Abstract
Scientific explanations for the cause of spontaneous processes can be understood from the energy perspective. The second law of thermodynamics shows that all real spontaneous processes lead to an increase in the total entropy of the universe. Entropy is closely related to energy, though the two concepts are distinct. In K-12 science education, students should gain familiarity with explanations for the cause of spontaneous processes. This includes a qualitative understanding of the second law of thermodynamics and entropy, as well as an understanding of the relationship between entropy and energy. Here, we review the energy dispersal metaphor for entropy and the energy spreading metaphor for entropy change, and describe how these metaphors can provide students with a scientific understanding of spontaneous processes from the energy perspective. We also discuss advantages of using this metaphor over others for teaching and learning about entropy in K-12 science education, such as the metaphor’s compliance with the features of entropy, the avoidance of common misconceptions when using the metaphor, the metaphor’s accessibility to students at a young age, and the metaphor’s amenability to updating for increasingly sophisticated understandings of entropy. We also discuss why the commonly used disorder metaphor for entropy should be avoided. Finally, we propose a potential K-12 learning progression for understanding spontaneous processes from the energy perspective.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
“Why do some physical processes and chemical reactions happen spontaneously while others do not?” This is a fundamental question in the physical sciences (National Research Council [NRC] 2012). Answering it can explain everything from why an object falls to the Earth’s surface to what determines chemical equilibrium (Lambert 2002b). The question also has deep significance for the life sciences, earth sciences, and engineering. For example, the conditions necessary for spontaneity explain the need for energetic coupling of spontaneous reactions with non-spontaneous reactions in living things, a fundamental characteristic of metabolism (Reece et al. 2011). Convection cycles, which play a key role in every major non-living Earth system, are perhaps best explained from the lens of spontaneous processes (Chen et al. 2010). Maximum theoretical efficiency in modern mechanical engineering is also inextricably linked, historically and presently, to this question (Dincer and Cengel 2001).
Given the significance of the question of spontaneity, one might conclude that after completing the 12th-grade, students should at least have familiarity with how this question is answered in contemporary science, recognizing that the answer is related to energy. Ideally, students should be able to go beyond that, and explain spontaneous processes in the context of the second law of thermodynamics (henceforth the second law). However, a comparison of K-12 science education standards from seven countries shows that only the standards from three countries involve entropy, the second law, and the direction of chemical reactions. In stark contrast, all of the standards place strong emphasis on the first law of thermodynamics (energy conservation) and the forms of energy (Wang et al. 2014). Perhaps this is why even at the university level and among chemistry majors, research evidence suggests that students are by and large unable to adequately explain why some processes and reactions are spontaneous, whereas others are not (Sözbilir and Bennett 2007; Boo 1998).
Because some sort of energy change or transfer always accompanies spontaneous processes, energy is used for explaining in a generalized manner why such processes occur. Yet typical energy constructs, such as the forms of energy, the transfer and transformation of energy, and the conservation of energy are inadequate for making such an explanation. Still, perhaps because of the emphasis on these constructs in K-12 curricula, students often attempt to use them in explaining spontaneous processes. For example, many students believe that a spontaneous process occurs when the energy of a system decreases (Carson and Watson 2002). Of course, because of the conservation of energy, any decrease in the energy of a system is offset by an increase in the energy of its surroundings, and vice versa. There is no logical justification for prioritizing an energy decrease in the system under study over an energy decrease in its surroundings (Atkins 2006). Thus, spontaneous endothermic chemical reactions provide a counter example of the common misconception that these processes come about because of a decrease in energy of the system. Both the system under study and its surroundings must be taken into consideration when analyzing the cause of spontaneous processes in the system.
Other documented misconceptions for why spontaneous processes occur include the correct idea that the concept of entropy can be used to explain spontaneity. However, in these misconceptions, entropy is considered either without reference to energy at all or simply as another form of energy in a way similar to thermal or kinetic energy or enthalpy (Sözbilir and Bennett 2007). The latter belief is not surprising in light of the common understanding of spontaneous events in terms of negative changes in Gibbs free energy (∆G), a construct that can be used as a proxy for the total entropy change of the universe (the system plus its surroundings) but is often presented without this context made explicit. Another source of this misunderstanding is that T∆S, in ∆G = ∆H − T∆S, is often considered as energy that is not useful, or as dissipated heat, which is related to energy, while ∆H is often considered as the total energy change of the system. The common metaphor for entropy as a measure of disorder also contributes to this misunderstanding. This occurs when students, not unreasonably, consider the increased motion and collisions of particles that accompany increased thermal energy as an increase in disorder. Other students will persistently use, despite being instructed to do otherwise, vague or spatial understandings of disorder to describe entropy, without connecting the concept in any way to energy (Carson and Watson 2002). Of course, for many reasons, not least because that entropy is not conserved for real processes, it cannot be considered as yet another form of energy. However, as we argue in this chapter, entropy should be considered from an energy perspective.
Ultimately, it is entropy that is the key concept for explaining spontaneous processes. The second law, put in one way, states that systems spontaneously evolve toward the state of maximum entropy of the universe. Thus, it can be said that spontaneous processes are those that increase the total entropy of the universe. But what is entropy? And what is the best way to approach teaching the concept in K-12 science courses? Entropy is perceived as a difficult concept for students to grasp. We propose that a key reason for this is a lack of a widely accepted, accessible, and effective means of teaching entropy, or a specific set of learning progressions for understanding spontaneous processes across K-12 science education and multiple disciplines.
In this chapter, we suggest a greater emphasis in K-12 education on understanding spontaneous processes by analyzing entropy from an energy perspective. Our proposal for accomplishing this draws heavily on a widely discussed but underused metaphor—entropy as a measure of the extent of energy dispersal in space (Lambert 1999, 2002a, b, 2005, 2006a, b, 2007, 2011; Lambert and Leff 2009; Leff 1996, 2007, 2012a, b, c, d, e; Kozliak and Lambert 2005, 2008). With this metaphor, the second law can be restated as “energy spreads out spontaneously if not hindered from doing so.” We also critically examine what remains a common method for introducing entropy in K-12 curricula, as represented by high school chemistry textbooks today—through the metaphor of entropy as “disorder” (e.g. Gao and Wang 2007; Song and He 2004; Wang 2007; Wilbraham et al. 2012). We analyze the advantages and disadvantages of the disorder and energy dispersal metaphors for entropy, with an emphasis on the metaphors’ fidelity to key features of the entropy concept, relationship to the energy perspective, accessibility to younger students, and openness to updating in a learning progression. We conclude by reiterating what students should know about entropy and spontaneous processes as well as common student misconceptions, while describing challenges for widespread and successful adoption of teaching entropy from an energy perspective in K-12 education. Finally, we propose an outline of a program to develop a K-12 learning progression for understanding spontaneous processes from an energy perspective.
2 Understanding Entropy from the Energy Perspective
The two most widely used quantitative expressions for entropy, which can be shown to be equivalent, are the macroscopic Clausius formulation and the microscopic (and quantum) Boltzmann formulation. In both formulations, entropy connects intimately to energy. Here, we begin by discussing the Clausius formulation, which clearly shows that entropy is a function of energy. We then outline the major features of the metaphor for entropy as the dispersal of energy. In a subsequent section, we show how to update this metaphor to comply with Boltzmann’s definition of entropy.
2.1 Clausius’ Definition of Entropy and the Dispersal of Energy Metaphor
Clausius’ mathematical definition of entropy change, dS = dq rev/T, could be expressed verbally as “entropy change equals the amount of energy dispersed reversibly at a specific temperature T.” This clearly does not imply that entropy is “disorder,” and in fact Clausius himself used the term “transformation” to describe entropy. This “transformation” in the simplest sense is energy’s dispersal from a source that is almost imperceptibly at a temperature above T to a receptor that is at T. More generally, Clausius’ equation can be thought of as an index of the amount of energy dispersal at a specific temperature (q rev/T). Though the specific temperature is clearly important, to a first approximation it follows that “entropy as the dispersal of energy” is a useful metaphor. When entropy increases, energy becomes more dispersed in space (Lambert 2002b).
2.2 Total Entropy and the Dispersal of Energy Between the System and Its Surroundings
It is crucial that useful entropy analysis takes into account both the system under study and its surroundings, because the second law holds that the total entropy of the universe never decreases. The second law tells us nothing about what can or cannot happen to the entropy of a system in the absence of its surroundings. Yet students often hold the misconception that spontaneous processes can be determined by changes in the system under study alone, without taking its surroundings into consideration (Sözbilir and Bennett 2007). This is not surprising, given that so many concepts in science concern themselves with the system alone. It is therefore useful to consider how our metaphor might be adapted when discussing the change of total entropy, such that the system and its surroundings are explicitly taken into consideration. We can simply say that when total entropy increases, energy becomes more dispersed between the system and its surroundings.
2.3 Connecting the Energy Dispersal Metaphor for Entropy to Spontaneous Processes
Total entropy is maximized in spontaneous processes. In other words, there will be more dispersal of energy between the system and its surroundings whenever a real spontaneous process occurs. This provides another formulation of the second law: “energy spreads out spontaneously if not hindered from doing so.” For the purposes of this discussion, and for clarity in using these metaphors in K-12 courses, we propose that we consider energy “dispersal” to be a static function describing energy distribution—that is, entropy. Energy “spreading,” on the other hand, is taken to be a dynamic process involving a change in entropy—in other words, the process of broadening the dispersal of energy between the system and its surroundings. A major advantage of framing an increase in entropy described by the second law in terms of energy spreading is that the surroundings are taken into consideration, which is often not explicit.
2.4 Using the Energy Dispersal Metaphor to Qualitatively Describe Spontaneous Processes
The spontaneous transfer of heat from higher temperature to lower temperature bodies follows the second law. In a more qualitative (and metaphorical) sense, even mathematically naïve students can understand that for energy to spread out maximally, the thermal energy of a hot object must be distributed to its surroundings, or the thermal energy of the hot surroundings must be distributed to a cool object. Similarly, particles carrying thermal energy will spread into a vacuum in order to maximize energy spreading (carrying their thermal energy over a larger space—a similar argument can be made for why mixed gases have more entropy than separated gases). The gravitational potential energy stored in the system of a suspended rock and the earth will, upon release of the rock, spread out as kinetic energy and eventually, upon impact on a surface, as heat to its surroundings. It should be noted that although this last example describes a mechanical rather than a thermodynamic system, the energy dispersal metaphor is still instructive in explaining why this spontaneous event occurs. In each of these contexts, even students that are still developing an understanding—of energy transfer, the conservation of energy, and energy transformation—should be able to use the idea of energy spreading to determine why certain spontaneous events occur. The dispersal of energy metaphor therefore provides accessibility to students for use in accurate entropy analysis and provides a natural avenue for taking both the system and its surroundings into account.
3 Movement to Replace the Disorder Metaphor with the Dispersal of Energy Metaphor
Lambert, an organic chemist, and Leff, a physicist, among others, have led a movement to shift the prevailing metaphor for entropy in introductory college chemistry and physics texts from the disorder metaphor to the dispersal of energy metaphor discussed above. Equivalent to this metaphor for entropy, and more relevant for K-12 educators, the Framework and Next Generation Science Standards (NGSS) use language of “toward more uniform energy distribution” to describe the evolution of uncontrolled systems (or the second law) (NRC 2012). In this section, we begin by discussing the success of the disorder metaphor, and then outline some major problems with using the disorder metaphor in K-12 education. In this context, we suggest some criteria for a successful replacement metaphor, and finally describe how the dispersal of energy metaphor meets these criteria.
3.1 Disorder Metaphor Has Been Pervasive
Since the formulation of the macroscopic entropy concept by Clausius and later the microscopic entropy concept by Boltzmann in the nineteenth century, many metaphors have been proposed to describe entropy (Leff 2007). To be sure, the disorder metaphor must be considered one of the most pervasive; it has been both long lasting and widely used. There are many good reasons for this. First, increased spatial disorder is in fact observed in many spontaneous processes—from the mixing of two ideal fluids to the expansion of gas in a vacuum. Second, the idea of disorder on some level is readily accessible, and lends a workable way to approach teaching and discussing the Boltzmann’s statistical mechanical formulation of entropy. Third, popular culture associations with ever-increasing disorder as a fundamental law of the universe are pervasive and appealing. Finally, the disorder metaphor has been so widely used for so long that it is difficult to quickly remove it from the discourse and curricula concerning entropy. The conception of entropy as disorder is also difficult to replace once incorporated by individuals (Sözbilir and Bennett 2007).
3.2 Entropy as Disorder: What’s the Problem?
It is widely accepted that metaphors, by mapping abstract concepts to relatable everyday phenomena, can help students better understand and use science concepts (Duit 1991). If metaphors are considered as models of scientific phenomena, then it holds that all metaphors are somewhat limited in reflecting the phenomena they represent. Thus, for students to demonstrate adequate understanding of how a metaphor is used, they must explicitly consider the advantages and shortcomings of the metaphor in describing the phenomenon or concept it represents (Glynn and Takahashi 1998). This is an especially difficult task for a highly abstract concept such as entropy, and it is nearly impossible if the metaphor used for understanding that concept in the first place fails to approximate key features of the concept itself. Unfortunately, this is the case for the disorder metaphor for entropy. Here, we discuss three major problems with the disorder metaphor.
3.2.1 “Disorder” Is Vague
The first problem with the disorder metaphor is the vagueness of the term itself. Typical dictionary definitions for disorder are: “lack of order or regular arrangement,” “confusion,” and “(in medicine) a disturbance of normal functioning.” The first of these definitions has a strong spatial connotation, which we discuss below. This is, to be fair, the definition that is typically emphasized in high school textbooks, though this does not guarantee that it will be the definition incorporated by students, and it does not reflect the more appropriate interpretation of entropy as it relates to available energy microstates. Using the “confusion” definition, a typical high school student might relate disorder to an inability to decide on a particular route when lost, for example. This, then, can be related to the disorder that some associate with higher temperatures, envisioned in terms of increased particle agitation. The variable definition of disorder makes the term itself confusing (Leff 2007).
There is also a problem that describing entropy as a measure of disorder does not in itself specify the level of analysis at which that disorder occurs. For example, ice cubes flying in space appear disordered macroscopically, though of course the ice cubes themselves are neatly ordered at the molecular level. Below the molecular level, the subatomic particles that make up the ice cube then have a higher degree of disorder. Which level of organization does “disorder” refer to (Donaldson 2011)?
3.2.2 Spatial Disorder Does Not Represent the Features of the Entropy Concept Well
The spatial disorder metaphor is related historically to the Boltzmann formulation of entropy. This particular formulation, known as the microscopic or statistical mechanical definition, holds that entropy increases logarithmically with the number of available energy microstates for a particular system (or, alternatively, with the number of ways of realizing the most probable microstates). In the modern quantum mechanical view of the Boltzmann formulation, microstates are possible ways of energy distribution, rather than spatial particle disorder (though particle disorder can be related to an increase in energy distribution). Clearly, entropy is an energy-related concept. Yet, the connection to energy using the spatial disorder metaphor of entropy is not explicit, and students may fail to recognize it (Granville 1985; cited in Cooper et al. 2014).
Another important and often ignored issue is that spatial disorder can just refer to a single “snapshot” of a particular system (i.e. one microstate), while according to the Boltzmann formulation entropy increases when there are more available microstates (i.e. the number of microstates), through which the system dynamically moves with an equal probability of being in each microstate at any particular instant. By simply considering snapshots, even experienced chemists can easily be fooled into naming what amounts to a lower entropy system as having higher entropy because one particular snapshot of that system is more likely to appear “disordered” (Styer 2000). So the disorder of one microstate and the number of available microstates must be differentiated in the teaching of entropy.
Avoiding a detailed discussion of the meaning of energy-microstate and the number of microstates, this brings us to the problem that we will draw wrong conclusions while analyzing entropy changes of some processes with the spatial disorder metaphor. In some situations, visual disorder actually decreases as entropy increases, such as spontaneous crystal formation in a supersaturated sodium sulfate solution. From a spatial viewpoint, there is more visual order after crystallization, so that the entropy of the system seems to decrease. But considering that the temperature actually decreases in this process, i.e. the system absorbs energy from its surroundings and the entropy of the surroundings decreases, the entropy of the system has to increase so as to make the total entropy increase. Thus the visual order of this case refers to higher entropy. There are other processes that increase entropy yet do not lead to more disorderly visual states. For example, within certain temperature bands, increasing the temperature of some liquid crystals leads to more alignment of the crystals, while entropy has increased (Leff 2007; Lambert 2002a).
Another issue is that the number of microstates available even for a relatively low entropy system (or considered as “more ordered” with this metaphor), such as a small ice cube relative to an equivalent amount of liquid water, is so staggeringly large that it cannot be called orderly in human terms (Kozliak and Lambert 2005).
Most of the above issues are beyond the normal realm of K-12 education. Still, the metaphors used in K-12 education should avoid perpetuating misconceptions in students who may pursue further study of the physical sciences at the university level. The final problem with the disorder metaphor, discussed below, reflects the perpetuation of misconceptions about entropy and spontaneous events that directly interfere with the goals of learning these concepts in K-12 education.
3.2.3 Disorder Metaphor Does Not in Itself Integrate the Entropy of the System and Its Surroundings
The disorder metaphor fails to approach entropy in such a way that considers the total entropy of the universe. While it is possible to consider the “disorder” of the system and the “disorder” of its surroundings, this must be done with additional effort, and is therefore often neglected, especially when giving qualitative explanations (Sözbilir and Bennett 2007). The formula for Gibbs free energy implicitly takes the entropy of the surroundings into consideration (through the enthalpy term), but students using this formula often miss a key feature of the second law—that the total entropy of the universe, not of a single system in the absence of its surroundings, never decreases (Carson and Watson 2002).
Typical life experiences which reflect common understandings of spatial disorder are, in fact, from the perspective of just the system under consideration, not indicative of entropy increases or spontaneous processes (Lambert 1999). A bedroom does not spontaneously, in the physical sense, become messy. In fact, the movement of clothes and other items around the room represents a non-spontaneous process only made possible by the agency of the individual (and, ultimately, associated spontaneous chemical reactions that increase the entropy of the universe). Buildings may rust spontaneously, but they do not typically fall apart spontaneously—instead this is brought on by weathering.
Yet, it is problematic that these connections are often made explicitly in textbooks. For example, the action of a pack of dogs running around after becoming unleashed is used to illustrate entropy in a recent high school textbook (Wilbraham et al. 2012). Two popular textbooks in mainland China (Song and He 2004; Wang 2007) use illustrations of scattered matches or a messy room as depictions of “increased entropy”. These events only indirectly, and through complicated mechanisms that consider the system and its surroundings as a whole, follow the second law.
It might be argued that the metaphor should not be required to comply with all the constraints of the concept it represents. However, for the purposes of pedagogy, a metaphor should not directly contradict the meaning of the entropy concept where it can be used in meaningful contexts (such as living organisms or Earth systems), leading to more misconceptions than it prevents. This is especially true if the metaphor does not do a particularly good job of representing the salient features of the concept.
3.3 Criteria for a Successful Replacement Metaphor and How Dispersal of Energy Meets These Criteria
Replacement of the disorder metaphor by a new metaphor or set of metaphors in K-12 education is past due. Whatever good that the disorder metaphor does for helping students to understand spontaneous processes, it does a greater amount of harm in misleading students and preventing broader incorporation of the second law into the everyday thinking of lay people. A replacement metaphor must accomplish many of the things that entropy as disorder fails to do.
3.3.1 Criterion I: The Entropy Metaphor Should Comply with the Features of the Concept
The metaphor must be reasonably precise in meaning, must not contradict the principles of entropy analysis if it takes the form of everyday macroscopic experience, and must comply with as many as possible of the features, whether theoretical or empirical, of the contemporary formulations of entropy.
How does the energy dispersal or spreading metaphor for entropy improve the situation over the disorder metaphor? First, from the perspective of the second law, energy “spreading” is unambiguous. Whereas “dispersal” or “distribution” may be more challenging for younger students to grasp, those words also carry far less ambiguity than “disorder.” Second, unlike “disorder,” many of the everyday experiences that students are likely to associate with “energy spreading” (such as diffusion and heat transfer) are consistent with entropy increases from the perspective of the system used to illustrate the metaphor. Another major advantage of the “dispersal of energy at a given temperature” metaphor is that it fits Clausius’ macroscopic formulation of entropy (from classical thermodynamics) quite well. The concept of “energy spreading” driving spontaneous processes also clearly relates the second law to energy. At the same time, the metaphor simplifies incorporation of many of the most relevant uses and consequences of the second law, among them the inclusion of both the system and its surroundings in analyzing event spontaneity, the determination of chemical equilibrium state, and the use of Gibbs free energy to analyze chemical reactions.
3.3.2 Criterion II: The Entropy Metaphor Should Prevent Potential Misconceptions
The metaphor should have a fundamental connection with other energy constructs, such that it makes the relationship between energy and entropy clear without furthering the misconception that entropy is another form of energy. Describing entropy explicitly as a measure of the dispersal of energy checks students’ misunderstanding of entropy as another form of energy.
It should also work to prevent users of the metaphor from thinking of entropy from a limited perspective, such as that only the system or only its surroundings is considered. As students study chemical systems in more depth, the temptation for them to explain spontaneous reactions by ignoring the surroundings will be minimized because the energy spreading metaphor looks beyond the single system.
3.3.3 Criterion III: The Entropy Metaphor Should Be Accessible to Students
The metaphor used for understanding entropy should be accessible to young students, particularly as it relates to its use in explaining spontaneous processes. Many familiar instances of spreading, related to diffusion or heat transfer, for example, demonstrate entropy straightforwardly so that it is not difficult for students to grasp its meaning. In well-designed reading materials with the energy dispersal metaphor, entropy and the second law will be readily understood by chemistry teachers- even by beginners in chemistry- and be accessible to students not majoring in science (Lambert 2005, 2006a, 2011).
Though not suggested by the Framework or the NGSS, we propose that upon completing of the 8th-grade, students should have an understanding that spontaneous events are determined by the maximal possible spreading of energy. In this way, students will be prepared for the reinforcing and deepening of their understanding of these ideas in high school. For example, students can much more quickly develop a qualitative understanding of chemical equilibrium from the perspective of energy spreading. Many reactions go to equilibrium rather than completion. This is because the mixture of products and reactants distributes the energy still contained within the chemical system, which will contribute to higher entropy of the system, and thus also contribute to higher total entropy (Lambert 2002b). It should be noted here that the point of maximum mixing state does not exactly correspond to equilibrium state, because the total entropy will be affected not only by the change in entropy of mixing of system, but also by the change in non-mixing entropy of system and the entropy change of surroundings (Gary 2004).
Another example of the advantages of an earlier understanding of spontaneity through energy spreading is that an earlier and more persistent qualitative understanding of the second law will demystify Gibbs free energy if it is presented in advanced classes or university. Students are better off understanding Gibbs free energy as a proxy for the degree of energy spreading in a reaction than they are puzzling through how a constructed form of energy could determine the direction of chemical reactions.
3.3.4 Criterion IV: The Entropy Metaphor Should Be Amenable to Updating and Modification
Like any metaphor, entropy as the dispersal of energy has its limitations. However, in evaluating the relative merits of different metaphors for science concepts for K-12 learners, top criteria should include the extent to which a metaphor is amenable to updating for modification in light of new evidence or more complex formulations of the concept. This is especially true when considering how to build on the concept across grade levels, or in such a way that lays a solid foundation for advanced study. The spatial disorder metaphor is difficult to update for reflecting the connections between entropy, spontaneous reactions, and energy. In fact, because it appears that the disorder metaphor itself (but not a sophisticated understanding of how it relates to entropy) is fairly easily incorporated by students, it can be difficult to replace once it has been established (Sözbilir and Bennett 2007). The dispersal of energy metaphor, on the other hand, is amenable to updating for reflecting the statistical mechanical formulation of entropy.
In considering chemical systems, the energy dispersal metaphor is a good metaphor for new learners because it works well to relate entropy to energy and leads to a view of reactions that considers both the system and its surroundings. This makes understanding why either exothermic reactions or endothermic reactions can take place spontaneously simpler for students. But the energy dispersal metaphor can lead to confusion in certain cases, for instance, in considering a constant-volume spontaneous chemical reaction in an isolated system where the distribution of energy in space is maximized both before and after the reaction. Below, we illustrate three levels in the route of updating and modifying the understanding of entropy from the perspective of energy dispersal, which will extend the usefulness of the metaphor to account for all chemical systems.
The first level understanding is the dispersal of energy between the system and its surroundings at the macroscale. This idea, which is discussed in Sect. 18.2 above and is elaborated on in Fig. 18.4 of Sect. 18.4.4, focuses on the energy redistribution in space when a reaction takes place.
The second level of understanding is the dispersal of energy across multiple energy levels at the microscale. To achieve this understanding, more advanced students can be led further to an understanding of the distribution of molecular energies across many discrete energy levels. On average, the distribution of particles across multiple energy levels, which can be expressed as in Fig. 18.1, does not change significantly for constant conditions (i.e.: pressure, volume, and temperature). Thus students can understand easily why higher entropy means that energy is more dispersed (energy distribution is in a broader band) from a molecular perspective, and vice versa. Because of the change in the possible energy levels which molecules in the system can occupy that accompanies a chemical reaction, as well as the change of the number and kinds of molecules, the distribution of energy across multiple energy levels also changes, even though the energy does not flow between the system and its surroundings (spreading in space).
The third level of understanding is that of entropy from the perspective of the number of energy microstates. The most fundamental contemporary view of entropy (S) is that it is logarithmically proportional to the number of available energy microstates of a system (W), such that S = k BlnW, where k B is Boltzmann’s constant. An energy microstate precisely describes one of many of these possible distributions of energy throughout all particles in a system. Because of collisions and other exchanges of energy between particles, the microstate of a system constantly changes. But as long as the number of available microstates stays the same, the entropy stays the same. According to the second law, the number of microstates of the universe (system + surroundings) can only remain the same or increase. A process will be spontaneous if it increases the total number of microstates of the universe.
If this statistical mechanical formulation is introduced to students, they could attempt to fit it within the context of energy dispersal and energy spreading metaphors. Students can make a connection between “energy spreading” and “energy having more ways of distributing itself” (that is, more energy microstates being available). It should be noted that in this case students must explicitly recognize that the energy is always in one microstate at a time, so it is incorrect to view energy as being “spread out” among microstates; energy can just be more dispersed among different energy levels in the energy distribution on average (see Fig. 18.1), which is caused by increasing the number of energy microstates. Extending the energy spreading metaphor in this way has the added benefit of reinforcing the probabilistic nature of entropy.
4 Conclusions
4.1 What Should Students Know About Entropy?
Students should be able to explain spontaneous processes qualitatively and to some extent be able to predict in which situations spontaneous processes will and will not occur. While the NGSS focuses primarily on heat transfer processes, we believe that students should be given more generalized tools that can apply to other physical processes as well as chemical reactions, along with applications in living organisms, Earth systems, and engineering. In order to make these explanations in a way that is consistent with the contemporary scientific view, students must at least implicitly involve entropy and the second law in their explanations, and should explicitly involve energy as well.
4.2 What Are the Challenges Teachers Face in Teaching Students This Knowledge?
There are both conceptual and structural problems in giving students the means to explain why spontaneous processes occur. Conceptually, entropy adds another layer of abstraction to already abstract energy concepts, such as transfer, transformation, and conservation. Both microscopic and macroscopic quantitative formulations of entropy are difficult, especially when applied to most meaningful situations to analyze entropy change. Entropy’s application to “the universe” rather than a particular system under study sets it apart from the way that teachers approach many other scientific concepts, including energy concepts. Related to this, there are many ways in which physical and chemical systems interact such that entropy often decreases for particular systems, seemingly in violation of the general principle of the second law.
Beyond the conceptual problems, however, there are structural problems in teaching students about spontaneous processes. While the situation is slowly improving, for years teaching rules for analyzing spontaneous processes have been relegated by both standards and textbooks as optional or something reserved for the end of a high school chemistry course. While other ideas, including the first law of thermodynamics, are typically seen as important across grade levels, entropy and the second law in any form have perhaps been regarded as too difficult to even approach or approximate with younger students, with the possible exception of through heat transfer in the middle grades. This limited prioritization has led to a relatively limited work on student learning progressions and misconceptions, as well as fewer curricular resources than might otherwise be expected.
Ultimately, however, it is the pervasive yet inappropriate use of the disorder metaphor for entropy that has prevented more widespread incorporation of the second law into student thinking. Entropy had been described as disorder for a long time (American Association for Advancement of Science [AAAS] 1990, 1993/2009; National Academy of Sciences [NAS] 1996), although it is no longer described this way in the newest US national standards documents (NRC 2012), and has been eliminated from many college-level textbooks (a list of textbooks can be found on http://entropysite.oxy.edu/). This metaphor still persists, however, in recently published and widely used high school textbooks, both in China and the United States (e.g. Gao and Wang 2007; Song and He 2004; Wang 2007; Wilbraham et al. 2012). It is equally important that although the disadvantages of the disorder metaphor have been all but settled in certain academic/pedagogical debates, this message has not, by and large, reached teachers. In China (and we suspect we would find similar data in the United States), of 3,833 high school chemistry teachers that we surveyed, 61 % considered entropy as disorder, whereas only 17 % considered entropy as the dispersal of energy.
Because the metaphor of entropy as disorder has been so pervasive, most of students’ misconceptions—that have been documented regarding entropy, the second law, and spontaneous processes—are directly or indirectly related to this metaphor. Common misconceptions include: entropy is another form of energy, related to thermal or kinetic energy; macroscale objects become spontaneously disordered; entropy increases whenever visible order decreases; disorder at the microscale refers to mixed-upness at any one instant, rather than an increase in available microstates; the physical imperative to increase entropy applies to a single system, rather than to the universe; entropy refers to instability; Gibbs free energy is not related to an increase in total entropy; and spontaneous processes are determined by a decrease in energy of the system under consideration (Boo 1998; Carson and Watson 2002; Sözbilir and Bennett 2007).
4.3 What Should Be Done to Meet These Challenges?
There have been several fruitful approaches to teaching entropy at the high school level in ways that go beyond the disorder metaphor (Bindel 2004; Hanson and Michalek 2006). However, we propose that the best way of addressing students’ conceptual difficulties with explaining spontaneous processes is to develop a successful framework for teaching about energy that includes both entropy and the second law. This framework should include ways of teaching that are accessible to students both in terms of making concrete connections between the entropy concept and their everyday experience and in terms of making meaningful connections between entropy and other energy topics that students have studied. The framework should also avoid many of the misconceptions that are brought upon by using the disorder metaphor for entropy. Finally, it should be amenable to building student ideas about spontaneous processes, such that these foundations can begin in the elementary grades, progress through the middle grades, and conclude in high school with a strong basis for increasingly complex applications, including quantitative applications. We believe that the entropy component of such a framework can be built around the energy dispersal metaphor for entropy and the energy spreading metaphor for explaining what drives spontaneous processes.
In order to successfully enact such a framework that aids in teaching about spontaneous processes, we propose that standards, written curricula, and teachers need to incorporate the framework. Because most active teachers themselves have likely been taught that entropy is disorder, and because their disorder schema is so persistent, teacher education—from pre-service to professional development—must focus on actively discrediting the disorder metaphor, at least as the best or only way to teach entropy. An effective means of doing this will be to provide teachers with concrete counterexamples of the disorder metaphor. Fortunately, as discussed in Sect. 18.3.2 above, there are many specific counterexamples that illustrate how visual or microscopic spatial disorder can decrease while entropy increases (Lambert 2002a; Leff 2007). Of course, teachers must also be presented with a positive alternative metaphor meant to replace the disorder metaphor.
It is also fortunate that the Framework and NGSS adopted the view that “uncontrolled systems evolve toward more uniform energy distribution (NRC 2012, p 125).” Although this view is stated in clear language and is simply another way of stating that spontaneous processes occur when energy dispersal is maximized, one concern may be that because the view does not explicitly draw a link with entropy, many teachers will not make this connection. The discussion of “more uniform energy distribution” is only a single paragraph in the Framework (NRC 2012, p 125), with a single corresponding Performance Expectation (which is at the high school level) in the NGSS. However, we believe that this minimal inclusion of the second law in the Framework and NGSS provides an opportunity for educators and curriculum developers to build a new framework for teaching entropy.
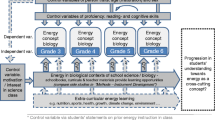
Because entropy is so intimately connected with energy, we believe that the basis for learning about spontaneous processes must begin as soon as energy transfer and transformation (along with conservation) are explored in science education. We see the need for the integration of entropy into the energy concept system through modified energy learning progressions. This integration needs empirical backing for what students are capable of understanding at any particular age, and how some of the more complicated ideas relating to entropy can be supported by specific earlier learning experiences and understandings. Below, we provide an outline for developing a potential K-12 learning progression for understanding spontaneous processes, which we hope can form a starting point for a broader redesign of energy learning progressions. It is important to emphasize that this learning progression requires much further research, especially as it relates to students’ prior ideas and the success of their incorporation of these ideas at different ages.
A few features of this learning progression are important to mention here. First, it prioritizes qualitative explanations over quantitative ones, because even extensive quantitative instructional practice with entropy-related concepts often does not necessarily have a meaningful effect on student understanding of spontaneous processes (Carson and Watson 2002). Second, the second law (though not by this name) is introduced before entropy. This is because the concept of “energy spreading” as a means of explaining spontaneous processes is actually conceptually less challenging than “the dispersal of energy at a specific temperature”—the metaphor for entropy itself. Third, technical vocabulary to describe any of these concepts, including “spontaneous,” “entropy,” and “the second law,” is not introduced until these concepts have been thoroughly established otherwise in students’ understanding.
4.4 A Proposed Outline of a K-12 Learning Progression for Explaining Spontaneous Processes
-
1.
Some physical events happen naturally (i.e., heat transfer from hot to cold substances) while others apparently never happen naturally (i.e., heat transfer from cold to hot substances). [Early elementary]
-
2.
Thermal energy can transfer into or out of a system (see Fig. 18.2). [Late elementary]
-
3.
The part outside of a system can be thought of as its surroundings, or as another system. Thermal energy released from a system will transfer into its surroundings, and thermal energy released from the surroundings will transfer into the system. In the process of energy transfer between two systems, total energy is always conserved (see Fig. 18.3). [Middle school]
-
4.
Thermal energy tends to spread from where it is concentrated to where it is less so. For example, if energy is concentrated in the system, it tends to spread into its surroundings. Otherwise, energy will transfer into the system. In this way it becomes maximally dispersed (see Fig. 18.4). [Middle school]
-
5.
There can be barriers to prevent this energy spreading from happening rapidly (such as insulating material), but even with barriers slowing it down, thermal energy still tends to spread. [Middle school]
-
6.
Forms of energy other than thermal energy also tend to spread from where they are concentrated to where they are less so. For example, in a chemical reaction, if the chemical energy stored in the system is more concentrated than its surroundings, the chemical energy will be transformed into thermal energy or other forms of energy to spread out of the system. Otherwise, other forms of energy from the surroundings will be transformed into chemical energy stored in the system. [High school]
-
7.
Energy spreading is what determines whether or not simple events happen on their own (i.e., spontaneously). If energy is already dispersed as far as possible, events will not happen on their own. If an event causes energy to be less dispersed, it will not happen on its own. [High school]
References
American Association for the Advancement of Science. (1990). Science for all Americans. New York: Oxford University Press. Retrieved from, http://www.project2061.org/publications/sfaa/online/chap4.htm#26
American Association for the Advancement of Science. (1993). Benchmarks for science literacy. New York: Oxford University Press. Revised web version (2009) http://www.project2061.org/publications/bsl/online/index.php
Atkins, P. W. (2006). Atkins’ physical chemistry. New York: Oxford University Press.
Bindel, T. H. (2004). Teaching entropy analysis in the first-year high school course and beyond. Journal of Chemical Education, 81, 1585–1594.
Boo, H. K. (1998). Students’ understandings of chemical bonds and the energetics of chemical reactions. Journal of Research in Science Teaching, 35, 569–581.
Carson, J., & Watson, E. M. (2002). Undergraduate students’ understandings of entropy and Gibbs free energy. University Chemistry Education, 6, 4–12.
Chen, S., Liu, Z., Bao, S., & Zheng, C. (2010). Natural convection and entropy generation in a vertically concentric annular space. International Journal of Thermal Sciences, 49, 2439–2452.
Cooper, M. M., Klymkowsky, M. W., & Becker, N. M. (2014). Energy in chemical systems: An integrated approach. In B. Chen, A. Eisenkraft, D. Fortus, J. Krajcik, K. Neumann, J. Nordine, & A. Scheff (Eds.), Teaching and learning of energy in K – 12 education (pp. 301–316). New York: Springer.
Dincer, I., & Cengel, Y. A. (2001). Energy, entropy and exergy concepts and their roles in thermal engineering. Entropy, 3, 116–149.
Donaldson, S. (2011). Entropy is not disorder. Retrieved from http://www.science20.com/train_thought/blog/entropy_not_disorder-75081
Duit, R. (1991). On the role of analogies and metaphors in learning science. Science Education, 75, 649–672.
Gao, P. L., & Wang, M. Z. (2007). Huaxue fanying yuanli [Principles of chemical reactions]. Jinan: Shandong Science and Technology Press.
Gary, R. K. (2004). The concentration dependence of the ∆S term in the Gibbs free energy function: Application to reversible reactions in biochemistry. Journal of Chemical Education, 81, 1599–1604.
Glynn, S. M., & Takahashi, T. (1998). Learning from analogy-enhanced science text. Journal of Research in Science Teaching, 35, 1129–1149.
Hanson, R. M., & Michalek, B. (2006). Give them money: The Boltzmann game, a classroom or laboratory activity modeling entropy changes and the distribution of energy in chemical systems. Journal of Chemical Education, 83, 581–588.
Kozliak, E. I., & Lambert, F. L. (2005). “Order-to-disorder” for entropy change? Consider the numbers! The Chemical Educator, 10, 24–25.
Kozliak, E. I., & Lambert, F. L. (2008). Residual entropy, the third law and latent heat. Entropy, 10, 274–284.
Lambert, F. L. (1999). Shuffled cards, messy desks, and disorderly dorm rooms – Examples of entropy increase? Nonsense! Journal of Chemical Education, 76, 1385–1387.
Lambert, F. L. (2002a). Disorder—A cracked crutch for supporting entropy discussions. Journal of Chemical Education, 79, 187–192. Revised web version http://www.entropysite.com/cracked_crutch.html
Lambert, F. L. (2002b). Entropy is simple, qualitatively. Journal of Chemical Education, 79, 1241–1246. Revised web version http://entropysite.oxy.edu/entropy_is_simple/index.html
Lambert, F. L. (2005). Teaching entropy is simple—If you discard “disorder”. Retrieved from, http://entropysite.oxy.edu/teaching_entropy.html
Lambert, F. L. (2006a). The second law of thermodynamics. Retrieved from, http://entropysite.oxy.edu/wiki_secondlaw.html
Lambert, F. L. (2006b). A modern view of entropy. Khymia, The Bulgarian Journal of Chemistry, 15, 13–21.
Lambert, F. L. (2007). Configurational entropy revisited. Journal of Chemical Education, 84, 1548–1550.
Lambert, F. L. (2011). Entropy in general chemistry. Retrieved from, http://entropysite.oxy.edu/wiki_entropy.html
Lambert, F. L., & Leff, H. S. (2009). The correlation of standard entropy with enthalpy supplied from 0 to 298.15 K. Journal of Chemical Education, 86(1), 94–98.
Leff, H. S. (1996). Thermodynamic entropy: The spreading and sharing of energy. American Journal of Physics, 64, 1261–1271.
Leff, H. S. (2007). Entropy, its language and interpretation. Foundations of Physics, 37, 1744–1766.
Leff, H. S. (2012a). Removing the mystery of entropy and thermodynamics – Part I. The Physics Teacher, 50(1), 28–31. Retrieved from, http://www.csupomona.edu/~hsleff/selpubs.html
Leff, H. S. (2012b). Removing the mystery of entropy and thermodynamics – Part II. The Physics Teacher, 50, 87–90. Retrieved from, http://www.csupomona.edu/~hsleff/selpubs.html
Leff, H. S. (2012c). Removing the mystery of entropy and thermodynamics – Part III. The Physics Teacher, 50, 170–172. Retrieved from, http://www.csupomona.edu/~hsleff/selpubs.html
Leff, H. S. (2012d). Removing the mystery of entropy and thermodynamics – Part IV. The Physics Teacher, 50, 215–217. Retrieved from, http://www.csupomona.edu/~hsleff/selpubs.html
Leff, H. S. (2012e). Removing the mystery of entropy and thermodynamics – Part V. The Physics Teacher, 50, 274–276. Retrieved from, http://www.csupomona.edu/~hsleff/selpubs.html
National Academy of Sciences. (1996). National science education standards. Washington, DC: The National Academy Press. Retrieved from, http://www.nap.edu/catalog/4962.html
National Research Council. (2012). A framework for K-12 science education: Practices, crosscutting concepts, and core ideas. Washington, DC: The National Academies Press. Retrieved from, http://www.nap.edu/catalog.php?record_id=13165
Reece, J. B., Urry, L., Cain, M., Wasserman, S., Minorsky, P., & Jackson, R. (2011). Campbell biology (9th ed.; International ed.). Harlow: Pearson Education.
Song, X. Q., & He, S. H. (2004). Huaxue fanying yuanli [Principles of chemical reactions]. Beijing: People’s Education Press.
Sözbilir, M., & Bennett, J. M. (2007). A study of Turkish chemistry undergraduates’ understanding of entropy. Journal of Chemical Education, 84, 1204–1208.
Styer, D. F. (2000). Insight into entropy. American Journal of Physics, 68, 1090–1096.
Wang, Z. H. (2007). Huaxue fanying yuanli [Principles of chemical reactions]. Hangzhou: Jiangsu Education Publishing House.
Wang, L., Wang, W., & Wei, R. (2014). What knowledge and ability should high school students have for understanding energy in chemical reactions? An analysis of chemistry curriculum standards in seven countries and regions. In B. Chen, A. Eisenkraft, D. Fortus, J. Krajcik, K. Neumann, J. Nordine, & A. Scheff (Eds.), Teaching and learning of energy in K – 12 education (pp. 87–102). New York: Springer.
Wilbraham, A., Staley, D., Matta, M., & Waterman, E. (2012). Pearson chemistry. Boston: Pearson Education.
Acknowledgments
We would like to thank Jeff Nordine, Knut Neumann, Tobin Roger, and Hui Jin for their detailed reviews of the drafts of this chapter and their brilliant suggestions. Our thanks go to Frank L. Lambert for his correspondence discussing entropy with us. Thanks to Chi-Yan Tsui for his careful edit on the paper. The Chicago Transformation Teacher Institutes (NSF DRL 0928669) provided funding and support. We are also grateful to many other friends for kindly offering us their help.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Wei, R., Reed, W., Hu, J., Xu, C. (2014). Energy Spreading or Disorder? Understanding Entropy from the Perspective of Energy. In: Chen, R., et al. Teaching and Learning of Energy in K – 12 Education. Springer, Cham. https://doi.org/10.1007/978-3-319-05017-1_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-05017-1_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05016-4
Online ISBN: 978-3-319-05017-1
eBook Packages: Humanities, Social Sciences and LawEducation (R0)