. 91, 365 (2008)
R. Kelly, A. Miotello, Comments on explosive mechanisms of laser sputtering. Appl. Surf. Sci. 96–98, 205 (1996)
A. Miotello, R. Kelly, Laser-induced phase explosion: new physical problems when a condensed phase approaches the thermodynamic critical temperature. Appl. Phys. A. 69, 67 (1999)
L. Sajti, R. Sattari, B. Chichkov, S. Barcikowski, Gram scale synthesis of pure ceramic nanoparticles by laser ablation in liquid. J. Phys. Chem. C. 114, 2421 (2010)
C.L. Sajti, R. Sattari, B. Chichkov, S. Barcikowski, Ablation efficiency of alpha-Al\(_2\)O\(_3\) in liquid phase and ambient air by nanosecond laser irradiation. Appl. Phys. A. 100, 203–206 (2010)
M. Bruchez, M. Moronne, P. Gin, S. Weiss, A.P. Alivisatos, Semiconductor nanocrystals as fluorescent biological labels. Science 281, 2013 (1998)
K. Sokolov, J. Aaron, B. Hsu, D. Nida, A. Gillenwater, M. Follen, Optical systems for in vivo molecular imaging of cancer. Technol. Cancer Res. Treat. 2, 491 (2003)
J.P. Sylvestre, S. Poulin, A.V. Kabashin, E. Sacher, M. Meunier, J.H.T. Luong, Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J. Phys. Chem. B. 108, 16864 (2004)
L. Sajti, S. Petersen, A. Menéndez-Manjón, S. Barcikowski, In situ bioconjugation in stationary media and in liquid flow by femtosecond laser ablation in liquid. Appl. Phys. A. 101, 259–264 (2010)
L. Sajti, A. Barchanski, P. Wagener, S. Klein, S. Barcikowski, Delay time and concentration effects during bioconjugation of nanosecond laser-generated nanoparticle in liquid flow. J. Phys. Chem. C. 115(12), 5094–5101 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Barchanski, A. et al. (2014). Laser Generation and Printing of Nanoparticles. In: Veiko, V., Konov, V. (eds) Fundamentals of Laser-Assisted Micro- and Nanotechnologies. Springer Series in Materials Science, vol 195. Springer, Cham. https://doi.org/10.1007/978-3-319-05987-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-05987-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05986-0
Online ISBN: 978-3-319-05987-7
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)