Abstract
TRP channels comprise one of the most rapid growing research topics in ion channel research, in fields related to ion channels including channelopathies and translational medicine. We provide here a critical survey on our current knowledge of TRP channels and highlight some of the still open or controversial questions. This comprises questions related to evolution of TRP channels; biophysics, i.e., permeation; pore properties and gating; modulation; the still-elusive 3D structure; and channel subunits but also their role as general sensory channels and in human diseases. We will conclude that our knowledge on TRP channels is still at the very beginning of an exciting research journey.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- TRP channels
- Evolution
- Pores
- Gating
- Associated proteins
- Subunits
- Modulation by calcium
- Sensory physiology
- Phosphoinositides
- Channelopathy
- TRPs in networks
1 Introduction
There is no doubt: The discovery of the trp gene superfamily which encodes TRP (transient receptor potential) cation channels has opened a highly exciting field to understand physiological and pathophysiological basic mechanisms of cell functions which were until this time inaccessible. This book reviews many examples reaching from sensory physiology, such as vision, hearing, and touch, to homeostatic functions such as Ca2+ and Mg2+ (re)absorption and thermo-regulation and to hereditary diseases ranging from stationary night blindness to neurodegenerative disease, kidney diseases, chronic pain, and skeletal abnormalities (reviewed in Nilius and Owsianik 2010b, 2011; Nilius et al. 2007). If we date the advent of “TRP research” with the isolation of the Drosophila “trp” (transient receptor potential) mutant (Cosens and Manning 1969), approximately 20 years passed until the Drosophila trp gene was cloned (Montell and Rubin 1989) and a role of TRP ion channels was explicitly mentioned (Hardie and Minke 1992, 1993). Now, again 20 years later, the progress in this field is overwhelming, but there are still many open questions, controversies, and surprises. Only some of them can be addressed in such a review, and we do not claim any complete view but will provide a few snapshots.
2 The Basics: Nomenclature
As always in the beginning of a rapidly extending research field, some Babylonian language disasters happened. Some TRP clones received up to five different names, e.g., OTRPC4, VR-OAC, TRP12, VRL-2, and presently TRPV4 (Everaerts et al. 2010). Fortunately, this Babylonian disaster was at least partially solved by agreeing on a unifying nomenclature based on homology (Montell et al. 2002). All 8 subfamilies were given the name TRP (for transient receptor potential, maybe not the best decision) followed by the subfamily indicators, C, V, M, A, P, ML, and N.
However, one problem recently appeared: The polycystin family comprises 5 TRPPs (TRPP1–5). However, only three are really channel proteins. Therefore, it was suggested by a recent review from the Clapham group that this family only contains the three channel members, TRPP1 (previously known as TRPP2 or PKD2, PC2 (polycystin 2) derived from the second gene which causes autosomal dominant polycystic kidney disease (ADPKD)), TRPP2 (previously TRPP3, polycystin-like (PCL) or PKD2-like 1 (PKD2L1)), and TRPP3 (also known as TRPP5 or PKD2L2) (Wu et al. 2010). The former TRPP1 (PKD1, PC1) and TRPP4 (PKD1L1) have been eliminated because they are indeed glycoproteins which contain a large N-terminal extracellular region and multiple transmembrane domains and have no homology with ion channels and also do not function as channels. This seems to be more logical but confuses again the ADPKD community. The HUGO nomenclature still considers the old description as TRPP2, TRPP3, and TRPP5 as the TRP family polycystin members. So we stick to the HUGO nomenclature throughout this volume.
3 Some Evolutionary Puzzles
TRPs appear relatively late in the evolution. No TRPs are expressed in bacteria in contrast to most of the well-described voltage-dependent ion channels (Fig. 1). They are expressed in some algae, yeast, and other fungi. Expression occurs in the unicellular choanoflagellate Monosiga brevicollis and importantly in many mammalian parasites. Five metazoan TRP subfamily members (TRPA, TRPC, TRPM, TRPML, and TRPV) were identified in choanoflagellates, demonstrating that they evolved before the emergence of multicellular animals (Fig. 1). One puzzle is the complete absence in plants (see for details Cai 2008; Prole and Taylor 2011, 2012). But why make these plants drugs for TRPs, like capsaicin, tetrahydrocannabinol, nicotine, and all the other spices being effective TRP modulators (Nilius and Appendino 2011, 2013)? They make them as “secondary metabolites” which are not essential for normal growth and development of the plant but appear to function in the defense of plants against herbivores and pathogens. TRPs are widely expressed in insects which may be targeted by these plant compounds (Matsuura et al. 2009). For example, a plant can produce substances that result in adverse physiological effects in the insect, such as a bitter taste or even poisoning. Ironically, the medicinal applications and pharmacological effects of secondary metabolites in animals (insects) and humans are better understood than their functions in the plants that produce them (Liscombe and Facchini 2008).
4 Do We Have the Correct Clones? Contribution of Endogenous TRPs?
There is a certain discrepancy between annotated TRP entries in protein and gene databases (e.g., UniProtKB and Ensembl Genome Browser), TRP cDNAs used for functional expression studies in vitro, and, apparently, TRP proteins expressed in vivo. For example, for human TRPC3, most in vitro studies have been performed with a cDNA encoding an 848-amino acid (aa) protein which is different to the 836-aa TRPC3 UniProtKB entry and the predicted 921-aa TRPC3 entry in the Ensembl Genome Browser. For human TRPC1, most in vitro studies have been done with the TRPC1beta variant (759 aa, Zitt et al. 1996) or with the TRPC1alfa variant (793 aa, Zhu et al. 1995), although the endogenous human TRPC1 protein may well cover an additional ~100 aa extension at its N-terminus (Ong et al. 2013). In fact, the identity of the endogenous TRP proteins is only known to a certain level of approximation: There is little direct experimental evidence to what extent the DNA sequences are translated to real proteins. This limitation refers not only to TRP channel genes but to most protein-coding genes in humans and many other organisms. In 2013, more than 95 % of protein sequences provided by the UniProt resource came from translations of coding sequences generated by gene prediction programs. Only less than 5 % of the protein entries rely on sequence data obtained by direct protein sequencing, by Edman degradation or MS/MS experiments (http://www.uniprot.org). New developments in protein analytical methods including mass spectrometry-based targeted proteomics will improve this situation in the future; but current approaches heavily rely on antibodies to pull out the protein of interest from complex biological samples. Using three independent antibodies, the endogenous protein sequence of TRPV6 could be obtained by this approach (Fecher-Trost et al. 2013). Compared to the 725-amino acid (aa) annotated sequence, the “real” TRPV6 protein contained a 40-aa extension within its N-terminus. However, adequate antibodies for most TRP proteins are not generally available (Wu et al. 2010), and therefore protein sequence information on most TRPs using mass spectrometry based has still to be shown.
Because of their convenience, many laboratories use HEK293 cells for functional expression of TRP cDNAs. Trpc1 (Zhu et al. 1995) and the Trpc3 cDNAs (Zhu et al. 1996) were originally cloned from HEK293 mRNA. It cannot be avoided but is hardly considered in the interpretation of data that the endogenous TRP proteins of HEK cells (TRPC1,TRPC3, and, maybe, TRPC7 and TRPM4) associate with the TRP proteins of expressed cDNAs to form functional channel complexes (Zagranichnaya et al. 2005). Considering the assumed broad expression of Trp genes, a similar situation will exist in most cell lines used for heterologous expression studies.
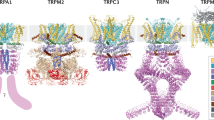
5 The Still-Elusive 3D Structure
It is agreed that all TRP consists of intracellular N- and C-termini and has six transmembrane spanning helices (TM) in which TM5 and TM6 probably form a classical channel pore. So far, only parts of TRP proteins have been crystallized, and the whole protein structure for some TRPs can be approached in electron cryomicroscopy pictures in a not very high resolution, e.g., for TRPV1 in a 19 Å resolution (Moiseenkova-Bell et al. 2008). Only 30 % of the channel protein is located in the plasma membrane (transmembrane domain TM); the other part forms a large cytosolic bulk. Both parts are connected by a fenestrated domain which might be important as an intracellular access to the TM region. Typical for the TRPV family and for TRPA1 is the N-terminal ankyrin repeat domain (ARD) (Fig. 3). TRPVs have an ARD formed by 6 ankyrin repeats (ARs) consisting of an inner and outer helix connected by unusual long “fingers” at the ARD’s “concave” side (Gaudet 2008a, b) which are considered to be quite flexible. TRPA1 is proposed to have an ARD consisting of 14–19 ARs (Wang et al. 2012). It is highly speculative whether this domain is involved in gating (probably for TRPA1 via covalent cys/lys modification) or whether it is mainly required for channel trafficking and insertion in the plasma membrane (Nilius et al. 2011) (Fig. 2).
Structure of TRPV1. (a) 3D reconstruction of TRPV1 obtained from cryo-electron microscopy. Inserted are in the plasma membrane part the atomic structures of Kv1.2 potassium channel (TM1–TM4, pink) and in the cytosolic part the TRPV1 ankyrin repeats (green). See the nice match of these structures. (b) High-resolution structure of Kv1.2 transmembrane domains. (c) TRPV1 ankyrin domains. Indicated is the ATP-binding site in TRPV1 [adapted from Lishko et al. (2007); Long et al. (2005); Moiseenkova-Bell et al. (2008) with permission]
Thus, the functional role of the ARD is still unknown. It provides ATP and calmodulin binding which interferes in the TRPV family with channel activation and desensitization (inactivation) (Lishko et al. 2007; Phelps et al. 2007, 2008, 2010). Characteristically, ankyrin repeats are connected by large “fingers” which might be important for channel–protein interactions. The ARD is involved in TRPV4 dysfunction in several channelopathies (Inada et al. 2012). Interestingly, the TM1-4 domains in TRPV1, TRPM4, and TRPM8 have some structural similarities with the TM domain of Kv1.2 including its voltage sensor (Nilius et al. 2005b). These TMs also nicely fit into the 3D structure of the transmembrane part of TRPV1 (Moiseenkova-Bell et al. 2008). It is extremely important to decipher a role of a potential voltage sensor in the TM region and the functional role of ARD in channel gating and trafficking. We refer to new and exciting data obtained from a 3D analysis of TRPV1 with a 3.4 A resolution to Chap. 11 in this book. “High-resolution views of TRPV1 and their implications for the TRP superfamily” by Ute. A. Hellmicu and Rachelle Gaudet.
All TRPM channels have coiled-coil domains, i.e., α-helices which form helical bundles of a preferred stoichiometry. This domain was crystallized in the C-terminus of TRPM7 where it is proposed to trigger tetramerization (Fujiwara and Minor 2008; Tsuruda et al. 2006). A similar domain in TRPP2 (PKD2) has been crystallographically analyzed, and the structure provides insight into TRPP2–PKD1 heteromerization (Yu et al. 2009; Zhu et al. 2011). Also the 3D structure of the TRPM7 kinase domain was analyzed by crystallography (Yamaguchi et al. 2001). However, these are only some snapshots into the TRP structure. Thus, the high-resolution 3D structure is highly expected to solve one of the mysteries of TRP channel gating.
6 α-Subunits and Elusive β-Subunits
Most of the TRP channels are most probably tetramers as shown first for TRPV1 (Kedei et al. 2001) and later for TRPV5 and TRPV6 (Hoenderop et al. 2003). Probably, nearly all TRPs form tetrahomomers. Several heteromeric TRP channels have been described. TRPP2 forms a trimer with PKD1 (for a review see Zheng 2013). Heteromeric channels are still a matter of debate, and their functional role must be evaluated. In the classical view, these tetramers are formed from the pore-carrying α-subunit. The mechanism of co-assembly is still not solved, although several mechanisms have been proposed. N-terminal ARD domains are protein–protein interaction sites (Sedgwick and Smerdon 1999). However, its role for α-subunit assembly is very much uncertain because isolated ARDs from TRPVs do not interact (Lishko et al. 2007; Phelps et al. 2007, 2008, 2010; Zhang et al. 2011) and TRPV1 and TRPV2 crystals do not multimerize (Jin et al. 2006; Lishko et al. 2007). Also for TRPA1, evidence has been reported that the ARD probably controls membrane insertion of the channels rather than tetramerization (Nilius et al. 2011). The role of coiled-coil domains for multimerization of TRPM channels has been discussed above. It is also reported that for TRPVs a region proximal of the TRP box might be required for subunit assembly (Zhang et al. 2011; Garcia-Sanz et al. 2004). Obviously, we do not yet understand the assembly of TRP subunits to form functional channel.
Another pertinent urgent problem to solve is the existence and possible function of β-subunits, i.e., proteins which bind to the α-subunit and modulate channel function by direct interference with channel gating or regulation of membrane insertion as studied extensively for voltage-operated Ca2+ channels (Richards et al. 2004; Hofmann et al. 2014). Such subunits are not yet discovered for TRP channels. A putative β-subunit, although not yet confirmed in detail, might be PACSIN 3, a member of a protein family that has been implicated in synaptic vesicular membrane trafficking and regulation of dynamin-mediated endocytotic processes. PACSIN 3 binds via its SH3 domain to a site in the proline-rich domain (PRD) proximal to the ARD in TRPV4 and not only increases channel insertion in the plasma membrane but also selects the activation mode of this channel, i.e., it suppressed activation by cell swelling which is thought to be an EET-dependent process (Cuajungco et al. 2006; D’hoedt et al. 2007).
A pertinent question is not yet answered: Is TRPC1 α-subunit? Originally described as a cation channel activated by store depletion (Mori et al. 2002; Zitt et al. 1996), it was later shown that TRPC1 proteins do not form functional channels by themselves (Storch et al. 2012; Strubing et al. 2001) but contribute to heterotetrameric TRPC channels, preferably with TRPC4 and TRPC5 (Hofmann et al. 2002; Strubing et al. 2001). Apparently, TRPC4 and TRPC5 but also TRPP2 (Tsiokas et al. 1999) and TRPV4 (Ma et al. 2010, 2011) pick up TRPC1 from the ER to the plasma membrane and the primary cilium (Bai et al. 2008), whereas in their absence TRPC1 may act as a leak channel of the ER (Berbey et al. 2009). Recently, it was shown that the full-length endogenous TRPC1 amplifies Ca2+ release-activated Ca2+ currents mediated by Orai channels (Ong et al. 2013) by direct interactions with components of the store-operated Ca2+ entry machinery (Liao et al. 2008; Singh et al. 2002; Yuan et al. 2007). The Trpc1 gene is supposed to be ubiquitously expressed, but how can one tell considering that there are no TRPC1 ion currents nor appropriate antibodies?
Another surprising example concerns the Xenopus TRPV6 homologue. xTRPC1 interacts with xTRPV6, thereby inhibiting the channel. TRPC1 is probably not part of the channel but regulates the activity of TRPV6 under physiological conditions (Schindl et al. 2012; Courjaret et al. 2013). Obviously, the search for TRP β-subunits is another high priority in TRP channel research.
Recently, it has been shown that an alternative splice variant of the mouse Trpa1 gene (TRPA1b) can physically interact with the main variant Trpa1 and increases the expression of TRPA1a in the plasma membrane. TRPA1a and TRPA1b co-expression significantly increases current density in response to different agonists without affecting their single-channel conductance. TRPA1 is obviously regulated through alternative splicing under physiological and also pathological conditions (Zhou et al. 2013). Thus, can such a variant in fact be considered as a β-subunit?
Another example came as a surprise. TRPM4 interacts with the sulfonylurea receptor 1 SUR1 and may confer the sensitivity of glibenclamide to this channel (Sala-Rabanal et al. 2012; Woo et al. 2013).
If a β-subunit is mainly defined as non-pore forming but with a positive effect on plasma membrane insertion of the α-subunit, then a β-subunit of voltage-dependent K+ channels, Kvβ2, must be considered as a novel TRP β-subunit. Kvβ2 interacts with TRPV1, increases the cell surface expression levels of this channel, and results in a significant increase of TRPV1 sensitivity to capsaicin, as functionally measured with patch clamp. Thus, Kvβ2 plays a role in TRPV1 channel trafficking to the plasma membrane (Bavassano et al. 2013). It is now intriguing to ask whether β-subunits among several channel families have a certain degree of promiscuity.
7 The Correct Expression Pattern
This is one of the main but still not completely solved problems. To solve this problem, specific TRP antibodies must be available, and transgenic models and reporter strategies must be employed to identify the correct cellular localization of TRP. The poor quality of available antibodies for TRPs has caused considerable frustration and may have led to publication and perpetuation of erroneous research results. It is true that to generate an antibody for a low abundant membrane protein like a TRP is a painstaking effort (Meissner et al. 2011) requiring months of laborious benchwork and money for synthesizing, expressing, and purifying the antigen, for hosting the suitable animals to be immunized, for generating and selecting hybridoma cells, and for purifying and characterizing the final antibodies. Especially the analysis of the antibody specificity is crucial. Only in very few cases, this major ordeal is accomplished by the many commercial suppliers that have proliferated and sell antibodies directed at a wide range of proteins including TRPs (Flockerzi et al. 2005; Ong et al. 2002, 2013; Wu et al. 2010).
In addition, we need to know the subcellular, e.g., intra-organelle, localization because intracellular TRPs are important functional players in cell organelles such as the endosomes, lysosomes, SR and ER, and Golgi apparatus (for a reviews see Cheng et al. 2010; Gees et al. 2010). Probably, also mitochondria may have TRP channels. TRPC3 is probably localized in mitochondria and carries a significant fraction of mitochondrial Ca2+ uptake that relies on extramitochondrial Ca2+ concentration. Up- and downregulation of TRPC3 expression influences also the mitochondrial membrane potential (Feng et al. 2013). It has to be noted that substantial discrepancies exist in the literature concerning TRP channel distribution. The use of mRNA detection is an indicator but has several drawbacks (Anderson and Seilhamer 1997; Anderson and Anderson 1998; Gygi et al. 1999; Pradet-Balade et al. 2001; Tew et al. 1996).
Immunohistochemistry relies on selective antibodies, which might be different from the ones used for protein detection in Western blotting. Radioactive ligands might be a complementary tool for detection of TRP channels. Now, promising is the genetic modification of a TRP channel locus which allows introduction of reporter genes such as lacZ or fluorescent proteins into living animals. Expression of these genes reflects the TRP distribution. Just as an example, many functions have been attributed to TRPV1 in the central nervous system, e.g., the development of cerebellar Purkinje cells and hippocampal pyramidal neurons and its contribution to glutamatergic synapse stabilization as important player in the neonatal cerebellar cortex and in the refinement of synaptic plasticity (Vennekens et al. 2012). It came as a big surprise that TRPV1 is probably even not expressed in these brain areas (Cavanaugh et al. 2011a, b). This causes another problem. Do we really know where the TRP channels that we wish to target are really located? A crucial test is always the detection of ion current through identified TRP channels (see below).
8 News About the Pore
As required for a channel protein, we do not understand yet the regulation of permeation. Is the pore of many TRPs in fact a dynamic structure contributing to gating much more than the known α-pore of classical channels (between TM5 and TM6)?
One of the most important functional features of many TRP pores is their permeability for Mg2+ and other heavy metals. The Mg2+ permeation is still a biophysical puzzle. So far, all ion channels refuse Mg2+ permeation because of its very high dehydration energy. TRP channels therefore provide a unique Mg2+ entry pathway. However, the biophysics behind is not yet unraveled (for a review see Owsianik et al. 2006).
There is now growing evidence that upon agonist stimulation some TRP channels such as TRPV1 (Chung et al. 2008) and TRPA1 (Karashima et al. 2010) show a dynamic pore behavior, i.e., a dilatation of the pore upon agonist binding. This dilatation is in the range of several Å. Mechanistically important seems to be the fact that this dilation of the TRPV1 pore decreases after cholesterol depletion of the plasma membrane (Jansson et al. 2013). In TRPA1, it causes an increase in divalent cation selectivity and fractional Ca2+ current and is therefore functionally very important. How does an agonist dilate the pore? Pore dilation has been also described in the TRPC family. Activation of TRPC4β with the G protein Gαi2 as compared with activation by GTPγS causes a small but significant dilation of the channel’s pore and increases Ca2+ permeability like the dilation of the TRPA1 pore (Jeon et al. 2013). This dynamic pore behavior needs to be explained soon because it will shed some light in the gating behavior of TRP channels. Of note, a pore dilation from 3 to 12 Å occurs by binding of G proteins to open the G-loop gate in GIRK2 potassium channels, a mechanism that has been explained by changes of the pore structure (Whorton and MacKinnon 2011).
Another route to follow is even more spectacular. TRP channels might posses and exploit under certain circumstances other pathways for ion conduction as shown for voltage-activated potassium and sodium channels. This pathways comprise a permeation gate through the voltage-sensing domain (TM1–TM4) of the channel, the ω-pore (Starace and Bezanilla 2004; Tombola et al. 2007; Gamal El-Din et al. 2011; Jurkat-Rott et al. 2010; Prütting and Grissmer 2011). It might be possible that TRP channels have a similar pathway which is active during distinct modes of activation (Vriens et al. 2014).
9 Fractional Ca2+ Currents in TRPs
Importantly, most of the TRP channels have in fact a small Ca2+ selectivity, and the “fractional Ca2+ current” is often less than 5 %. However, for understanding the functional impact of TRP channels, we need to know which partition of the current under physiological condition is carried by Ca2+ compared with monovalent cation. We need to measure this “fraction” to check which amount of Ca2+ is really contributing to the current (Karashima et al. 2010; Samways and Egan 2011). Unfortunately, such measurements are rare (done for TRPV1, TRPA1, TRPM3, and TRPM8). TRPV5 and TRPV6 are the only “real” Ca2+ channels with fractional Ca2+ currents of ~100 %. TRPA1 and TRPM3, with a fractional Ca2+ current of ~20 %, can be considered as relatively efficient Ca2+ channels. Probably, all other TRP channels have fractional Ca2+ currents of less than 10 % (Gees et al. 2010). Importantly, TRPV1 is known as a TRP channel with a relatively high Ca2+ permeability (PCa/PNa ~ 10) (Owsianik et al. 2006). However, its fractional Ca2+ current is only in the range of 5 % of the total current. High fractional Ca2+ current so far is only measured for TRPV5, TRPV6, TRPM3, and TRPA1 (reviewed in Gees et al. 2010). Even for the Drosophila TRP and TRPL channel, which are regarded as highly Ca2+-selective channels, the fractional Ca2+ current is only in the range of 29 % and 17 %, respectively (Chu et al. 2013).
Knowing the fractional Ca2+ current will also at least partially answer the questions: which increases in [Ca2+]i can be expected under physiological conditions and whether it is likely that the TRP-induced depolarization is essential rather than the Ca2+ influx. Obviously, TRP channels are functionally coupled with Ca2+-sensing effector proteins and may therefore achieve a high signal gain. It is therefore probably an important feature of TRP channels to provide only a low fractional Ca2+ current or in the case of high fractional Ca2+ current have only a low-density expression.
Obviously, these issues have to be urgently addressed for TRPC channels.
10 Gating
TRP channels have been widely described as receptor-activated channels, ligand-activated channels, and “directly” gated channels. However, some TRP channels are constitutively open, at least in several heterologous expression systems (e.g., TRPV5, TRPV6, TRPC3, TRPA1, and others) raising the question whether a crucial gating partner is missing in the expression system. This question of constitutive open TRP channels seems to be especially intriguing in the brain: TRPV4 is active at physiological brain temperature in hippocampal neurons and thereby controls their excitability in vitro. Local changes in temperature, which occur in the brain, cause modulation of brain activity, e.g., local cooling might reduce neuronal electric excitability via a decreased TRPV4 activity and thereby contribute to brain functions (Shibasaki et al. 2007; Tominaga 2013, personal communication).
The question whether TRP channels like some K2P channels are responsible for background currents is unanswered.
Receptor activation comprises G protein-coupled receptors (GPCRs) and receptor tyrosine kinases that activate phospholipases C (PLCs) which gate/modulate TRP channel activity by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), production of diacylglycerol (DAG) or inositol 1,4,5-trisphosphate (IP3), and subsequent liberation of Ca2+ from intracellular stores. Ligand activation is due to exogenous small organic molecules, e.g., capsaicin, endogenous lipids, or products of lipid metabolism (diacylglycerols, phosphoinositides, eicosanoids, anandamide), and purine nucleotides and their metabolites (adenosine diphosphoribose (ADP-ribose), βNAD) but also by proteins (like G proteins for TRPCs).
Direct activation can occur via changes of temperature, changes in the membrane potential (voltage activation), and maybe also mechanical forces (Nilius and Owsianik 2011; Ramsey et al. 2006; Wu et al. 2010).
Thus, TRP channels are polymodal channels, opposite to voltage-gated channels, which only sense changes in the transmembrane electric field. TRP channel might be considered as integrators of multiple cellular signals and might be preferred coincidence detectors (Ramsey et al. 2006). However, this makes the identification of a basic gating mechanism difficult if not impossible, and probably each channel exploits multiple mechanisms to open the gate. So far, we do not have any structural insight how the gating process is initiated (Nieto-Posadas et al. 2011).
Let’s first consider possibilities of direct gating. Maybe the most intriguing mechanism is TRP gating by depolarization. In this view, gating would share some similarities with the activation mechanism of classical voltage-dependent channels. However, half maximal activation occurs at very positive membrane potentials, which are physiologically irrelevant. However, several signals, like temperature changes and agonist binding, cause a shift in the activation curve toward more negative potentials, thereby increasing the fraction of open channels under steady state conditions (Nilius et al. 2005b; Voets et al. 2004). This paradigm “gating by shift” has been described for several channel types (TRPV1, TRPA1, TRPM4, TRPM8, etc.) but is mechanistically not yet understood. The shift is supported by a small apparent gating charge of TRP channels which is in the range of 0.5–0.8e (Nilius et al. 2005b; Voets et al. 2007; Zheng 2013). A proposed voltage-sensing role of basic residues in TM4 and the TM4–TM5 linker of TRPM8 might be considered as a first hint (Voets et al. 2007). Although the architectural and pharmacological similarities exist between TRP channels and Shaker K+ channels, replacing previously identified domains and critical structural motifs of the membrane-spanning portions of Kv2.1 with corresponding regions of two TRP channels, TRPM8 and TRPV1, did not allow the formation of hybrid channels, i.e., the TM3b–TM4 paddle motif of Kv2.1 can be replaced by the analogous regions of both TRP channels without abolishing voltage activation, but swapping putative voltage-sensing TRP channel regions with the Kv2.1 voltage sensor yields nonfunctional channels (Kalia and Swartz 2013). Obviously, such studies should be extended. Obviously, the true voltage sensors need to be pinned down. Probably, the measurement of gating currents should be envisaged. Because of the small gating charge of TRP channels [0.4–0.9e (Nilius et al. 2005b)], better methods must be applied, e.g., the limited slope method (Almers 1978; Sigg and Bezanilla 1997), to measure in a low open probability range the effective gating charge or changes in gating charge caused by mutations in a putative voltage sensor (see Voets et al. 2007). From the voltage dependence point of view, temperature and agonists are modulators of the voltage-dependent opening of the channel gate. The true nature of this shift is still elusive. However, the existence of the open probability shift is a clear fact that needs to be explained (for a critical discussion and challenges of this simplified view see, e.g., Nieto-Posadas et al. 2011; Zheng 2013).
Direct channel gating is caused by the covalent modification of cysteine and lysine residues. Such covalent modifications of Cys and Lys residues in the N-terminus of TRPA1 by electrophiles have been extensively described (for a comprehensive review, see Nilius et al. 2012). However, there are many issues to consider. First, covalent modification by electrophilic compounds may result in the formation of Michael adducts, thiocarbamates, alkylation products of cysteine, and disulfides. The chemistry of these modified residues is not known, i.e., the subsequent intracellular modification to make the channel again available. Second, it is not yet completely clear which cysteines are really required for gating. Critical in mouse TRPA1 are C415, C422 (in the ARD), and C622 (between ARD and TM1) and in human C622, C642, and C666 (between ARD and TM1); in addition, modification of K710 is involved. However, 30 from the 31 cysteine are reactive 174, 193, 415, 422, 463, 609, 622, 634, 642, 666, and 859, and in addition four sulfide intra-chain bridges are formed between cys666-662, cys666-463, cys622-609, and cys666-193. Eleven cysteines have been identified to be possibly involved in gating (Fig. 3).
(a) Two-dimensional representation of a TRPA1 dimer indicating the reactive cysteine residues. The cytoplasmic N- and C-termini are separated by six transmembrane helices. Each monomer contains 31 cysteine residues (blue circles), and the N-terminus has an extended ankyrin repeat domain (box). (b) TRPA1 is shown in the cryo-electron microscopic 3D structure. The modeled TRPV1 TM domain fits into the TRPA1 structure (blue ribbon). The TRPA1 N-terminus (green) and the C-terminus are shown. (c) Conserved cysteines involved in electrophilic activation in the mouse (Cys-415, Cys-422, Cys-622), human (Cys-622, Cys-642, Cys-666), and Drosophila (Cys-622, Cys-642) homologues are highlighted in the zoomed-in view (ball-and-stick structures and labeled, right panel). Histidines involved in zinc activation are also indicated in yellow (adapted from Cvetkov et al. (2011) with permission)
This disulfide bonding might be also involved in channel gating via an unknown mechanism. Probably, critical N-terminal cysteine residues involved in electrophilic activation are located at the interface between neighboring subunits, and conformational changes at this region might lead to unknown conformational changes resulting in channel activation (Cvetkov et al. 2011; Wang et al. 2012). How the covalent modification of the intracellular core signals to the channel gate is completely unknown. Third, surprisingly, that activation of the channel in inside-out patches is difficult and requires the presence of MgATP or polyphosphates (Kim and Cavanaugh 2007; Karashima et al. 2008). However, channel gating by non-electrophilic agonists such as Δ9-THC and menthol is still possible (Cavanaugh et al. 2008). It seems, yet not at all understood, that some intracellular factors, such as polyphosphates, may act as a scaffold to stabilize the channel in an available conformation.
Another direct form of gating remains mechanistically also enigmatic and came as a surprise. Heavy metals such as Zn2+, Cd2+, and Cu2+ can directly gate TRPA1. Activating Zn2+ binds to the C-terminal His983 and Cys1021 (Andersson et al. 2009; Banke and Wickenden 2009; Gu and Lin 2010; Hu et al. 2009b).
Another gating puzzle emerged with the identification of an obvious role of the pore region as part of the gating machinery. Activation of TRPV1 occurs by low pH and requires the pore residues E648 (E600 for potentiation of TRPV1 activity by low pH). Surprisingly, the non-charged T633 is also required for proton activation but not for activation by agonists (Ryu et al. 2007). In addition, TRPV1 is also activated by the double-knot toxin DkTx from the Earth tiger tarantula. This activation requires binding of the toxin to residues in the outer pore (Bohlen et al. 2010). This binding probably causes conformational changes in the pore region, thereby opening the channel gate (Bright and Sansom 2004). Also the inner pore of some TRP channels, TRPV1, TRPV3, TRPV4, and TRPA1, seems to be involved in direct channel gating as evidenced from mutagenesis experiments. TM6 in TRPA1 may possess two points of flexibility which could function as a gating hinge similar to Kv channels (Benedikt et al. 2009; Bright and Sansom 2004). Specific point mutations within the cytosolic TM4–TM5 linker of TRPA1 (N885S) (Kremeyer et al. 2010) or TRPV3 (G573C or G573S) (Asakawa et al. 2006; Xiao et al. 2008c) or the introduced G503S (TRPC4) and G504S (TRPC5) mutations in the corresponding sequences of TRPC4 and TRPC5 (Beck et al. 2013) lead to constitutively active channels indicating that the non-mutated amino acid residues are essential to keep the channels in a gateable configuration.
Obviously, many TRP channels are activated by binding of agonists. In this view, they can be considered as “ligand”-activated channels. Such ligands function endogenously or are externally applied. The most studied ligand is certainly capsaicin, a TRPV1 agonist. Capsaicin activates TRPV1 with K1/2 values varying with temperature and voltage. Capsaicin binding occurs to the closed channel state probably at several binding sites mapped in the TM2–TM4 region (Jordt and Julius 2002), but other sites have been identified at the TRP box, the pore domain, and the N- and C-termini TRPV1. The docking of the capsaicin molecule to the interface between two channel monomers in the TM3/TM4 region, i.e., the probably voltage-sensing domain, has been analyzed in detail. The vanillyl moiety forms a π−π stacking and hydrophobic interactions with Tyr511 and H-bonding with Ser512. In addition, the carbonyl group (B region) made H-bonding interactions with Tyr511 and Lys571. This binding favors an open-channel configuration (Lee et al. 2011). Although this structural modeling is intriguing, we still have no clue, even for this most advanced case, how the channel gates by ligand binding. The existence of probably more binding sites with positive cooperativity makes the underlying mechanism of channel opening still more uncertain not to speak of the still-elusive docking sites for endogenous agonists like the endocannabinoid anandamide and 2-arachidonoylglycerol (2-AG) and the lipid 12-HPETE. The situation for other TRP channels is still much less clear. Certainly, we have to fill the gap how ligand binding gates a TRP channel and binding studies have to be connected with functional data.
The situation of pinning down clear-cut gating mechanisms for TRPCs seems especially difficult. What do we really understand of TRPC gating? Obviously, Gi,o and Gq,11 are required at the same time to activate TRPC4/TRPC5. Simultaneously, an elevation of [Ca2+]i is facilitating. Activation is also voltage dependent, and PI(4,5)P2 is a unique negative gating modulator (Otsuguro et al. 2008; Tsvilovskyy et al. 2009; Zholos et al. 2004). The complexity of these gating mechanisms is striking and puzzling. In addition, it has also been shown that TRPC4/TRPC5 are activated by Gαi rather than Gq/11. But what about the endogenous environment of the cell systems used? Gi,o and Gq,11 appear to be present in most cell lines used for Trp cDNA expression, but what about the receptors activating these G proteins (http://www.uni-leipzig.de/~strotm/molbio/endogenous_gpcr.htm)? In another approach, interaction of C-terminal SEC14-like and spectrin-type domain SESTD with Gαi was required for gating. What is the mechanism (Jeon et al. 2008, 2012, 2013)?
11 Positive and Negative Ca2+ Feedback
Probably all TRP channels are modulated by Ca2+. Most TRPs show an activity-dependent inactivation which is mediated by Ca2+ and involves as possible mechanisms Ca2+-dependent kinases, Ca2+-dependent phosphatases, Ca2+-regulated PLCs, and especially calmodulin. The mechanisms of this modulation is not clear (Gordon-Shaag et al. 2008); often Ca2+-dependent PI(4,5)P2 depletion is involved (Rohacs 2013; Rohacs and Nilius 2007). In many cases, this modulation is bimodal, e.g., comprises activation and negative feedback inhibition. As for all Ca2+-permeable channels, negative feedback inhibition is extremely useful to avoid cellular Ca2+ overload. But do we really understand this mechanism? Some TRP channels such as TRPM4 and TRPM5 are directly activated by Ca2+. Indeed, an increase in [Ca2+]i is required to activate these channels in a voltage-dependent manner. Without Ca2+, even very large depolarizations cannot activate the channel. Thus, this situation is different from, e.g., BKCa channels. Unfortunately, an activating binding site for Ca2+ has not been reliably determined. However, it has been shown that the presence of calmodulin which binds to probably several C-terminal sites shifts the voltage-dependent activation toward much more negative potentials (Nilius et al. 2005a). Again, the gating mechanism remains unclear. Both Ca2+-activated channels desensitize/inactivate upon prolonged exposure to elevated [Ca2+]i. This effect can be nicely explained by a Ca2+-dependent PI(4,5)P2 depletion via activation of a Ca2+-activated PLCβ4 (Nilius et al. 2006, 2008; Rohacs and Nilius 2007). A pleckstrin-like homology domain is required for PI(4,5)P2 binding. Another intriguing example for the dual regulation by Ca2+ is TRPA1. Channel activity, if it is activated, is strongly potentiated by extracellular Ca2+ followed by channel desensitization. Thus, Ca2+ is one of the most important endogenous modulators of TRPA1. Both mechanisms are not understood. TRPA1 is affected by [Ca2+]e due to entry through TRPA1 and subsequent elevation of intracellular calcium. The mutation of Asp918 in the putative TRPA1 pore greatly reduces Ca2+ permeability. Extracellular Ca2+ alone produced neither potentiation nor inactivation, e.g., channel activation is required. Application of Ca2+ to the cytosolic face of excised patches is sufficient to produce both potentiation and inactivation of TRPA1 channels. Moreover, in whole-cell recordings, elevation of intracellular Ca2+ potentiated, but did not inactivate TRPA1. Thus, potentiation and inactivation are two independent mechanistically not understood processes (Nilius et al. 2011, 2012; Wang et al. 2008). In this case, desensitization is probably much less attributed to an increased PI(4,5)P2 breakdown as for other channels such as TRPM4, TRPM8, TRPV5, and TRPV6 (Karashima et al. 2008; Rohacs and Nilius 2007) or is even opposite (Kim et al. 2009). These are only examples for the probably universal modulatory activity of Ca2+ on TRP channels. Because of the bimodal effect of even extracellular Ca2+ on TRPA1, the use of a TRP-current readout in a Ca2+-free solutions screening of channel modulators can easily generate false-negative (missing potentiation) or false-positive (lacking inactivation) results.
Because of the extremely important regulation of many TRP channels by Ca2+, it is indicated to search downstream of Ca2+ for effects of Ca2+-binding signaling proteins, e.g., calmodulin (CaM). CaM in most cases exerts an inhibitory effect on the channel gating. This negative feedback loop works locally and rapidly to regulate the level of channel activity suitable for cellular physiology. Activation of CaM by Ca2+ influx following TRP channel activation can also affect channel function indirectly, e.g., by binding of CaM to other proteins such as CaM-sensitive protein kinases. Given the diversity of TRP channels, it is perhaps not surprising that the sites of CaM binding are also quite diverse and multiple sites have been detected on many TRP channels at the N- and C-terminus (Zhu 2005). Because of this diversity, clear-cut functional data related to CaM should always substantiate such binding studies. As one striking example, the crystal form of the ARD of TRPV1 exists as a complex with CaM (Lishko et al. 2007). Thus, the ARD of TRPV1 (TRPV3, TRPV4) serves as a CaM-binding site. ATP was also found to bind to the CaM-binding site of TRPV1 and competes. CaM binding occurs in the absence of Ca2+. The functional impact of the ATP–CaM competition is accelerated channel desensitization in the presence of CaM and a delay in the presence of ATP and absence of CaM. The diversity of CaM action is also shown in the surprising findings that Ca2+ binding to CaM may underlie the Ca2+-dependent activation of TRPM4 (Nilius et al. 2005a) and the Ca2+-dependent potentiation of TRPV3 (Xiao et al. 2008b) and TRPV4 (Strotmann et al. 2003, 2010). Again, this diversity of CaM action needs to be understood and underpinned by more functional and structural analysis.
12 The PI(4,5)P2 Puzzle
One exciting aspect of TRP channel regulation is the interaction with and modulation by plasma membrane phosphatidylinositol phosphates (PIPs) and in particular by phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). Probably all TRPs are regulated by PIPs. PI(4,5)P2-binding sites in nearly all PIP-modulated TRPs have been proposed and functionally tested via mutagenesis (Nilius et al. 2008). PI(4,5)P2 positively modulates the activity of TRPM4, TRPM5, TRPM7, TRPM8, TRPV5, and TRPV6 but inhibits TRPC4α. Effects on TRPA1 are still somewhat controversial (reviewed in Rohacs 2013; Rohacs and Nilius 2007). Probably the most intriguing yet important problem to solve refers to a complex regulation of TRPV1 by PI(4,5)P2. It has been first shown that nerve growth factor (NGF) and bradykinin activate PLC, which hydrolyzes PI(4,5)P2 and subsequently sensitizes the channel through interactions with a region in the C-terminus (Chuang et al. 2001; Prescott and Julius 2003). The opposite effect has been later described. Indeed, depletion of PI(4,5)P2 in inside-out patches leads to channel inhibition, and direct application of PI(4,5)P2 activated the channel (Klein et al. 2008). Again somewhat later, a new transmembrane protein has been found, Pirt (phosphoinositide interacting regulator of TRP), that interacts with both PI(4,5)P2 and TRPV1, thereby inducing an activating effect on TRPV1. Thus, PI(4,5)P2 only activated TRPV1 in the presence of Pirt (Kim et al. 2008). However, this mechanism has been recently challenged. Pirt is probably not required for PI(4,5)P2 modulation of TRPV1. Pirt does not alter the phosphoinositide sensitivity of TRPV1 in HEK293 cells. Dorsal root ganglion neurons from Pirt knockout mice have an apparent affinity for PI(4,5)P2 indistinguishable from that of their wild-type littermates. A proximal C-terminal region of TRPV1 is sufficient for PI(4,5)P2 binding. This proximal C-terminal region of TRPV1 can interact directly with PI(4,5)P2 and may play a key role in PIP2 regulation of the channel. Importantly, Pirt does probably not bind to TRPV1 (Ufret-Vincenty et al. 2011). However, Pirt might be an accessory protein for other channels, e.g., TRPM8, and may act as a positive modulator under conditions of channel activation (Tang et al. 2013). In another approach, PLC activation by bradykinin leads to a moderate decrease in PI(4,5)P2 ), but no sustained change in the levels of its precursor PI(4)P. Preventing this selective decrease in PI(4,5)P2 inhibited TRPV1 sensitization, while selectively decreasing PI(4,5)P2 independently of PLC potentiated the sensitizing effect of protein kinase C (PKC) on the channel, thereby inducing increased TRPV1 responsiveness. Maximal pharmacological TRPV1 stimulation leads to a large decrease of both PI(4,5)P2 and its precursor PI(4)P. Attenuating the decrease of either lipid significantly reduced desensitization, and simultaneous reduction of PI(4,5)P2 and PI(4)P independently of PLC inhibited TRPV1. In this view, differential changes in phosphoinositide levels are mediated by probably distinct PLC isoforms which can result in opposite effects on TRPV1 (Lukacs et al. 2013). Recently, TRPV1 was inserted into artificial liposomes. It can be activated by capsaicin, protons, and heat. Under these conditions, TRPV1 is fully functional in the absence of phosphoinositides, i.e., does not require PI(4,5)P2 for activation. PI(4,5)P2, PI(4)P, and phosphatidylinositol inhibit TRPV1 (Cao et al. 2013). Obviously, there is still no unifying view how PIPs act on the probably best studied TRP channel, TRPV1. However, this modulation is critical for the understanding of TRPV1 function under conditions of inflammation and other disease-causing influences. In addition, we are still at the beginning of the beginning to understand specific actions of different PIPs on TRP channels. For the TRPML family, a completely different modulation pattern as for plasma membrane TRPs has been described. In endolysosome, PI(3)P and specifically PI(3,5)P2 are positive regulators for TRPML1–3. These lipids are also required for the correct channel trafficking. In late endosomes, PI(3,5)P2 is the positive regulator, whereas PI(4,5)P2 is a negative regulator, and its depletion activated TRPML1 (Cheng et al. 2010; Zhang et al. 2012; Dong et al. 2010) indicating a probably highly diverse modulation pattern of TRPs by PIPs.
13 Understanding Networks
The last example on modulation of TRPV1 by PI(4,5)P2 points to an integrated action of Ca2+ influx via the channel and activation of different isoforms of PLC (PLCδ, PLCβ4) and PKCε. Obviously, this is an example for a functional network which causes extremely variable channel reaction. Submaximal activation via bradykinin receptors (coupled to PLCβ4) causes a restricted PI(4,5)P2 depletion and no change in PI(4)P; activation of TRPV1 by PKCε activation via the Ca2+ influx overwrites the negative effect of PI(4,5)P2 depletion. On the other hand, maximal TRPV1 activation, e.g., by high concentrations of capsaicin, causes a large PI(4,5)P2 and PI(4)P depletion via PLCδ activation which overwrites activating effects of PKCε (Lukacs et al. 2013).
Another striking example for a network modulating function of TRPC1 has been recently published and is discussed here as another example. Protein kinase C (PKC) and PI(4,5)P2 are obligatory for the activation of TRPC1 in vascular smooth muscle cells (VSMCs). This activation is coordinated by myristoylated alanine-rich C kinase substrate (MARCKS) which is required for the TRPC1 channel’s activation by PKC and PI(4,5)P2. TRPC1 channels and MARCKS form signaling complexes. If PI(4,5)P2 is bound to MARCKS, TRPC1 is closed. Activators of TRPC1 channels induce PKC phosphorylation of TRPC1 proteins, which causes dissociation of TRPC1 subunits from MARCKS and a release of the bound PI(4,5)P2. The released PI(4,5)P2 may now bind to TRPC1 and causes channel opening. Thus, MARCKS regulates the native TRPC1 in VSMCs by acting as a reversible PI(4,5)P2 buffer, which in turn is regulated by PKC-mediated TRPC1 phosphorylation, and PI(4,5)P2 acts finally as gating ligand of TRPC1 (Shi et al. 2013a). Because of the complexity of such a mechanism in native cells, it is important to verify similar mechanisms in other TRPC1 expressing cell types!
These are only a very simple example that TRPs function in complex networks. Probably, network-based approaches may facilitate the understanding of TRP channel biology and their function in disease. Using databases of protein–protein interaction (PPI) (Chun et al. 2013), the multifunctionality of TRP channels might be better understandable. An example is given in Fig. 4a showing the number of interacting TRP channels with several proteins and in Fig. 4b representing the network of the interfering interaction of TRP channels with 3 proteins (ITPR3, inositol 1,4,5-trisphosphate receptor, type 3; CALM1, calmodulin 1; and the SRC tyrosine-protein kinase). Interestingly, comparing the similarity of TRP subfamilies based on PPI or sequence data may give additional information (Fig. 4c). We refer to a review on network-based approaches in biomedical science, which describes the current state of TRP channel network biology and which discusses a promising future direction of TRP channel research (Chun et al. 2013). However, as always with conclusions drawn from databases, they heavily rely on the data present in the databases. So far, no rigorous and comprehensive targeted high-resolution proteomic study is available on the nano-environment of any endogenous TRP channel. Such studies are available for other ion channels such as voltage-activated Cav2 channels (Muller et al. 2010) or AMPA receptors (Schwenk et al. 2012). The very few published studies on TRP proteomes even failed to identify the “targeted” TRP. As long as we do not know details of the specific makeup of endogenous TRP channel complexes, conclusions on networks may be premature.
(a) TRP channel-interacting proteins published to bind to multiple TRP channels. (b) CALM1, SRC, and ITPR3 interact with at least eight TRP channel isotypes within or across the subfamilies (light blue edges). Gray line marks the interactions among TRP channels. (c) Discordance between protein–protein interactions (PPI) and sequence similarities. PPI similarity denotes here how many interacting proteins are shared among TRPC channels. Sequence similarity is calculated using a standard protein BLAST tool (from Chun et al. (2013) with permission)
14 The Quest for Selective Tools
The pharmaceutical industry focuses on generation of small molecules, which are synthesized in vitro and screened with high-throughput methods. Although there is now considerable progress in the synthesis of highly selective and also reversible TRP channel modulators (like for TRPV1, TRPV4, TRPA1), this is not yet the case for all TRP channels. It is highly desirable to ask the pharmaceutical industry for distributing such selective tools especially if they are not golden bullets for the therapeutically useful TRP targets. Obviously, the progress in the research on TRPV4 was mainly triggered by the early release of the relatively selective agonist 4αPDD from Glaxo (Watanabe et al. 2002).
15 The Use of Natural Compounds
Natural compounds teach us a lot about TRP channels. The problem seems to be that they are difficult to synthesize, but is there not a huge intellectual quest for natural compounds? Since probably more than 5,000 years, mankind fights for spices added to our food. Many of them are just efficient TRP channel modulators (Nilius and Appendino 2011, 2013). Interestingly, some natural compounds have been used in traditional medicine, e.g., as antidepressants have TRP channels as targets. One of these compounds is incensole acetate which is released by the burning of resin from the Boswellia plant and has been used for religious and cultural ceremonies for millennia. It activates TRPV3, which is expressed in the brain and causes anxiolytic-like and antidepressant-like behavioral effects (Moussaieff and Mechoulam 2009; Moussaieff et al. 2008). St. John’s wort has been used medicinally for over 5,000 years. Relatively recently, one of its phloroglucinol derivatives, hyperforin, an antidepressant compound, has been identified as effective activator of TRPC6 (Leuner et al. 2007, 2010). Hyperforin has been shown to have cognitive-enhancing, memory-facilitating properties and has probably neuroprotective effects (Griffith et al. 2010). This example is only mentioned to open the interest of TRPists for natural compounds. Estimations are done that less than 1 % of all blooming plants have never been tested for natural compounds which might be useful as medical compounds. One has to remember that these molecules were evaluated during more than 3.5 billions of years in evolution! A striking example how optimized screening natural compounds, i.e., of flavanone derivatives from citrus plants, has been successfully used to identify highly potent blockers of TRPM3, a channel with a rather elusive and intractable pharmacology (Straub et al. 2013a, b).
16 Why Is It So Difficult to Measure TRP Currents in Native Cells?
TRP proteins form channels. Obviously, the most direct evidence that such a channel generates physiological signals is the detection of a current via TRP channels in native cells. It turned out that this is a difficult task. First, for reasons explained above, TRP channel expression might be low, and especially only few channels are located in the plasma membrane where they form available ion channels. Second, although the single-channel conductance of several TRPs might be large under conditions of permeation of monovalent ions, under physiological conditions in the presence of Ca2+, this conductance might be low. However, because of all difficulties with an exact localization of TRP channels in the plasma membrane, such a functional readout is unavoidable and necessary. As a striking example, when TRPV5 and TRPV6 were first described as Ca2+-selective ion channels (Nilius et al. 2000), in heterologous expression systems, whole-cell currents and channel current (in the absence of Ca2+) could be measured but not in the physiological most relevant native cell system, the Ca2+-reabsorbing cells in the rabbit kidney connecting tubule and cortical collecting duct epithelial cells from which the trpv5 gene was cloned (Vennekens et al. 2000); also TRPV5-dependent45Ca2+ fluxes could be measured. This is probably due to the low single-channel conductance if Ca2+ is the charge carrier (in contrast to a high conductance of 77pS if monovalent cations are the charge carrier). Currents are probably too small to be detected indicating the need of using more refined methods such as single-channel measurements and fluctuation analysis. On the other hand, the amount of eGFP-tagged TRPV5 (den Dekker et al. 2005) or TRPV6 protein (Fecher-Trost et al. 2013) routed to the plasma membrane after cDNA expression was too small to be detectable by confocal laser scanning microscopy. That means that also after cDNA expression the amount of functional channels in the plasma membrane is very low, like in native cells, but sufficient for current recording in one system but not in the other. May measuring native currents under divalent-free conditions improve this situation?
The measurement of currents through TRP channel is also very much complicated because of the lack of selective and reversible inhibitory tools. Also for Ca2+-activated TRP channels, TRM4 and TRPM5, identification of those currents is possible by selective activation of those channel by Ca2+ uncaging (Ullrich et al. 2005). However, in native cells, there are always the problems to dissect these currents through TRP channels from other Ca2+-activated currents. In addition, using “selective” tools like siRNA or comparison of currents in native cells from wild-type and knockout animals is difficult and often based on unreliable readouts. Thus, any functional mechanism involving TRP channels should address this problem of measuring TRP-mediated currents in native cells by the use of identified pharmacological agonist and antagonists which act reversible and selective. The problem of tiny current should be tried to overcome by fluctuation analysis and single-channel measurements, e.g., TRPM4 identification in inside-out patches from sinoatrial nodes (Demion et al. 2007).
17 Species Dependence
There are plenty of examples that activation and modulation of TRP channels are highly species dependent, also within mammalian species. Only in some examples, in the presence of saturating capsaicin, rat TRPV1 reaches full activation, with no further stimulation by protons. In contrast, human TRPV1 (hTRPV1) is potentiated by extracellular protons and magnesium, even at saturating capsaicin (probably due to differences in negatively charged residues in the TM3–TM4 linker) (Wang et al. 2010). Also in the search for effective TRPV1 antagonists, the significant lack of consistency of the pharmacology of many TRPV1 antagonists across different species has created a substantial obstacle (probably due to differences in the pore) (Papakosta et al. 2011). In the search for TRPA1 antagonists, trichloro(sulfanyl)ethyl benzamides were discovered to be active in humans but had no or even activating activity in rodents (Klionsky et al. 2007). Both rodent and rhesus monkey TRPA1 do not respond to extracellular acidosis, and protons even inhibited rodent TRPA1, but human TRPA1 is activated by protons (an effect probably caused by differences in TM5, TM6) (de la Roche et al. 2013). Electrophilic thiamines block human TRPA1 but activate rat TRPA1 (Chen et al. 2008). Non-electrophilic menthol is an activator of mouse TRPA1 at low concentrations and a blocker at high concentrations; but it only activates human TRPA1 (differences in the TM5–TM6 pore region) (Xiao et al. 2008a). Caffeine activates mouse TRPA1 but blocks human TRPA1 (Met268Pro point mutation in the mouse TRPA1 N-terminus changes activation into inhibition) (Nagatomo et al. 2010). Many more of such examples could be mentioned. Even more important, in invertebrates and ancestral vertebrates, TRPA1 serves as a heat receptor. In mammals, TRPA1 is probably a receptor for noxious cold. This is, however, controversial. Cold activates rat and mouse TRPA1 but not human or rhesus monkey TRPA1. At the molecular level, a single residue within the S5 transmembrane domain (G878 in rodent but V875 in primate) seems to account for the observed difference in cold sensitivity (Chen et al. 2013).
It might be important to consider that such species-dependent effects may give some insight into structural requirements of channel activation and modulation. It should also be mentioned that of course basic functional differences in organ function, e.g., the beat frequency in rodents as compared to human hearts, might substantially alter effects of TRP activation raising, e.g., the problem whether rodent models are in general appropriate for an extrapolation to humans (e.g., the TRPM4 effect in mice might be underestimated as compared to humans).
18 Are TRPs Really the Aristotle’s Main Sensory Players? Surprises?
Since their discovery, TRP channels have been always considered as the main players in the sensory system and were even considered as the main signal transducers providing via Aristotle’s classical five-sense recognition of the outer world (Damann et al. 2008). Vision is in Aristotle’s view the noblest of all five senses. So far, TRPs were only considered as photoactive channel in invertebrates. In fact, TRP and TRPL in Drosophila are main players in vision as many times excellently reviewed (Hardie and Postma 2008; Montell 2012). A more recently evolving topic in mammals concerns intrinsically photosensitive retinal ganglion cells (ipRGCs) which are discovered photoreceptors in the mammalian eye which mediate primarily nonimage visual functions, such as pupillary light reflex and circadian photo-entrainment, and are expected to respond to the absolute light intensity (for a recent reviews see Lucas 2013; Pickard and Sollars 2012). It is intriguing to unravel the functioning of this photo-sensing including possible TRP channels and its modulation by different chromophores (Schmidt et al. 2011). A first surprise, melanopsin-expressing photosensitive retinal ganglion cells (pRGCs) express TRPM1 and TRPM3 which are involved in the nonimage-forming responses to light (Hughes et al. 2012). Melanopsin signaling depends also on TRPC6 and TRPC7. Iris muscles isolated from nocturnal mammals such as mice contract when exposed to light through the action of a melanopsin-based signaling pathway. Ablating in mouse the expression of both TRPC6 and TRPC7 also eliminated the light response in the M1 subtype of melanopsin-expressing, intrinsically photosensitive retinal ganglion cells (M1 ipRGCs) (Xue et al. 2011).
At note, very surprisingly, it was recently shown that gating of Drosophila TRP involved not only a photosensitive step coupled to light sensing by rhodopsin but also a mechano-sensitive step (Hardie and Franze 2012). In general, we have to expect that polymodal stimuli, such as light, heat, and force, converge on chromophores which in turn modulate various TRP channels, as shown now in Drosophila (Kirkwood and Albert 2013).
Highly hypothetical but intriguing to consider is the possible existence of “deep brain photoreception” by extraocular light detectors also in mammals, e.g., in dopaminergic amacrine neurons, which may again settled by a melanopsin–TRP interaction. Such sensing may still form a connection with neuroendocrine cells for the control of mood (Fernandes et al. 2012, 2013).
TRPM1 has now been also described as a direct player in image-visual function. It is TRPM1 expressed on ON bipolar cells in the retina which invert the sign of light responses from hyperpolarizing to depolarizing before passing them on to ganglion cells (ON response). TRPM1 colocalizes with metabotropic glutamate receptor mGluR6 (GRM6) coupled to Gαo and Gβ3 proteins. Light responses are generated when TRPM1 is activated. The gating mode of this channel is still obscure: Unbinding of glutamate, which is released from adjacent rod photoreceptor cells, from mGluR6 activates TRPM1. Binding of glutamate to mGluR6 closes the channel. The signaling cascade is still not known. After activation, TRPM1 desensitizes and the light response decays (Koike et al. 2010; Morgans et al. 2009). Underlining the important role of this novel TRP-mediated vision pathway, TRPM1 is linked to the autosomal-recessive congenital stationary night blindness type 1C (CSNB1C), a retinal disorder characterized by nonprogressive impaired night vision and decreased visual activity due to ON bipolar cell dysfunction (Audo et al. 2009; Nakamura et al. 2010).
The question remains: Are other TRP channels involved in vision (Gilliam and Wense 2011)? TRPC1 has been detected in cones and rods which may hint to still unknown function beyond light sensing but regulation of Ca2+ homeostasis in photoreceptors (Molnar et al. 2012).
TRPs have now been more and more involved in light sensing in cells outside the visual system. Melanocytes sense UV light (mainly UVB light) via TRPM1-mediating melanogenesis (Devi et al. 2009). TRPA1 is also involved in UVB sensing and early melanogenesis; a retinal-dependent G protein-coupled signaling pathway is involved in TRPA1 activation (Bellono et al. 2013; Bellono and Oancea 2013). UVB light is also supposed to activate TRPV4 (Moore et al. 2013). Obviously, the mechanism underlying activation of TRP channels by light is of high interest.
It came also as a surprise that at least in rattlesnake and in bats TRPA1 can be activated by infrared light (Gracheva et al. 2010, 2011; Panzano et al. 2010; Geng et al. 2012; Yokoyama et al. 2011). Activation is reportedly due to heat response of TRPA1 above a temperature threshold for activation at ~28 °C. TRPA1 is a heat-activated channel in these species. Again, the underlying activation mechanism of TRPA1 is highly interesting. If snakes would detect temperature differences of 0.01 °C, which is probably even an underestimation (Ebert and Westhoff 2006), currents through TRPA1 would exhibit a Q10 of >1015. Obviously, this temperature sensing must involve a nonlinear signal amplification process upstream of channel opening. Vice versa, considering a Q10 of ~10, as typical for thermoTRPs, the increase in open probability induced by a warming of 0.003 °C would be only ~0.2 %! It is very unlikely that this would be enough to generate sufficient depolarization to trigger action potentials in the snake trigeminal neurons. One has also to consider that the radiation energy decays with the fourth power of the distance (Stefan–Boltzmann law), which would predict very small activation energies for TRPA1 in the snake’s pit organ. Of note, it has never been shown that TRPA1 antagonists impair the infrared sensing of snake or bats! However, infrared radiation sensing by TRP channels is a highly exciting, yet unsolved, topic.
In Aristotle’s view, touch, or now more generally described as “somato-sensitivity,” was the most primitive sense. However, somato-sensitivity is the most essential and necessary sense for survival, far beyond the other four senses. This has now become one of the most exciting topics in the TRP field. We will only refer to two examples: mechano- and temperature sensing.
How are TRP channels involved in mechano-sensation? There are several examples which are all reviewed in detail (described in the issue of HEP Plant and in Eijkelkamp et al. 2013). However, with the advent of completely unexpected mechano-sensing channels, Piezo1 and Piezo2, some of the earlier mechano-TRP concepts have certainly to be revised (for a critical discussion, see Nilius and Honore 2013). One recent finding might be mentioned to open our interest novel forms of mechano-sensing. Probably TRPV4 in glia cells can mechanically sense infrasound (16 Hz) which can even be involved in infrasound-caused neurodegeneration (Shi et al. 2013b).
Another important part of somato-sensing is temperature (less correct “thermo-”) sensing. It has been one of the most accomplishments in the TRP field to identify the so-called thermoTRPs which are supposed as the main temperature sensors. Without any doubt, some TRPs have a very high temperature sensitivity as expressed in Q10 values >10. Mechanistic aspects of thermosensing by TRPs have been addressed in many excellent reviews and will not be further mentioned (Dhaka et al. 2006; Ferrer-Montiel et al. 2012; Patapoutian et al. 2003; Vay et al. 2011; Voets 2012; Wetsel 2011) (also described in this volume by Voets, T. “TRP channels and thermosensation”). The list of thermoTRPs is constantly changing, e.g., TRPV2, TRPV3, and TRPV4 ( reviewed by Voets 2012; Nilius et al. 2014) are not anymore considered as functionally involved in temperature sensing, but TRPM3 must be added (Vriens et al. 2011). Several “thermo”TRPs are located in thermosensitive regions such as in skin keratinocytes, in thermosensory nerve endings, and in blood vessels. However, many other cell types are of course also thermosensitive and may not exploit TRP channels. As an important surprise for the coupling of temperature and metabolism, fat cells sense temperature TRP independent (Ye et al. 2013). Temperature sensing has probably many other faces such as metabolic control of many cell functions and homeostasis. At note, TRP channels must be considered as only one player in temperature sensing, as many other channels have been recognized to play an important role in determining the action potential frequency and pattern upon heat or cold stimulation in sensory nerve fibers, in mediating thermoregulatory response, or in triggering temperature-mediated response from the cellular to the behavioral level. Many other proteins, not only ion channel, are involved in orchestrating temperature sensing in temperature-mediated responses. Rhodopsin can function as a temperature sensor and might be involved as an amplifier for thermoTRP activation (Shen et al. 2011). The melatonin-related orphan receptor GPR50 is involved in determining the basal metabolic rate and is a temperature sensor (Swoap 2013). The Ca2+ sensor STIM1 is a thermosensor (Xiao et al. 2011). Nav1.7, Nav1.8, IKD, Kv1, Kv7 (M-current), K2P channels, the proton channel Hv1 and TMEM16A (Ano1, a Ca2+-activated Cl- channel), HCN channels, and probably several more function as temperature sensors (see recent reviews Cho et al. 2012; Fujiwara et al. 2012; Madrid et al. 2009; Orio et al. 2013; Voets 2012). This all indicates that TRP will not be the only clue for understanding temperature sensing. Interestingly and illustrating the still mediocre understanding of temperature sensing as a whole physiopsychological event, we refer to the fact that classical phenomena related to thermosensation, including “Weber’s silver Thaler illusion,” the “thermal grill illusion,” and “Weber’s three-bowl experiment,” are not yet understood and cannot be explained at the level of TRPs. It is even difficult to find a quantitative model involving several classes of ion channels to describe the fast and slow adaptation in thermosensory fibers and the mostly unimodal (bell-shaped) steady state discharge.
We will not refer to many unsolved problems in taste, olfaction, and hearing. Many open questions have been addressed in this volume of HEP, e.g., the controversial role of PKD2L1 (TRPP3), the existence of a salt receptor in the TRP family (TRPML3, filed as Senomyx Patent, 2009Footnote 1), the role of TRPV1 in taste rather than chemesthesis, the real function of the many TRP channels in the inner ear [e.g., TRPC3, TRPC5; TRPV1, TRPV4, TRPV5; TRPA1; TRPML3; TRPP3; etc. (Asai et al. 2010)] beyond hearing after identification of the probably main mechano-transducer channels TMC1 and TMC2 (Pan et al. 2013), and a still somewhat elusive role of TRPs in olfaction (Dong et al. 2012).
19 TRPs Go Metabolic
We all agree that TRPs have a huge impact on many sensory functions and behave like unique cellular sensors which are activated by polymodal stimuli. However, more and more evidence defines a role of TRP channels in the regulation of metabolic networks and metabolic diseases, e.g., type 2 diabetes, obesity, dyslipidemia, metabolic syndrome, atherosclerosis, metabolic bone diseases, and electrolyte disturbances. This is certainly a line which deserves a high consideration. Intriguing examples are as follows: (1) The weight of Trpv1 -/-mice heavily exceeds that of controls, which is coupled with a predisposition to age-associated overweight (Garami et al. 2010, 2011); (2) dietary TRPV1 activation improves energy metabolism, muscle growth, and exercise endurance by upregulating PGC-1α and nNOS/mTOR in skeletal muscles (Ito et al. 2012; Luo et al. 2012); (3) TRPV4 antagonists elevate thermogenesis in adipose tissues and protect from diet-induced obesity, adipose inflammation, and insulin resistance (Ye et al. 2012); (4) TRPC1, TRPC5, and TRPC6 expression is altered in a more humanlike metabolic syndrome model, the Ossabaw miniature pigs (Hu et al. 2009a); (5) brown fat activity is, besides β3-adrenergic stimulation, modulated by peroxisome proliferator-activated receptor γ (PPARγ) and the connected stimulation of TRPV1 by dietary capsaicin and monoacylglycerols (Birerdinc et al. 2012); (6) TRPV1 activation prevents nonalcoholic fatty liver disease (Li et al. 2012, 2013); and much more published evidence (for detailed reviews see Nilius and Appendino 2011, 2013; Zhu et al. 2010). Concerning these obviously exciting novel approaches to metabolic diseases, some pertinent questions have to be answered: What are the precise signaling pathways up- and downstream of TRPs in adipocytes and in the hypothalamus?
How do TRPs such as TRPV1 exert tissue-specific, positive, and negative effects on metabolism? Do TRPs participate in metabolic diseases, and do known genetic variants (SNPs) in TRP genes influence disease prevalence? Are TRPs useful pharmacological targets for treating obesity and insulin resistance (see for reviews Liu et al. 2008; Nilius and Appendino 2011, 2013; Zhu et al. 2010)?
20 TRPs and Disease
We have to accept a general scheme: TRP channels are not highly expressed on most native cells, and the current density will be low. Therefore, a pertinent question is whether these obviously more “modulatory” ion channels will have such a striking functional impact to cause diseases. Importantly, although many knockout models do not show per se very striking phenotypes, the number of channelopathies connected to dysfunctional TRP channels is amazingly high (for reviews see Cornell et al. 2008; Kiselyov et al. 2007; Nilius 2007; Nilius and Owsianik 2010b; Nilius et al. 2007). One of the probably most exciting puzzles that we have to solve in understanding TRP channelopathies is related to TRPV4 (Nilius and Owsianik 2010a). The Trpv4 gene is prone to many mutations a surprising number of different diseases. Familial digital arthropathy–brachydactyly only affects fingers and toes. Mutations in Trpv4 have also been identified in motor/sensory neuropathies, such as the congenital distal spinal muscle atrophy, scapuloperoneal spinal muscle atrophy, and Charcot–Marie–Tooth disease type 2C,with highly variable phenotypes, i.e., skeletal dysplasias (brachyolmia, spondylometaphyseal dysplasia Kozlowski, spondyloepimetaphyseal dysplasia Maroteaux pseudo-Morquio type 2, metatropic dysplasia, and parastremmatic dysplasia with a unifying feature short trunk with vertebral platyspondyly and scoliosis), skeletal diseases (familial digital arthropathy–brachydactyly only affecting fingers and toes), and motor/sensory neuropathies (congenital distal spinal muscle atrophy, scapuloperoneal spinal muscle atrophy, and Charcot–Marie–Tooth disease type 2C characterized by degeneration of motor and sensory neurons and peripheral nerves) (for a review, see Nilius and Voets 2013). More than 50 disease-causing mutants have been so far identified. They are distributed over the whole channel protein, with only two hot spots, the ADR (mainly neuropathies) and a region around the MAP-binding site in the C-terminus (skeletal dysplasias) (Nilius and Voets 2013). Another puzzle appeared concerning TRPV4 channelopathies: It is widely discussed that TRPV4 plays an important role in endothelium, contributes to the EDHF mechanism, and subsequently is an endothelial player for the regulation of blood pressure (Earley et al. 2009; Filosa et al. 2013; Kassmann et al. 2013; Kohler et al. 2006; Loot et al. 2008; Rath et al. 2009; Saliez et al. 2008; Vriens et al. 2005; Zhang and Gutterman 2010). However, any vascular phenotype in the TRPV4 patients is missing. The striking challenge is that mutations which are often located in the same domains of the channel proteins and even three identical mutations cause different diseases.
Most TRP-caused diseases have a dominant inheritance pattern, which implies that the patients carry both a mutant and a wild-type allele, which creates a general often overlooked problem: TRPs function probably as tetramers; thus, patients are expected to express TRP channels with variable stoichiometry of WT and mutant subunits. Assuming that wild-type and mutant subunits are equally expressed and assemble into tetrameric channels in a random fashion, one would expect that the TRP channels in a patient’s cell consist of 1/16 (6.25 %) pure wild-type channels and 1/16 pure mutant channels and that the 14/16 (87.5 %) of functional channels are heteromultimeric channels consisting of a mixture of wild-type and mutant subunits. This obviously requires the understanding of the properties of such heteromultimeric channels, which have often even not been studied. Understanding a disease needs the functional analysis of such heteromers! It is very likely that they exhibit properties that differ strongly from either pure wild-type or pure mutant homotetramers.
Understanding of the mechanistic background of these channel malfunctions not only is essential for a treatment of these diseases but may also help to understand the functioning of these exciting proteins and their involvement in different signaling cascades.
Obviously, TRP channels are connected to acquired diseases. In these cases, a critical mechanistic understanding is often missing, and far-reaching extrapolations and hopes are focused on TRP channels. We will also refer to a puzzle, seen the huge social impact: TRP causes diseases in the brain. Many discussions have been started about age as a drug target, and TRPs are considered as main actors. TRPM7 and TRPM2 have been put forward as potential factors in neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, stroke, and diseases associated with oxidant-mediated neuronal damage (McNulty and Fonfria 2005). Especially TRPM2, which is highly expressed in the striatum (caudate nucleus and putamen), is supposed to play a key role in bipolar disorders (Uemura et al. 2005; Xu et al. 2006). Recent case–control studies implicate TRPM2 conferring risk for bipolar disorder (BD), and genetic variants of TRPM2 have been identified to be coupled with BD supporting a role for this channel in the pathogenesis of this disorder (see for a review Chahl 2007; Xu et al. 2009). Depletion of intracellular Mg2+, a symptom of ischemic brain injury, and a reduction of extracellular Ca2+ are both associated with poor neurological outcome and are both conditions which activate TRPM7 and possibly increase the Ca2+ load of neuronal cells. This leads to a secondary injury processes and to cell death (Cook et al. 2009). Downregulation of TRPM2 and TRPM7 causes probably neuroprotective effect in the cerebral cortex and hippocampus after brain injury, oxidative stress, inflammation, and neuronal death (Cook et al. 2010). Therefore, TRPM7 might be a potential target for neuroprotection after brain injury. Suppressing the expression of TRPM7 in hippocampal CA1, neurons conferred resistance to ischemic cell death, preserved cell function, and prevented ischemia-induced deficits in memory (Rempe et al. 2009; Sun et al. 2009). Is this an antiaging strategy? On the other hand, mutations in TRPM2 have been discovered which result in rapidly inactivated channels which are unable to maintain sustained Mg2+ influx (Hermosura et al. 2002, 2005, 2008; Hermosura and Garruto 2007). Unfortunately, although quite a lot of evidence has been published, we face now clinical studies which completely refuse this even mechanistically underpinned view (Hara et al. 2010). However, still, TRPM7 might be involved in the familial Alzheimer’s disease (FAD) but mechanistically linked to aberrant PI(4,5)P2 causing TRPM7 dysfunction (Landman et al. 2006). In another view as the abovementioned role for “switching on” of light responses or as tumor suppressor, TRPM1 could be linked to a complex neurodevelopmental disorder characterized by severe visual impairment, intellectual disability, and refractory epilepsy. This disease is caused by a microdeletion in chromosome 15q13.3 carrying the trpm1 gene (Lepichon et al. 2010). Aging increases also a risk for ischemic brain injury by oxidative stress, edema, inflammation, and excitotoxicity. TRPM4 is reportedly involved in the damaging secondary events that accompany brain injury. Inhibition of TRPM4 channels provides beneficial effects in ischemia-associated cerebral edema and secondary hemorrhage (Simard et al. 2010) and has been considered as a neuroprotective target in the brain or spinal cord lesion (Gerzanich et al. 2009). However, the pharmacological evidence that TRPM4 is really blocked by KATP (SUR1 bound) inhibitors is still controversial. Also the question arises whether SUR1 is indeed a TRPM4 subunit as suggested (Woo et al. 2013).
This is just to say that although brain injury confers a major burden to Western society and effective treatments are urgently required, the enthusiasm that TRP channel may solve many of such problems should be qualified by a more rigorous investigation of TRP channel functions in more refined and suitable models! But which mechanisms are really involved? How can we really understand that TRP channels are causative in all the sketched diseases?
Obviously, we have to admit that in many diseases the role of TRPs might be somewhat overinterpreted/overstressed. Better pathophysiological models which consider the difficulties to handle TRP must be developed. We are still in the very beginning of understanding disease-causing dysfunctions of TRP channels.
Because of all the controversies and gaps in our understanding on TRP, we have to confess that we have opened an exciting field, but the current state of TRP channel research, as described in this book, is still in its infancy!
Notes
- 1.
Moyer B, Zlotnik A, Hevezi P, Soto H, Lu M, Gao N, Servant G, Brust P, Williams M, Kalabat D, et al. (2009) Identification of Trpml3 (Mcoln3) as a salty taste receptor and use in assays for identifying taste (salty) modulators and/or therapeutics that modulate sodium transport, absorption or excretion and/or aldosterone and/or vasopressin production or release. SENOMYX patent.
References
Almers W (1978) Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol 82:96–190
Anderson NL, Anderson NG (1998) Proteome and proteomics: new technologies, new concepts, and new words. Electrophoresis 19:1853–1861
Anderson L, Seilhamer J (1997) A comparison of selected mRNA and protein abundances in human liver. Electrophoresis 18:533–537
Andersson DA, Gentry C, Moss S, Bevan S (2009) Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+. Proc Natl Acad Sci U S A 106:8374–8379
Asai Y, Holt JR, Géléoc GS (2010) A quantitative analysis of the spatiotemporal pattern of transient receptor potential gene expression in the developing mouse cochlea. J Assoc Res Otolaryngol 11:27–37
Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, Tsukahara K, Arimura A, Horikawa T, Hirasawa T, Sakata T (2006) Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol 126:2664–2672
Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Said S, Bujakowska K, Nandrot EF, Lorenz B, Preising M et al (2009) TRPM1 Is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 85:720–729
Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P (2008) Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9:472–479
Banke TG, Wickenden AD (2009) Intracellular zinc irritates TRPA1. Nat Chem Biol 5:141–142
Bavassano C, Marvaldi L, Langeslag M, Sarg B, Lindner H, Klimaschewski L, Kress M, Ferrer-Montiel A, Knaus HG (2013) Identification of voltage-gated K channel beta 2 (Kvbeta2) subunit as a novel interaction partner of the pain transducer Transient Receptor Potential Vanilloid 1 channel (TRPV1). Biochimica et biophysica acta 1833:3166–3175
Beck A, Speicher T, Stoerger C, Sell T, Dettmer V, Jusoh SA, Abdulmughni A, Cavali Eacute A, Philipp SE, Zhu MX et al (2013) Conserved gating elements in TRPC4 and TRPC5 channels. J Biol Chem 288:19471–19483
Bellono NW, Oancea E (2013) UV light phototransduction depolarizes human melanocytes. Channels (Austin) 7(3), ahead of pring, PMID 23764911
Bellono NW, Kammel LG, Zimmerman AL, Oancea E (2013) UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci U S A 110:2383–2388
Benedikt J, Samad A, Ettrich R, Teisinger J, Vlachova V (2009) Essential role for the putative S6 inner pore region in the activation gating of the human TRPA1 channel. Biochim Biophys Acta 1793:1279–1288
Berbey C, Weiss N, Legrand C, Allard B (2009) Transient receptor potential canonical type 1 (TRPC1) operates as a sarcoplasmic reticulum calcium leak channel in skeletal muscle. J Biol Chem 284:36387–36394
Birerdinc A, Jarrar M, Stotish T, Randhawa M, Baranova A (2012) Manipulating molecular switches in brown adipocytes and their precursors: a therapeutic potential. Prog Lipid Res 52:51–61
Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D (2010) A bivalent Tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141:834–845
Bright JN, Sansom MS (2004) Kv channel S6 helix as a molecular switch: simulation studies. IEE Proc Nanobiotechnol 151:17–27
Cai X (2008) Unicellular Ca2+ signaling ‘Toolkit’ at the origin of metazoa. Mol Biol Evol 25:1357–1361
Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D (2013) TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77:667–679
Cavanaugh EJ, Simkin D, Kim D (2008) Activation of TRPA1 by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional states of TRPA1. Neuroscience 154:1467–1476
Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI (2011a) Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci 31:10119–10127
Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI (2011b) Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31:5067–5077
Chahl LA (2007) TRP’s: links to schizophrenia? Biochim Biophys Acta 1772:968–977
Chen J, Zhang XF, Kort ME, Huth JR, Sun C, Miesbauer LJ, Cassar SC, Neelands T, Scott VE, Moreland RB et al (2008) Molecular determinants of species-specific activation or blockade of TRPA1 channels. J Neurosci 28:5063–5071
Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D (2013) Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 4:2501
Cheng X, Shen D, Samie M, Xu H (2010) Mucolipins: intracellular TRPML1-3 channels. FEBS Lett 584:2013–2021
Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F et al (2012) The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci 15:1015–1021
Chu B, Postma M, Hardie RC (2013) Fractional Ca2+ currents through TRP and TRPL channels in Drosophila photoreceptors. Biophys J 104:1905–1916
Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D (2001) Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411:957–962
Chun JN, Lim JM, Kang Y, Kim EH, Shin YC, Kim HG, Jang D, Kwon D, Shin SY, So I, Jeon JH (2013) A network perspective on unraveling the role of TRP channels in biology and disease. Pflugers Arch 466:173–182
Chung MK, Guler AD, Caterina MJ (2008) TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci 11:555–564
Cook NL, Van Den Heuvel C, Vink R (2009) Are the transient receptor potential melastatin (TRPM) channels important in magnesium homeostasis following traumatic brain injury? Magnes Res 22:225–234
Cook NL, Vink R, Helps SC, Manavis J, van den Heuvel C (2010) Transient receptor potential melastatin 2 expression is increased following experimental traumatic brain injury in rats. J Mol Neurosci 42:192–199
Cornell RA, Aarts M, Bautista D, Garcia-Anoveros J, Kiselyov K, Liman ER (2008) A double TRPtych: six views of transient receptor potential channels in disease and health. J Neurosci 28:11778–11784
Cosens DJ, Manning A (1969) Abnormal electroretinogram from a Drosophila mutant. Nature 224:285–287
Courjaret R, Hubrack S, Daalis A, Dib M, Machaca K (2013) The Xenopus TRPV6 homolog encodes a Mg-permeant channel that is inhibited by interaction with TRPC1. J Cell Physiol 228:2386–2398
Cuajungco MP, Grimm C, Oshima K, D’Hoedt D, Nilius B, Mensenkamp AR, Bindels RJ, Plomann M, Heller S (2006) PACSINs bind to the TRPV4 cation channel: PACSIN 3 modulates the subcellular localization of TRPV4. J Biol Chem 281:18753–18762
Cvetkov TL, Huynh KW, Cohen MR, Moiseenkova-Bell VY (2011) Molecular architecture and subunit organization of TRPA1 channel revealed by electron microscopy. J Biol Chem 286:38168–38176
D’hoedt D, Owsianik G, Prenen P, Cuajungco MP, Grimm G, Heller S, Voets T, Nilius B (2007) Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J Biol Chem 283:6272–6280
Damann N, Voets T, Nilius B (2008) TRPs in our senses. Curr Biol 18:R880–889
de la Roche J, Eberhardt MJ, Klinger AB, Stanslowksy N, Wegner F, Koppert W, Reeh PW, Lampert A, Fischer MJ, Leffler A (2013) The molecular basis for species-specific activation of human TRPA1 by protons involves poorly conserved residues within transmembrane domains 5 and 6. J Biol Chem 288:20280–20292
Demion M, Bois P, Launay P, Guinamard R (2007) TRPM4, a Ca(2+)-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res 73:531–538
den Dekker E, Schoeber J, Topala CN, van de Graaf SF, Hoenderop JG, Bindels RJ (2005) Characterization of a Madin-Darby canine kidney cell line stably expressing TRPV5. Pflugers Arch 450:236–244
Devi S, Kedlaya R, Maddodi N, Bhat KM, Weber CS, Valdivia HH, Setaluri V (2009) Calcium homeostasis in human melanocytes: role of transient receptor potential melastatin 1 (TRPM1) and its regulation by ultraviolet light. Am J Physiol Cell Physiol 297:C679–C687
Dhaka A, Viswanath V, Patapoutian A (2006) TRP ion channels and temperature sensation. Annu Rev Neurosci 29:135–161
Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H (2010) PI(3,5)P(2) controls membrane traffic by direct activation of mucolipin Ca release channels in the endolysosome. Nat Commun 1:38
Dong HW, Davis JC, Ding SY, Nai Q, Zhou FM, Ennis M (2012) Expression of transient receptor potential (TRP) channel mRNAs in the mouse olfactory bulb. Neurosci Lett 524:49–54
Earley S, Pauy T, Drapp R, Tavares MJ, Liedtke W, Brayden JE (2009) TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol 297:H1096–H1102
Ebert J, Westhoff G (2006) Behavioural examination of the infrared sensitivity of rattlesnakes (Crotalus atrox). J Comp Physiol A 192:941–947
Eijkelkamp N, Quick K, Wood JN (2013) Transient receptor potential channels and mechanosensation. Annu Rev Neurosci 36:519–546
Everaerts W, Nilius B, Owsianik G (2010) The vanilloid transient receptor potential channel Trpv4: From structure to disease. Prog Biophys Mol Biol 103:2–17
Fecher-Trost C, Wissenbach U, Beck A, Schalkowsky P, Stoerger C, Doerr J, Dembek A, Simon-Thomas M, Weber A, Wollenberg P et al (2013) The in vivo TRPV6 protein starts at a non-AUG triplet decoded as methionine upstream the canonical initiation at AUG. J Biol Chem 288:16629–16644
Feng S, Li H, Tai Y, Huang J, Su Y, Abramowitz J, Zhu MX, Birnbaumer L, Wang Y (2013) Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc Natl Acad Sci U S A 110:11011–11016
Fernandes AM, Fero K, Arrenberg AB, Bergeron SA, Driever W, Burgess HA (2012) Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr Biol 22:2042–2047
Fernandes AM, Fero K, Driever W, Burgess HA (2013) Enlightening the brain: linking deep brain photoreception with behavior and physiology. BioEssays 35:775–779
Ferrer-Montiel A, Fernández-Carvajal A, Planells-Cases R, Fernández-Ballester G, González-Ros JM, Messeguer A, González-Muñiz R (2012) Advances in modulating thermosensory TRP channels. Expert Opin Ther Pat 22:999–1017
Filosa JA, Yao X, Rath G (2013) TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol 61:113–119
Flockerzi V, Jung C, Aberle T, Meissner M, Freichel M, Philipp SE, Nastainczyk W, Maurer P, Zimmermann R (2005) Specific detection and semi-quantitative analysis of TRPC4 protein expression by antibodies. Pflugers Arch 451:81–86
Fujiwara Y, Minor DL Jr (2008) X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J Mol Biol 383:854–870
Fujiwara Y, Kurokawa T, Takeshita K, Kobayashi M, Okochi Y, Nakagawa A, Okamura Y (2012) The cytoplasmic coiled-coil mediates cooperative gating temperature sensitivity in the voltage-gated H(+) channel Hv1. Nat Commun 3:816
Gamal El-Din TM, Heldstab H, Lehmann C, Greeff NG (2011) Double gaps along Shaker S4 demonstrate omega currents at three different closed states. Channels (Austin) 4:93–100
Garami A, Shimansky YP, Pakai E, Oliveira DL, Gavva NR, Romanovsky AA (2010) Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J Neurosci 30:1435–1440
Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA (2011) Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci 31:721–173
Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernandez-Ballester G, Ferrer-Montiel A (2004) Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci 24:5307–5314
Gaudet R (2008a) A primer on ankyrin repeat function in TRP channels and beyond. Mol Biosyst 4:372–379
Gaudet R (2008b) TRP channels entering the structural era. J Physiol 586:3565–3575
Gees M, Colsoul B, Nilius B (2010) The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2:a003962
Geng J, Liang D, Jiang K, Zhang P (2012) Molecular evolution of the infrared sensory gene TRPA1 in snakes and implications for functional studies. PLoS One 6:e28644
Gerzanich V, Woo KS, Vennekens R, Orest Tsymbalyuk SO, Ivanova S, Ivanov A, Geng Z, Chen Z, Nilius B, Flockerzi V et al (2009) De novo expression of TRPM4 initiates secondary hemorrhage in spinal cord injury. Nat Med 15:185–191
Gilliam JC, Wense TG (2011) TRP channel gene expression in the mouse retina. Vision Research 51, 2440-2452.x. Vision Res 51:2440–2452
Gordon-Shaag A, Zagotta WN, Gordon SE (2008) Mechanism of Ca2+-dependent desensitization of TRP channels. Channels 2:125–129
Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D (2010) Molecular basis of infrared detection by snakes. Nature 464:1006–1012
Gracheva EO, Cordero-Morales JF, Gonzalez-Carcacia JA, Ingolia NT, Manno C, Aranguren CI, Weissman JS, Julius D (2011) Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 476:88–91
Griffith TN, Varela-Nallar L, Dinamarca MC, Inestrosa NC (2010) Neurobiological effects of hyperforin and its potential in Alzheimer’s disease therapy. Curr Med Chem 17:391–406
Gu Q, Lin RL (2010) Heavy metals zinc, cadmium and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J Appl Physiol 108:891–897
Gygi SP, Rochon Y, Franza BR, Aebersold R (1999) Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 19:1720–1730
Hara K, Kokubo Y, Ishiura H, Fukuda Y, Miyashita A, Kuwano R, Sasaki R, Goto J, Nishizawa M, Kuzuhara S, Tsuji S (2010) TRPM7 is not associated with amyotrophic lateral sclerosis-parkinsonism dementia complex in the Kii peninsula of Japan. Am J Med Genet B Neuropsychiatr Genet 153B:310–313
Hardie RC, Franze K (2012) Photomechanical responses in Drosophila photoreceptors. Science 338:260–263
Hardie RC, Minke B (1992) The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8:643–651
Hardie RC, Minke B (1993) Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci 16:371–376
Hardie RC, Postma M (2008) Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates. In: Basbaum AI, Shepherd GM, Westheimer G (eds) The senses: a comprehensive reference, Vision I. Academic, San Diego, pp 77–130
Hermosura MC, Garruto RM (2007) TRPM7 and TRPM2-CANDIDATE susceptibility genes for Western Pacific ALS and PD? Biochim Biophys Acta 1772:822–835
Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A (2002) Dissociation of the store-operated calcium current ICRAC and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol (Lond) 539:445–458
Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM (2005) A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci U S A 102:11510–11515
Hermosura M, Cui AM, Go G, Davenport B, Shetler C, Heizer J, Schmitz C, Mocz G, Garruto R, Perraud A-L (2008) Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc Natl Acad Sci U S A 105:18029–18034
Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ (2003) Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22:776–785
Hofmann T, Schaefer M, Schultz G, Gudermann T (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A 99:7461–7466
Hofmann F, Flockerz IV, Kahl S, Wegener JW (2014) L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol Rev 94:303–26
Hu G, Oboukhova EA, Kumar S, Sturek M, Obukhov AG (2009a) Canonical transient receptor potential channels expression is elevated in a porcine model of metabolic syndrome. Mol Endocrinol 23:689–699
Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A (2009b) Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol 5:183–190
Hughes S, Pothecary CA, Jagannath A, Foster RG, Hankins MW, Peirson SN (2012) Profound defects in pupillary responses to light in TRPM-channel null mice: a role for TRPM channels in non-image-forming photoreception. Eur J Neurosci 35:34–43
Ihara M, Hamamoto S, Miyanoiri Y, Takeda M, Kainosho M, Yabe I, Uozumi N, Yamashita A (2013) Molecular bases of multimodal regulation of a fungal transient receptor potential (TRP) channel. J Biol Chem 288
Inada H, Procko E, Sotomayor M, Gaudet R (2012) Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry 51:6195–6206
Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S (2012) Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med 19:101–106
Jansson ET, Trkulja CL, Ahemaiti A, Millingen M, Jeffries GD, Jardemark K, Orwar O (2013) Effect of cholesterol depletion on the pore dilation of TRPV1. Mol Pain 9:1
Jeon JP, Lee KP, Park EJ, Sung TS, Kim BJ, Jeon JH, So I (2008) The specific activation of TRPC4 by Gi protein subtype. Biochem Biophys Res Commun 377:538–543
Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, Choe H, Muallem S, Kim HJ, So I (2012) Selective Galphai subunits as novel direct activators of TRPC4 and TRPC5 channels. J Biol Chem 287:17029–17039
Jeon JP, Roh SE, Wie J, Kim J, Kim H, Lee KP, Yang D, Jeon JH, Cho NH, Kim IG et al (2013) Activation of TRPC4beta by Galpha subunit increases Ca selectivity and controls neurite morphogenesis in cultured hippocampal neuron. Cell Calcium 54:307–319
Jin X, Touhey J, Gaudet R (2006) Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem 281:25006–25010
Jordt SE, Julius D (2002) Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108:421–430
Jurkat-Rott K, Holzherr B, Fauler M, Lehmann-Horn F (2010) Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflugers Archiv 460:239–248
Kalia J, Swartz KJ (2013) Exploring structure-function relationships between TRP and Kv channels. Sci Rep 3:1523
Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B (2008) Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch 457:77–89
Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, Nilius B (2010) Agonist-induced changes in Ca(2+) permeation through the nociceptor cation channel TRPA1. Biophys J 98:773–783
Kassmann M, Harteneck C, Zhu Z, Nurnberg B, Tepel M, Gollasch M (2013) TRPV1, TRPV4, and the kidney. Acta Physiol (Oxf) 207:546–564
Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM (2001) Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem 276:28613–28619
Kim D, Cavanaugh EJ (2007) Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci 27:6500–6509
Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X (2008) Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 133:475–485
Kim D, Cavanaugh E, Simkin D (2009) Inhibition of transient receptor potential A1 by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol 295:C92–99
Kirkwood NK, Albert JT (2013) Sensory transduction: confusing the senses. Curr Biol 23:R22–23
Kiselyov K, Soyombo A, Muallem S (2007) TRPpathias. J Physiol 578:641–653
Klein RM, Ufret-Vincenty CA, Hua L, Gordon SE (2008) Determinants of molecular specificity in phosphoinositide regulation: PI(4,5)P2 is the endogenous lipid regulating TRPV1. J Biol Chem 283:26208–26216
Klionsky L, Tamir R, Gao B, Wang W, Immke DC, Nishimura N, Gavva NR (2007) Species-specific pharmacology of Trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain 3:39
Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J (2006) Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26:1495–1502
Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A et al (2010) TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A 107:332–337
Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR et al (2010) A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66:671–680
Landman N, Jeong SY, Shin SY, Voronov SV, Serban G, Kang MS, Park MK, Di Paolo G, Chung S, Kim T-W (2006) Presenilin mutations linked to familial Alzheimer’s disease cause an imbalance in phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Sci U S A 103:19524–19529
Lee JH, Lee Y, Ryu H, Kang DW, Lee J, Lazar J, Pearce LV, Pavlyukovets VA, Blumberg PM, Choi S (2011) Structural insights into transient receptor potential vanilloid type 1 (TRPV1) from homology modeling, flexible docking, and mutational studies. J Comput Aided Mol Des 25:317–327
Lepichon JB, Bittel DC, Graf WD, Yu S (2010) A 15q13.3 homozygous microdeletion associated with a severe neurodevelopmental disorder suggests putative functions of the TRPM1, CHRNA7, and other homozygously deleted genes. Am J Med Genet A 152A:1300–1304
Leuner K, Kazanski V, Muller M, Essin K, Henke B, Gollasch M, Harteneck C, Muller WE (2007) Hyperforin-a key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J 21:4101–4111
Leuner K, Heiser JH, Derksen S, Mladenov MI, Fehske CJ, Schubert R, Gollasch M, Schneider G, Harteneck C, Chatterjee SS, Muller WE (2010) Simple 2,4 diacylphloroglucinols as TRPC6 activators - identification of a novel pharmacophore. Mol Pharmacol 77:368–377
Li L, Chen J, Ni Y, Feng X, Zhao Z, Wang P, Sun J, Yu H, Yan Z, Liu D et al (2012) TRPV1 activation prevents nonalcoholic fatty liver through UCP2 upregulation in mice. Pflugers Archiv 463:727–732
Li Q, Li L, Wang F, Chen J, Zhao Y, Wang P, Nilius B, Liu D, Zhu Z (2013) Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor delta activation. Pflugers Archiv 465:1303–1316
Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L (2008) Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A 105:2895–2900
Liscombe DK, Facchini PJ (2008) Evolutionary and cellular webs in benzylisoquinoline alkaloid biosynthesis. Curr Opin Biotechnol 19:173–180
Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R (2007) The Ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54:905–918
Liu D, Zhu Z, Tepel M (2008) The role of transient receptor potential channels in metabolic syndrome. Hypertens Res 31:1989–1995
Long SB, Campbell EB, Mackinnon R (2005) Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309:897–903
Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I (2008) Role of cytochrome P450-dependent TRPV4 activation in flow-induced vasodilatation. Cardiovasc Res 80:445–452
Lucas RJ (2013) Mammalian inner retinal photoreception. Curr Biol 23:R125–133
Lukacs V, Yudin Y, Hammond GR, Sharma E, Fukami K, Rohacs T (2013) Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J Neurosci 33:11451–11463
Luo Z, Ma L, Zhao Z, He H, Yang D, Feng X, Ma S, Chen X, Zhu T, Cao T et al (2012) TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res 22:551–564
Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X (2010) Functional role of TRPV4-TRPC1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol 30:851–858
Ma X, Nilius B, Wong JW, Huang Y, Yao X (2011) Electrophysiological properties of heteromeric TRPV4-C1 channels. Biochim Biophys Acta 1808:2807–2818
Madrid R, de la Pen E, Donovan-Rodriguez T, Belmonte C, Viana F (2009) Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Physiol (Lond) 587:1961–1976
Matsuura H, Sokabe T, Kohno K, Tominaga M, Kadowaki T (2009) Evolutionary conservation and changes in insect TRP channels. BMC Evol Biol 9:228
McNulty S, Fonfria E (2005) The role of TRPM channels in cell death. Pflügers Arch 451:235–242
Meissner M, Obmann VC, Hoschke M, Link S, Jung M, Held G, Philipp SE, Zimmermann R, Flockerzi V (2011) Lessons of studying TRP channels with antibodies. In: Zhu MX (ed) TRP channels. CRC Press, Boca Raton, FL, pp 135–148
Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG (2008) Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci U S A 105:7451–7455
Molnar T, Barabas P, Birnbaumer L, Punzo C, Kefalov V, Krizaj D (2012) Store-operated channels regulate intracellular calcium in mammalian rods. J Physiol 590:3465–3481
Montell C (2012) Drosophila visual transduction. Trends Neurosci 35:356–363
Montell C, Rubin GM (1989) Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2:1313–1323
Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham D, Harteneck C, Heller S, Julius D et al (2002) A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 9:229–231
Moore C, Cevikbas F, Pasolli HA, Chen Y, Kong W, Kempkes C, Parekh P, Lee SH, Kontchoua N-A, Ye I et al (2013) UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci U S A 110:E3225–3234
Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL (2009) TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A 106:19174–19178
Mori Y, Wakamori M, Miyakawa T, Hermosura M, Hara Y, Nishida M, Hirose K, Mizushima A, Kurosaki M, Mori E et al (2002) Transient receptor potential 1 regulates capacitative Ca(2+) entry and Ca(2+) release from endoplasmic reticulum in B lymphocytes. J Exp Med 195:673–681
Moussaieff A, Mechoulam R (2009) Boswellia resin: from religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J Pharm Pharmacol 61:1281–1293
Moussaieff A, Rimmerman N, Bregman T, Straiker A, Felder CC, Shoham S, Kashman Y, Huang SM, Lee H, Shohami E et al (2008) Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J 22:3024–3034
Muller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, Schulte U (2010) Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci U S A 107:14950–14957
Nagatomo K, Ishii H, Yamamoto T, Nakajo K, Kubo Y (2010) The Met268Pro mutation of mouse TRPA1 changes the effect of caffeine from activation to suppression. Biophys J 99:3609–3618
Nakamura M, Sanuki R, Yasuma TR, Onishi A, Nishiguchi KM, Koike C, Kadowaki M, Kondo M, Miyake Y, Furukawa T (2010) TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol Vis 16:425–437
Nieto-Posadas A, Jara-Oseguera A, Rosenbaum T (2011) TRP channel gating physiology. Curr Top Med Chem 11:2131–2150
Nilius B (2007) TRP channels in disease. Biochim Biophys Acta 1772:805–812
Nilius B, Appendino G (2011) Tasty and healthy TR(i)PS: the human quest for culinary pungency. EMBO Rep 12:1094–1101
Nilius B, Appendino G (2013) Spices: the savory and beneficial science of pungency. Rev Physiol Biochem Pharmacol 164:1–76
Nilius B, Honore E (2013) Sensing pressure with ion channels. Trends Neurosci 35:477–486
Nilius B, Owsianik G (2010a) Channelopathies converge on TRPV4. Nat Genet 42:98–100
Nilius B, Owsianik G (2010b) Transient receptor potential channelopathies. Pflugers Archiv 460:437–450
Nilius B, Owsianik G (2011) The transient receptor potential family of ion channels. Genome Biol 12:218–229
Nilius B, Voets T (2013) The puzzle of TRPV4 channelopathies. EMBO Rep 14:152–163
Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G (2000) Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol (London) 527:239–248
Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX (2005a) Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 280:6423–6433
Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T (2005b) Gating of TRP channels: a voltage connection? J Physiol (Lond) 567:33–44
Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T (2006) The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 25:467–478
Nilius B, Owsianik G, Voets T, Peters JA (2007) Transient receptor potential channels in disease. Physiol Rev 87:165–217
Nilius B, Owsianik G, Voets T (2008) Transient receptor potential channels meet phosphoinositides. EMBO J 27:2809–2816
Nilius B, Prenen J, Owsianik G (2011) Irritating channels: the case of TRPA1. J Physiol 589:1543–1549
Nilius B, Appendino G, Owsianik G (2012) The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Archiv 464:425–458
Nilius B, Biro T, Owsianik G (2014) TRPV3: time to decipher a poorly understood family member! J Physiol 592:295–304
Ong HL, Chen J, Chataway T, Brereton H, Zhang L, Downs T, Tsiokas L, Barritt G (2002) Specific detection of the endogenous transient receptor potential (TRP)-1 protein in liver and airway smooth muscle cells using immunoprecipitation and Western-blot analysis. Biochem J 364:641–648
Ong EC, Nesin V, Long CL, Bai CX, Guz JL, Ivanov IP, Abramowitz J, Birnbaumer L, Humphrey MB, Tsiokas L (2013) A TRPC1-dependent pathway regulates osteoclast formation and function. J Biol Chem 288:22219–22232
Orio P, Parra A, Madrid R, Gonzalez O, Belmonte C, Viana F (2013) Role of Ih in the firing pattern of mammalian cold thermoreceptor endings. J Neurophysiol 108:3009–3023
Otsuguro KI, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV (2008) Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem 283:10026–10036
Owsianik G, Talavera K, Voets T, Nilius B (2006) Permeation and selectivity of trp channels. Annu Rev Physiol 68:685–717
Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR (2013) TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79:504–515
Panzano VC, Kang K, Garrity PA (2010) Infrared snake eyes: TRPA1 and the thermal sensitivity of the snake pit organ. Sci Signal 3:22
Papakosta M, Dalle C, Haythornthwaite A, Cao L, Stevens EB, Burgess G, Russell R, Cox PJ, Phillips SC, Grimm C (2011) Chimeric approach reveals that differences in the TRPV1 pore domain determine species-specific sensitivity to block of heat activation. J Biol Chem 286:39663–33967
Patapoutian A, Peier AM, Story GM, Viswanath V (2003) ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 4:529–539
Phelps CB, Procko E, Lishko PV, Wang RR, Gaudet R (2007) Insights into the roles of conserved and divergent residues in the ankyrin repeats of TRPV ion channels. Channels (Austin) 1:148–151
Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R (2008) Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 47:2476–2484
Phelps CB, Wang RR, Choo SS, Gaudet R (2010) Differential regulation of TRPV1, TRPV3 AND TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem 285:731–740
Pickard GE, Sollars PJ (2012) Intrinsically photosensitive retinal ganglion cells. Rev Physiol Biochem Pharmacol 162:59–90
Pradet-Balade B, Boulme F, Beug H, Mullner EW, Garcia-Sanz JA (2001) Translation control: bridging the gap between genomics and proteomics? Trends Biochem Sci 26:225–229
Prescott ED, Julius D (2003) A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300:1284–1288
Prole DL, Taylor CW (2011) Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS One 6:e26218
Prole DL, Taylor CW (2012) Identification and analysis of cation channel homologues in human pathogenic fungi. PLoS One 7:e42404
Prütting S, Grissmer S (2011) A novel current pathway parallel to the central pore in a mutant voltage-gated potassium channel. J Biol Chem 286:20031–20042
Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647
Rath G, Dessy C, Feron O (2009) Caveolae, caveolin and control of vascular tone: nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF) regulation. J Physiol Pharmacol 60(Suppl 4):105–109
Rempe DA, Takano T, Nedergaard M (2009) TR(I)Pping towards treatment for ischemia. Nat Neurosci 12:1215–1216
Richards MW, Butcher AJ, Dolphin AC (2004) Ca2+ channel beta-subunits: structural insights AID our understanding. Trends Pharmacol Sci 25:626–632
Rohacs T (2013) Regulation of transient receptor potential channels by the phospholipase C pathway. Adv Biol Regul 53:341–355
Rohacs T, Nilius B (2007) Regulation of transient receptor potential (trp) channels by phosphoinositides. Pflugers Archiv 455:157–168
Ryu S, Liu B, Yao J, Fu Q, Qin F (2007) Uncoupling proton activation of vanilloid receptor TRPV1. J Neurosci 27:12797–12807
Sala-Rabanal M, Wang S, Nichols CG (2012) On the potential interactions between non-selective cation channel TRPM4 and the sulfonylurea receptor SUR1. J Biol Chem 287:8746–8756
Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O et al (2008) Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117:1065–1074
Samways DS, Egan TM (2011) Calcium-dependent decrease in the single-channel conductance of TRPV1. Pflugers Archiv 462:681–691
Schindl R, Fritsch R, Jardin I, Frischauf I, Kahr H, Muik M, Riedl MC, Groschner K, Romanin C (2012) Canonical transient receptor potential (TRPC) 1 acts as a negative regulator for vanilloid TRPV6 mediated Ca2+ influx. J Biol Chem 287:35612–33520
Schmidt TM, Chen SK, Hattar S (2011) Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci 34:572–580
Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Muller CS, Bildl W, Baehrens D, Huber B, Kulik A et al (2012) High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74:621–633
Sedgwick SG, Smerdon SJ (1999) The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci 24:311–316
Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C (2011) Function of rhodopsin in temperature discrimination in Drosophila. Science 331:1333–1336
Shi J, Birnbaumer L, Large WA, Albert AP (2013a) Myristoylated alanine-rich C kinase substrate coordinates native TRPC1 channel activation by phosphatidylinositol 4,5-bisphosphate and protein kinase C in vascular smooth muscle. FASEB J 28:244–255
Shi M, Du F, Liu Y, Li L, Cai J, Zhang GF, Xu XF, Lin T, Cheng HR, Liu XD et al (2013b) Glial cell-expressed mechanosensitive channel TRPV4 mediates infrasound-induced neuronal impairment. Acta neuropathologica 126:725–739
Shibasaki K, Suzuki M, Mizuno A, Tominaga M (2007) Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci 27:1566–1575
Sigg D, Bezanilla F (1997) Total charge movement per channel. The relation between gating charge displacement and the voltage sensitivity of activation. J Gen Physiol 109:27–39
Simard JM, Kahle KT, Gerzanich V (2010) Molecular mechanisms of microvascular failure in central nervous system injury-synergistic roles of NKCC1 and SUR1/TRPM4. J Neurosurg 113:622–629
Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS (2002) Calmodulin regulates Ca(2+)-dependent feedback inhibition of store-operated Ca(2+) influx by interaction with a site in the C terminus of TrpC1. Mol Cell 9:739–750
Starace DM, Bezanilla F (2004) A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 427:548–553
Storch U, Forst AL, Philipp M, Gudermann T, Mederos YSM (2012) TRPC1 reduces the calcium permeability in heteromeric channel complexes. J Biol Chem 287:3530–3540
Straub I, Krugel U, Mohr F, Teichert J, Rizun O, Konrad M, Oberwinkler J, Schaefer M (2013a) Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol Pharmacol 84:736–750
Straub I, Mohr F, Stab J, Konrad M, Philipp S, Oberwinkler J, Schaefer M (2013b) Citrus fruit and fabacea secondary metabolites potently and selectively block TRPM3. Br J Pharmacol 168:1835–1850
Strotmann R, Schultz G, Plant TD (2003) Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a carboxy terminal calmodulin binding site. J Biol Chem 278:26541–26549
Strotmann R, Semtner M, Kepura F, Plant TD, Schoneberg T (2010) Interdomain interactions control Ca-dependent potentiation in the cation channel TRPV4. PLoS One 5:e10580
Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE (2001) TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29:645–655
Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP et al (2009) Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 12:1300–1307
Swoap SJ (2013) Thermoregulation: an orphan receptor finds its way in the cold. Curr Biol 22:R17–18
Tang Z, Kim A, Masuch T, Park K, Weng H, Wetzel C, Dong X (2013) Pirt functions as an endogenous regulator of TRPM8. Nat Commun 4:2179
Tew KD, Monks A, Barone L, Rosser D, Akerman G, Montali JA, Wheatley JB, Schmidt DE Jr (1996) Glutathione-associated enzymes in the human cell lines of the National Cancer Institute Drug Screening Program. Mol Pharmacol 50:149–159
Tombola F, Pathak MM, Gorostiza P, Isacoff EY (2007) The twisted ion-permeation pathway of a resting voltage-sensing domain. Nature 445:546–549
Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme V (1999) Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci U S A 96:3934–3939
Tsuruda PR, Julius D, Minor DL Jr (2006) Coiled coils direct assembly of a cold-activated TRP channel. Neuron 51:201–212
Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V (2009) Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137:1415–1424
Uemura T, Kudoh J, Noda S, Kanba S, Shimizu N (2005) Characterization of human and mouse TRPM2 genes: identification of a novel N-terminal truncated protein specifically expressed in human striatum. Biochem Biophys Res Commun 328:1232–1243
Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE (2011) Localization of the PIP2 sensor of TRPV1 ion channels. J Biol Chem 283:26208–26216
Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B (2005) Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 37:267–278
Vay L, Gu C, McNaughton PA (2011) The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol 165:787–801
Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ (2000) Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem 275:3963–3969
Vennekens R, Menigoz A, Nilius B (2012) TRPs in the brain. Rev Physiol Biochem Pharmacol 163:27–64
Voets T (2012) Quantifying and modeling the temperature-dependent gating of TRP channels. Rev Physiol Biochem Pharmacol 162:91–119
Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748–754
Voets T, Owsianik G, Janssens A, Talavera K, Nilius B (2007) TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 3:174–182
Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B (2005) Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97:908–915
Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S et al (2011) TRPM3 is a nociceptor channel INVOLVED in the detection of noxious heat. Neuron 70:482–494
Vriens J, Held K, Janssens A, Tóth BI, Kerselaers S, Nilius B, Vennekens R, Voets T (2014) Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nature Chem Biol 10:188–195
Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER (2008) The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem 283:32691–32703
Wang S, Poon K, Oswald RE, Chuang HH (2010) Distinct modulations of human capsaicin receptor by protons and magnesium through different domains. J Biol Chem 285:11547–11156
Wang L, Cvetkov TL, Chance MR, Moiseenkova-Bell VY (2012) Identification of in vivo disulfide conformation of the TRPA1 ion channel. J Biol Chem 287:6169–6176
Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J et al (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277:13569–13577
Wetsel WC (2011) Sensing hot and cold with TRP channels. Int J Hyperthermia 27:388–398
Wheeler GL, Brownlee C (2008) Ca(2+) signalling in plants and green algae - changing channels. Trends Plant Sci 13:506–514
Whorton MR, MacKinnon R (2011) Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147:199–208
Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM (2013) The sulfonylurea receptor 1 (Sur1) - transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem 288:3655–3667
Wu LJ, Sweet TB, Clapham DE (2010) International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62:381–404
Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A (2008a) Identification of the transmembrane domain five as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci 28:9640–9651
Xiao R, Tang J, Wang C, Colton CK, Tian J, Zhu MX (2008b) Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J Biol Chem 283:6162–6174
Xiao R, Tian J, Tang J, Zhu MX (2008c) The TRPV3 mutation associated with the hairless phenotype in rodents is constitutively active. Cell Calcium 43:334–343
Xiao B, Coste B, Mathur J, Patapoutian A (2011) Temperature-dependent STIM1 activation induces Ca(2+) influx and modulates gene expression. Nat Chem Biol 7:351–358
Xu C, Macciardi F, Li PP, Yoon IS, Cooke RG, Hughes B, Parikh SV, McIntyre RS, Kennedy JL, Warsh JJ (2006) Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 141:36–43
Xu C, Li PP, Cooke RG, Parikh SV, Wang K, Kennedy JL, Warsh JJ (2009) TRPM2 variants and bipolar disorder risk: confirmation in a family-based association study. Bipolar Disord 11:1–10
Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P et al (2011) Melanopsin signalling in mammalian iris and retina. Nature 479:67–73
Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J (2001) Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell 7:1047–1057
Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Bostrom P, Mepani RJ et al (2012) TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 151:96–110
Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM (2013) Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A 110:12480–12485
Yokoyama S, Altun A, Denardo DF (2011) Molecular convergence of infrared vision in snakes. Mol Biol Evol 28:45–48
Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, Tong L, Isacoff EY, Yang J (2009) Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci USA 106:11558–11563
Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S (2007) STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9:636–645
Zagranichnaya TK, Wu X, Villereal ML (2005) Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem 280:29559–29569
Zhang DX, Gutterman DD (2010) TRP channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol 52:133–139
Zhang F, Liu S, Yang F, Zheng J, Wang K (2011) Identification of a tetrameric assembly domain in the C-terminus of heat-activated TRPV1 channels. J Biol Chem 286:15308–15316
Zhang X, Li X, Xu H (2012) Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc Natl Acad Sci U S A 109:11384–11389
Zheng J (2013) Molecular mechanism of TRP channels. Compr Physiol 3:221–242
Zholos AV, Zholos AA, Bolton TB (2004) G-protein-gated TRP-like cationic channel activated by muscarinic receptors: effect of potential on single-channel gating. J Gen Physiol 123:581–598
Zhou Y, Suzuki Y, Uchida K, Tominaga M (2013) Identification of a splice variant of mouse TRPA1 that regulates TRPA1 activity. Nat Commun 4:2408
Zhu MX (2005) Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels. Pflugers Archiv 451:105–115
Zhu X, Chu PB, Peyton M, Birnbaumer L (1995) Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett 373:193–198
Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L (1996) trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 85:661–671
Zhu Z, Luo Z, Ma S, Liu D (2010) TRP channels and their implications in metabolic diseases. Pflugers Arch 461:211–223
Zhu J, Yu Y, Ulbrich MH, Li MH, Isacoff EY, Honig B, Yang J (2011) Structural model of the TRPP2/PKD1 C-terminal coiled-coil complex produced by a combined computational and experimental approach. Proc Natl Acad Sci U S A 108:10133–10138
Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Lückhoff A, Schultz G (1996) Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron 16:1189–1196
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Nilius, B., Flockerzi, V. (2014). What Do We Really Know and What Do We Need to Know: Some Controversies, Perspectives, and Surprises. In: Nilius, B., Flockerzi, V. (eds) Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology, vol 223. Springer, Cham. https://doi.org/10.1007/978-3-319-05161-1_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-05161-1_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05160-4
Online ISBN: 978-3-319-05161-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)