Abstract
Cancer drug development is complex and associated with a high failure rate of new therapies in clinical trials. In particular, the history of cancer immunotherapy extends beyond a century with its most noteworthy successes having occurred just in the last 3 years. New drugs in this space – both approved and under investigation – demonstrate increased levels of benefit for patients, and their development is based on progress in basic science and investigational methods. Together these improvements created the environment for reproducible and biology-driven clinical investigation of new compounds and thus a higher success rate. This chapter summarizes the methodological improvements for immunotherapy drug development and their application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Background
Much progress has been made in basic oncologic science over the last decades, which has created high hopes to deliver more effective drugs and better success rates in clinical investigation. Nevertheless, clinical success rates did not keep up with reported scientific progress and thus did not meet expectations of academic or industry drug developers, patients, or society at large. A recent study by Begley and Ellis suggests the reproducibility of published landmark preclinical data from oncologic research to be as low as 11 % (Begley and Ellis 2012). The results were attributed to limited scrutiny regarding experimental controls and data interpretation as well as selection of nonrepresentative data for publication. While a similar analysis is not available for cancer immunotherapy, repeat observations such as limited reproducibility of data or limited correlation of findings between pharmacodynamic and clinical outcomes (e.g., immune monitoring) lead to the hypothesis that existing investigational methods used in cancer immunotherapy development would require adaptation, and the new methods would need to be added to the investigational toolbox to better reflect biology and achieve higher reproducibility (Hoos et al. 2007a).
Indeed, looking back at the history of cancer immunotherapy, which arguably began in the late nineteenth century, reveals the following: The first regressions of cancerous tumors due to an immune intervention were observed by William B. Coley in 1890 after inducing inflammation in these tumors through local injection of a bacterial cocktail also known as Coley’s toxins. In the subsequent 100 years, progress was limited to the scientific knowledge of its time, which was able to accelerate with the emergence of modern methods such as the process for making monoclonal antibodies. However, even in the modern era of controlled clinical trials between the 1970s and today, clinical progress in cancer immunotherapy trials remained rather disappointing, which has led to mostly negative assessments regarding the potential of this modality in the pharmaceutical, oncology, and investment communities (Lesterhuis et al. 2011; Parish 2003). It was only in the last decade that pivotal progress was made both on the basic science and methodological front, which culminated in 2010 and 2011 in the approval of two modern cancer immunotherapies, sipuleucel-T (Kantoff et al. 2010) and ipilimumab (Hodi et al. 2010), based on improved survival outcomes in randomized Phase 3 trials (Fig. 1).
Sipuleucel-T, a cell-based therapeutic cancer vaccine (Provenge®) approved for hormone-refractory prostate cancer (Kantoff et al. 2010), and ipilimumab, a T-cell potentiating monoclonal antibody blocking the cytotoxic T-cell antigen 4 (anti-CTLA-4; Yervoy) approved for unresectable or metastatic melanoma (Hodi et al. 2010; Hoos et al. 2010b), are two distinct types of immunotherapies, which achieved survival improvements for patients as monotherapies in two unrelated tumor entities. Clinical development of both agents were influenced by a new development paradigm (Hoos et al. 2011).
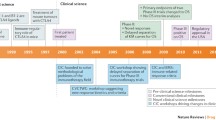
A key factor for this recent turn in the field is that – over the last decade – leading organizations in the cancer immunotherapy community began to systematically establish new methods for rational clinical investigation. These methods support increased data reproducibility and enabled clinical success (Goldman and DeFrancesco 2009; Finke et al. 2007) (Fig. 2).
This addresses the broader methodological concern for Oncology research raised by Begley and Ellis (2012). But it also addresses the more immunotherapy-specific concerns by Goldman and DeFrancesco that immunotherapy failures can be explained due to an inadequate approach to their development, suggesting “companies not doing their homework” and asking “what lessons from the list of failures will inform future practitioners in the field” (Goldman and DeFrancesco 2009) ? With the recent methodological advances, such lessons are now available (Finke et al. 2007; Hoos et al. 2007b, 2011) and are complementary with the basic scientific progress in the cancer immunotherapy field.
Methodological improvements were motivated by the struggle of the drug development industry and academic institutions devoted to inventing and developing cancer immunotherapies. Nonprofit groups such as the Cancer Immunotherapy Consortium (CIC; a program of the nonprofit Cancer Research Institute CRI) founded for the advancement of the cancer immunotherapy field systematically began to create a methodological framework that would provide the knowledge and tools needed for successful development programs. The US-based CIC created a partnership with the Association for Cancer Immunotherapy (CIMT) in Europe and, with broad contributions from the scientific and drug development communities, established this new framework encompassing the following: a biology-driven development paradigm for cancer immunotherapies (Hoos et al. 2007a), harmonized methods for detecting immune response to support immune biomarker development (Britten et al. 2007; van der Burg et al. 2011), improved clinical trial designs (Hoos et al. 2007b) and clinical endpoints (Hoos et al. 2010a; Wolchok et al. 2009), a publication framework for immune monitoring results from clinical trials (Janetzki et al. 2009; Britten et al. 2010), and scientific exchange and regulatory interactions to inform guidance document development by regulatory authorities (FDA 2009; EMA 2010).
This chapter provides a perspective on the recent methodological lessons in the immunotherapy space and summarizes the emerging framework that promises to enable greater and more reproducible success for future development programs (Fig. 3).
Immuno-oncology: An Evolving Area Within Oncology
Oncology, the clinical discipline of cancer therapy, has been an established medical specialty for several decades. Its hallmarks are the science of cancer biology as described by Hanahan and Weinberg (Hanahan and Weinberg 2011); a recognized clinical development paradigm (based on observations with chemotherapy) for investigation of new therapies in Phase 1, 2, and 3 clinical trials; defined criteria for measuring therapeutic effects such as RECIST (Response Evaluation Criteria in Solid Tumors) or WHO (World Health Organization) criteria for solid tumors; understood kinetics of therapeutic effects; established standards for publication of new scientific data; and the availability of effective therapies paired with a clear understanding of their use. All this is anchored in a defined community represented by organizations such as the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO). Together, these hallmarks create a framework of credibility in which patient care, scientific discovery, publication, clinical development, and regulatory review can take place.
Despite clear evidence that the whole class of cancer immunotherapies has critical unique features that are different from those of the established classical therapeutic approaches in oncology, the field did not initially respond to the need of creating an appropriate alternative methodological framework accommodating these class-specific characteristics. Rather, to minimize controversy, keep shorter timelines, and build recognition in oncology, investigations of immunotherapies utilized the existing development paradigm based on cytotoxic drugs. This ultimately may have contributed to a high fraction of failures made in past developments (Goldman and DeFrancesco 2009).
Between 2004 and today, CIC and CIMT filled this void by creating a systematic framework using broad community knowledge and providing needed tools for successful development of immunotherapies (Table 1).
A Development Paradigm for Cancer Immunotherapies
The first step was the proposal of a clinical development paradigm in 2004. At the time, much knowledge around developmental problems and potential solutions existed in the field with little consensus on how to uniformly utilize and translate it into a comprehensive new paradigm. CIC and its partner organization the Society for Immunotherapy of Cancer (SITC) formed the Cancer Vaccine Clinical Trial Working Group (CVCTWG) with stakeholders from academia, the biotechnology and pharmaceutical industry and the US FDA. Together they built a paradigm for development of cancer vaccines and related immunotherapies (Hoos et al. 2007a). It recognizes differences between chemotherapy and immunotherapy such as (1) the optimal biologic dose is often not the maximum tolerated dose; (2) a treatment effect is not proportionally linked to toxicity; (3) conventional pharmacokinetics may not solely determine dose and schedule; (4) antitumor response may not be the only predictor of survival; and (5) clinical effects can be delayed in time and can occur after tumor volume increase (often categorized as progression). The new paradigm categorizes clinical development into proof-of-principle trials and efficacy trials, where efficacy trials are recommended to be randomized (Phases 2 and 3). It also provides considerations for toxicity screening in early trials, concepts for measurement of biologic activity, criteria for the use of immune monitoring assays, dose and schedule investigation, decision points in development, clinical study design, biology-based clinical endpoints, and combination therapy. The main value of this paradigm lies in the consensus between all main constituents involved with cancer immunotherapy development, namely, academicians, pharmaceutical/biotech industry, and the US FDA (Hoos et al. 2007b; FDA 2009).
Improved Clinical Endpoints
Chemotherapy and targeted therapy have direct effects on tumor cells and typically induce a measurable impact on tumor growth within a few weeks of administration or demonstrate not to be effective at all. In contrast, therapies utilizing the immune system induce indirect antitumor effects by initially stimulating the immune system followed by a broader spectrum of clinical responses including delayed effects. Delayed effects on tumors may include shrinkage after initial volume increase of existing lesions and appearance of new lesions, which may both be caused by immune infiltrates or prolonged stabilization of lesions without any shrinkage (Wolchok et al. 2009). With some immunotherapies, the latter patterns appear to be more common than the conventional response. They likely reflect the interplay between the immune system and the tumor described as immunoediting (Dunn et al. 2002).
Delayed effects and stabilization of tumor lesions influence the standard efficacy endpoints of antitumor response and overall survival (Finke et al. 2007; Hoos et al. 2010a). Both endpoints need adjustment to address this biology. For survival, Kaplan-Meier curves from randomized immunotherapy trials may show a delayed separation after months, which directly influences the statistical power to determine treatment effects observed over the entire length of a given curve (Fine 2007). Statistical models used in randomized oncology trials, where separation of Kaplan-Meier (KM) curves is expected early after treatment initiation, typically assume proportional hazards reflected in a constant hazard ratio over time. In order to address the delayed separation, alternative statistical models need to consider that all events prior to the separation do not contribute to the differentiation between study arms after the separation, thus causing reduced statistical power. Compensation for such power reduction can occur through a split of the hazard ratio into an early and a late component before and after the separation (Hoos et al. 2010a). When planning interim analyses in randomized studies, the absence of early effects would need to be accounted for to determine timing of the analysis and the value of testing for futility.
Immune-Related Response Criteria to Characterize Antitumor Effects
Standard response criteria based on WHO (1979) or RECIST (Eisenhauer et al. 2009) for assessing clinical effects of anticancer agents were created with the experience from cytotoxic drugs using tumor shrinkage as their measure of activity. With the altered biology of immunotherapies, their response patterns are broader than those of chemotherapy and may manifest after a period of stable disease or after initial tumor burden increase or appearance of new lesions. This may represent influx of lymphocytes into the tumor (Wolchok et al. 2009; Ribas et al. 2009). Such patterns were commonly noted in past trials but were never systematically described due to lack of suitable criteria (Kruit et al. 2005; van Baren et al. 2005). Principles for development of new response criteria were derived from the described development paradigm (Hoos et al. 2007b) and immune-related response criteria and were refined using large data sets from the ipilimumab (anti-CTLA-4) development program with 487 advanced melanoma patients from Phase 2 trials (Wolchok et al. 2009). Four patterns of response were described: A: immediate response, B: durable stable disease with possible slow decline in tumor burden, C: response after tumor burden increase (possible lymphocyte infiltration), and D: response in the presence of new lesions. Immune-related response criteria (irRC) are generally based on WHO and RECIST criteria, describe tumor burden as a continuous variable, account for new lesions in the overall tumor burden, and require confirmation of progression similar to the established confirmation of response at a subsequent time point after first detection. Ipilimumab data suggest that irRC identify patients with previously unrecognized benefit as indicated by favorable survival outcomes. Such patients displayed novel response patterns (Wolchok et al. 2009). Since their creation in 2009, irRC are undergoing prospective validation and are being tested in countless trials with a broad spectrum of cancer immunotherapies.
Managing Data Variability in Immune Biomarker Development
The monitoring of treatment-induced immune responses is important for understanding the mechanism of action and the description of early biologic effects prior to reaching clinical endpoints. Such immune biomarkers depend on reliable and reproducible assays and may provide data on (1) whether the biological target was hit, (2) how to dose the agent, (3) whether synergies exist for therapeutic combinations, (4) how patient populations may be defined, (5) how biologic activity can be characterized, and (6) whether they predict clinical outcomes as surrogates for patient benefit (Wagner 2002). Common immune response assays used to determine function, phenotype, and frequency of antigen-specific T cells such as ELISPOT, intracellular cytokine staining, and HLA-peptide multimer staining have inherently high data variability (Janetzki et al. 2009). This variability has contributed to the abundant challenge of developing biomarkers for the above applications. After extensive efforts across more than 120 academic, industry, and government laboratories over close to a decade, a solution for this data variability has emerged: immune assay harmonization. Harmonized use of immune assays across laboratories provides an external quality-control mechanism and guidance for assay conduct that – if followed – can substantially increase assay performance and decrease data variability.
Harmonization criteria were established through large international proficiency panel programs conducted by the CIC and CIMT (Janetzki et al. 2008; Britten et al. 2008, 2009; Attig et al. 2011; Mander et al. 2010; Moodie et al. 2010]. Harmonization is a tool to improve data reliability for immune monitoring and enhance clinical development of immune therapies at any stage of assay evolution (Janetzki and Britten 2012; van der Burg et al. 2011). It reminds of the successful initiatives of ICH-GCP for clinical protocols (ICH 1996) and has the potential to bring immune monitoring to the forefront of immune biomarker development and provide support in guiding decision making in clinical development (van der Burg 2008).
Consistent Reporting of Immune Monitoring Data
Variability is not limited to immune monitoring. It extends to the presentation of methods and results in scientific publications. To date, many publications of T-cell assay experiments lack information critical variables known to impact assay performance. In its absence scientists reading these publications are not enabled to fully understand the content or reproduce the experiment. The solution lies in creation of a publication framework that determines a minimum set of critical variables a publication must contain to transparently summarize what experiment was done under which conditions and with which results. The scientific community faced this challenge with a series of modern bioassays and responded with the creation of the Minimum Information About Biological and Biomedical Investigations (MIBBI) concept (Taylor et al. 2008). Over the last decade MIBBI created transparency measures for more than 30 biological assays such as DNA microarrays, RNAi experiments, or cellular assays. CIC and CIMT started the Minimal Information About T-Cell Assays (MIATA) project in 2009, which established a framework for publication of T-cell assay results from clinical trials (Janetzki et al. 2009). MIATA is based on an extensive community-wide vetting process over approximately 2 years incorporating the expertise and concerns of more than 120 experts from all areas of clinical immunology and achieved wide acceptance (Britten et al. 2010). The final version of MIATA became available in 2012 (Britten et al. 2012) and is being tested now as part of the Materials and Methods sections of several peer-reviewed journals. Its impact will depend on the breadth of use across the community.
Regulatory Guidance
The described methodological advances for the growing immune-oncology space evolved under the auspices of CIC and CIMT and with the participation of all major stakeholders from academia, biotechnology and pharmaceutical industries, and the US FDA. When a scientific area reaches the point of producing drug candidates ready for regulatory review and possible approval, there is an accompanying need for regulatory guidance documents clarifying a uniform view of regulatory authorities on the subject. In the case of cancer immunotherapy, the FDA utilized the scientific lessons from the community, hosted a workshop where these topics were reviewed, and published a draft guidance on “Clinical Considerations for Therapeutic Cancer Vaccines” (2009). The FDA draft document contained many of the topics summarized above, went through public consultation, and was finalized 2011. Similarly, the European Medicines Agency (EMA) issued a concept paper soliciting public feedback on a proposed revision of the guidance on “evaluation of anticancer medicinal products in man.” EMA specifically requested community input regarding clinical endpoints for biologics and cancer vaccines (EMA 2010). CIMT and CIC jointly offered their integrated positions to EMA, which found inclusion in the updated guidance document. Overall, CIC and CIMT have created a process that addresses cutting-edge aspects of the field, create a uniform voice, and enable officials at FDA and EMA to more easily review and assess community positions.
Anti-CTLA-4 Antibody Development: Application of the Development Paradigm
The example of anti-CTLA-4 antibody development (Hoos et al. 2010b) illustrates the relevance of biology-based drug development as outlined in the new immunotherapy paradigm. Clinical trials with anti-CTLA-4 antibodies started at the biotechnology company Medarex in the year 2000 with Phase 1 and 2 trials suggesting an approximate 10 % response rate in patients with advanced melanoma. Interest from big pharmacy for developing anti-CTLA-4 blocking antibodies led to independent licensing deals with Pfizer and Bristol-Myers Squibb (BMS) for different antibody isoforms and sparked two parallel development programs in advanced melanoma with tremelimumab (Pfizer) and ipilimumab (BMS), respectively. As was standard in the industry, both programs initially used chemotherapy criteria to guide development (Hoos et al. 2010a; Finke et al. 2007). By its design, the tremelimumab program conducted an early interim analysis using conventional futility criteria for survival in its Phase 3 study. A survival difference was not observed, and, consequently, the Phase 3 trial was terminated for futility as per Data Monitoring Committee recommendations (Ribas et al. 2008). Two years downstream extended follow-up on the study population revealed a separation of survival curves (Ascierto et al. 2011). To the contrary, interaction of the ipilimumab development program with CIC enabled the program to adapt to new scientific information. This resulted in the change of the primary endpoint for two pivotal Phase 3 trials in advanced melanoma from response or progression-free survival to overall survival with no early interim analyses that may mislead the assessment (Hodi et al. 2010; Hoos et al. 2010b). Both Phase 3 studies demonstrated improved survival (HR 0.66 and HR 0.72, respectively) in their final analyses, thus supporting the regulatory approval for patients with unresectable and metastatic melanoma. Based on the matured knowledge about immunotherapy development, BMS acquired Medarex in 2009 in a transaction valued $2.4 billion and is now developing a pipeline of immuno-oncology agents resulting from the acquisition (2011).
The development programs for ipilimumab and tremelimumab and their respective results illustrate the importance of science-driven clinical development for immunotherapies and of collaboration across various constituents to direct scientific progress. These observations also suggest that the prospective application of the new paradigm may help avoid critical pitfalls for future immunotherapy programs.
Summary
The last decade has brought many methodological improvements that accompany our growing scientific understanding of tumor immunology (Finn 2008). Their application has enabled success in the space of immuno-oncology and allowed it to emerge as a successful new subspecialty within oncology. By addressing the obvious weak spot in immunotherapy drug development, namely, the absence of a biology-based clinical development paradigm and other associated methodological advances, the foundation for future progress in immuno-oncology has been created. The resulting methodological framework will likely expand with the now rapidly growing space.
References
Ascierto PA, Marincola FM, Ribas A (2011) Anti-CTLA4 monoclonal antibodies: the past and the future in clinical application. J Transl Med 9:196
Attig S, Price L, Janetzki S, Kalos M, Pride M, McNeil L, Clay T, Yuan J, Odunsi K, Hoos A, Romero P, Britten CM (2011) A critical assessment for the value of markers to gate-out undesired events in HLA-peptide multimer staining protocols. J Transl Med 9:108
Begley CG, Ellis LM (2012) Drug development: raise standards for preclinical cancer research. Nature 483:531–533
BMS press release (2011) Bristol-Myers Squibb to acquire Medarex. July 22 2009. http://www.bms.com/news/press_releases/pages/default.aspx
Britten CM, Janetzki S, van der Burg SH, Gouttefangeas C, Hoos A (2007) Toward the harmonization of immune monitoring in clinical trials: Quo vadis? Cancer Immunol Immunother 57:285–288
Britten CM, Gouttefangeas C, Welters MJ, Pawelec G, Koch S, Ottensmeier C, Mander A, Walter S, Paschen A, Muller-Berghaus J, Haas I, Mackensen A, Kollgaard T, Thor SP, Schmitt M, Giannopoulos K, Maier R, Veelken H, Bertinetti C, Konur A, Huber C, Stevanovic S, Wolfel T, van der Burg SH (2008) The CIMT-monitoring panel: a two-step approach to harmonize the enumeration of antigen-specific CD8+ T lymphocytes by structural and functional assays. Cancer Immunol Immunother 57:289–302
Britten CM, Janetzki S, Ben Porat L, Clay TM, Kalos M, Maecker H, Odunsi K, Pride M, Old L, Hoos A, Romero P (2009) Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Cancer Immunol Immunother 58:1701–1713
Britten CM, Janetzki S, van der Burg SH, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, O’Donnell-Tormey J, Odunsi K, Old LJ, Pawelec G, Roep BO, Romero P, Hoos A, Davis MM (2010) Minimal information about T cell assays: the process of reaching the community of T cell immunologists in cancer and beyond. Cancer Immunol Immunother 60:15–22
Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, O’Donnell-Tormey J, Odunsi K, Old LJ, Ottenhoff TH, Ottensmeier C, Pawelec G, Roederer M, Roep BO, Romero P, van der Burg SH, Walter S, Hoos A, Davis MM (2012) T cell assays and MIATA: the essential minimum for maximum impact. Immunity 37(1):1–2
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991–998
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
EMA (2010) Concept paper on the need to revise the guideline on the evaluation of anticancer medicinal products in man. European Medicines Agency. July 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/09/WC500096730.pdf
FDA (2009) Guidance for industry: clinical considerations for therapeutic cancer vaccines. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, September 2009. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM182826.pdf
Fine GD (2007) Consequences of delayed treatment effects on analysis of time-to-event endpoints. Drug Inf J 41:535–539
Finke LH, Wentworth K, Blumenstein B, Rudoplph NS, Hoos A (2007) Lessons from randomized phase III studies with active cancer immunotherapies-outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccine 25:B97–B109
Finn OJ (2008) Cancer immunology. N Engl J Med 358:2704–2715
Goldman B, DeFrancesco L (2009) The cancer vaccine roller coaster. Nat Biotechnol 27:129–139
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, Nichol G (2007a) A clinical development paradigm for cancer vaccines and related biologics. J Immunother 30:1–15
Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, Nichol G (2007b) A clinical development paradigm for cancer vaccines and related biologics. J Immunother 30:1–15
Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J (2010a) Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102:1388–1397
Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, Chin K, Canetta R, Humphrey R (2010b) Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol 37:533–546
Hoos A, Britten CM, Huber C, O’Donnell-Tormey J (2011) A methodological framework to enhance the clinical success of cancer immunotherapy. Nat Biotechnol 29:867–870
ICH (1996) Harmonised tripartite guideline: guideline for good clinical practice E6(R1). 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf
Janetzki S, Britten CM (2012) The impact of harmonization on ELISPOT assay performance. Methods Mol Biol 792:25–36
Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, Kast WM, Hoos A (2008) Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol Immunother 57:303–315
Janetzki S, Britten CM, Kalos M, Levitsky HI, Maecker HT, Melief CJ, Old LJ, Romero P, Hoos A, Davis MM (2009) “MIATA”-minimal information about T cell assays. Immunity 31:527–528
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422
Kruit WH, van Ojik HH, Brichard VG, Escudier B, Dorval T, Dreno B, Patel P, van Baren N, Avril MF, Piperno S, Khammari A, Stas M, Ritter G, Lethe B, Godelaine D, Brasseur F, Zhang Y, van der Bruggen P, Boon T, Eggermont AM, March M (2005) Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer 117:596–604
Lesterhuis WJ, Haanen JB, Punt CJ (2011) Cancer immunotherapy–revisited. Nat Rev Drug Discov 10:591–600
Mander A, Gouttefangeas C, Ottensmeier C, Welters MJ, Low L, van der Burg SH, Britten CM (2010) Serum is not required for ex vivo IFN-gamma ELISPOT: a collaborative study of different protocols from the European CIMT Immunoguiding Program. Cancer Immunol Immunother 59:619–627
Moodie Z, Price L, Gouttefangeas C, Mander A, Janetzki S, Lower M, Welters MJ, Ottensmeier C, van der Burg SH, Britten CM (2010) Response definition criteria for ELISPOT assays revisited. Cancer Immunol Immunother 59:1489–1501
Parish CR (2003) Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol 81:106–113
Ribas A, Hauschild A, Kefford R, Punt CJA, Haanen JB (2008) Phase III open-label, randomized, comparative study of tremelimumab (CP-675, 206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. American Society of Clinical Oncology, annual meeting 2008, abstract LBA 9011
Ribas A, Chmielowski B, Glaspy JA (2009) Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res 15:7116–7118
Taylor CF, Field D, Sansone SA, Aerts J, Apweiler R, Ashburner M, Ball CA, Binz PA, Bogue M, Booth T, Brazma A, Brinkman RR, Michael CA, Deutsch EW, Fiehn O, Fostel J, Ghazal P, Gibson F, Gray T, Grimes G, Hancock JM, Hardy NW, Hermjakob H, Julian RK Jr, Kane M, Kettner C, Kinsinger C, Kolker E, Kuiper M, Le Novere N, Leebens-Mack J, Lewis SE, Lord P, Mallon AM, Marthandan N, Masuya H, McNally R, Mehrle A, Morrison N, Orchard S, Quackenbush J, Reecy JM, Robertson DG, Rocca-Serra P, Rodriguez H, Rosenfelder H, Santoyo-Lopez J, Scheuermann RH, Schober D, Smith B, Snape J, Stoeckert CJ Jr, Tipton K, Sterk P, Untergasser A, Vandesompele J, Wiemann S (2008) Promoting coherent minimum reporting guidelines for biological and biomedical investigations: the MIBBI project. Nat Biotechnol 26:889–896
van Baren N, Bonnet MC, Dreno B, Khammari A, Dorval T, Piperno-Neumann S, Lienard D, Speiser D, Marchand M, Brichard VG, Escudier B, Negrier S, Dietrich PY, Maraninchi D, Osanto S, Meyer RG, Ritter G, Moingeon P, Tartaglia J, van der Bruggen P, Coulie PG, Boon T (2005) Tumoral and immunologic response after vaccination of melanoma patients with an ALVAC virus encoding MAGE antigens recognized by T cells. J Clin Oncol 23:9008–9021
van der Burg SH (2008) Therapeutic vaccines in cancer: moving from immunomonitoring to immunoguiding. Expert Rev Vaccines 7:1–5
van der Burg SH, Kalos M, Gouttefangeas C, Janetzki S, Ottensmeier C, Welters MJ, Romero P, Britten CM, Hoos A (2011) Harmonization of immune biomarker assays for clinical studies. Sci Transl Med 3:108ps44
Wagner JA (2002) Overview of biomarkers and surrogate endpoints in drug development. Dis Markers 18:41–46
WHO (1979) WHO handbook for reporting results of cancer treatment. World Health Organization Offset Publication No. 48, Geneva
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Acknowledgment
CIC and CIMT thank all participants of its workshops and community-wide initiatives for their contribution of knowledge, which have enabled the creation of the immune-oncology framework.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hoos, A. (2014). Developing Cancer Immunotherapies as Drugs: Setting the Stage Through Methodological Progress. In: Britten, C., Kreiter, S., Diken, M., Rammensee, HG. (eds) Cancer Immunotherapy Meets Oncology. Springer, Cham. https://doi.org/10.1007/978-3-319-05104-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-05104-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05103-1
Online ISBN: 978-3-319-05104-8
eBook Packages: MedicineMedicine (R0)