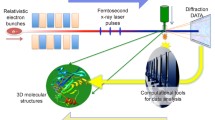
Abstract
Third-generation synchrotron radiation (SR) X-rays have transformed structural biology research in the past decades with the advent of high-brilliance undulator X-ray beams. Combined with developments in microfocus beams, area detectors, and data analysis software, it is now possible to determine large complex structures from extremely small (microns in all directions) crystals, as exemplified by the structures of potassium channels, G-protein coupled receptors (GPCRs), ribosomes, and RNA polymerase complexes. The number of depositions in PDB (the Protein Data Bank) is already more than 100,000, the majority of which have been determined by X-rays. Almost all biology, biochemistry, and molecular biology textbooks now contain protein structures to explain their functions in the cell. Now, X-ray free-electron lasers (XFELs) promise to provide new opportunities in structural biology research. They produce X-ray beams with extraordinary properties: 9 to 10 orders of magnitude higher peak brilliance and 1000 times shorter pulse lengths, compared to existing synchrotron radiation beamlines around the world. Diffraction patterns can be recorded from micro and nanocrystals before radiation damage starts to destroy the samples. Using this “diffract-before-destroy” concept, a new area of XFEL biology has arisen, wherein biologically important questions are tackled beyond what is possible using traditional SR. Ultrashort XFEL pulses allow for frozen-in-time fluctuation X-ray scattering with a possibility of expanding isotropic solution scattering. New modes of XFEL pulses such as two-pulse/two-color are being developed for pump-probe femtosecond time-resolved experiments and phasing. Additionally, hybrid methods are increasingly used for challenging biological questions, in which SR and XFEL methods are combined with other methods such as cryo-electron microscopy, NMR, DEER, and optical and atomic force microscopes. Taken together, SR and XFELs provide a portfolio of structural biology tools which continue to enable cutting edge structural biology research of complex molecular machineries.
Similar content being viewed by others
References
N. Alonso-Garc´ıa, I. Garc´ıa-Rubio, J.A. Manso, R.M. Buey, H. Urien, A. Sonnenberg, G. Jeschke, J.M. de Pereda, Combination of X-ray crystallography, SAXS and DEER to obtain the structure of the FnIII-3,4 domains of integrin α6β4. Acta Crystallogr. D71, 969–985 (2015)
J. Amann, W. Berg, V. Blank, F.-J. Decker, Y. Ding, P. Emma, Y. Feng, J. Frisch, D. Fritz, J. Hastings, Z. Huang, J. Krzywinski, R. Lindberg, H. Loos, A. Lutman, H.-D. Nuhn, D. Ratner, J. Rzepiela, D. Shu, Yu. Shvyd’ko, Shvyd’ko, S. Spampinati, S. Stoupin, S. Terentyev, E. Trakhtenberg, D. Walz, J. Welch, J. Wu, A. Zholents, D. Zhu, Demonstration of self-seeding in a hard-X-ray free-electron laser. Nat. Photonics 6, 693–698 (2012)
D. Arnlund, L.C. Johansson, C. Wickstrand, A. Barty, G.J. Williams, E. Malmerberg, J. Davidsson, D. Milathianaki, D.P. DePonte, R.L. Shoeman, D. Wang, D. James, G. Katona, S. Westenhoff, T.A. White, A. Aquila, S. Bari, P. Berntsen, M. Bogan, T.B. van Driel, R.B. Doak, K.S. Kjær, M. Frank, R. Fromme, I. Grotjohann, R. Henning, M.S. Hunter, R.A. Kirian, I. Kosheleva, C. Kupitz, M. Liang, A.V. Martin, M.M. Nielsen, M. Messerschmidt, M.M. M. Messerschmidt, M.M. Seibert, J. Sjöhamn, F. Stellato, U. Weierstall, N.A. Zatsepin, J.C. Spence, P. Fromme, I. Schlichting, S. Boutet, G. Groenhof, H.N. Chapman, R. Neutze, Visualizing a protein quake with time-resolved X-ray scattering at a free-electron laser. Nat. Methods 11, 923–926 (2014)
K. Ayyer, H.T. Philipp, M.W. Tate, J.L. Wierman, V. Elser, S.M. Gruner, Determination of crystallographic intensities from sparse data. IUCrJ. 2, 29–34 (2015)
T.R. Barends, L. Foucar, S. Botha, R.B. Doak, R.L. Shoeman, K. Nass, J.E. Koglin, G.J. Williams, S. Boutet, M. Messerschmidt, I. Schlichting, De novo protein crystal structure determination from X-ray free-electron laser data. Nature 505, 244–247 (2014)
T.R. Barends, L. Foucar, A. Ardevol, K. Nass, A. Aquila, S. Botha, R.B. Doak, K. Falahati, E. Hartmann, M. Hilpert, M. Heinz, M.C. Hoffmann, J. Kofinger, J.E. Koglin, G. Kovacsova, M. Liang, D. Milathianaki, H. Lemke, J. Reinstein, C.M. Roome, R.L. Shoeman, G.J. Williams, I. Burghardt, G. Hummer, S. Boutet, I. Schlichting, Direct observation of ultrafast collective motions in CO myoglobin upon ligand dissociation. Science. Epub ahead of print (2015a)
T. Barends, T.A. White, A. Barty, L. Foucar, M. Messerschmidt, R. Alonso-Mori, S. Botha, H. Chapman, R.B. Doak, L. Galli, C. Gati, M. Gutmann, J. Koglin, A. Markvardsen, K. Nass, D. Oberthur, R.L. Shoeman, I. Schlichting, S. Boutet, Effects of self-seeding and crystal post-selection on the quality of Monte Carlo-integrated SFX data. J Synchrotron Radiat. 22, 644–652 (2015b)
S. Botha, K. Nass, T.R. Barends, W. Kabsch, B. Latz, F. Dworkowski, L. Foucar, E. Panepucci, M. Wang, R.L. Shoeman, I. Schlichting, R.B. Doak, Room-temperature serial crystallography at synchrotron X-ray sources using slowly flowing free-standing high-viscosity microstreams. Acta Crystallogr. D Biol. Crystallogr. 71, 387–397 (2015)
S. Boutet, L. Lomb, G.J. Williams, T.R. Barends, A. Aquila, R.B. Doak, U. Weierstall, D.P. DePonte, J. Steinbrener, R.L. Shoeman, M. Messerschmidt, A. Barty, T.A. White, S. Kassemeyer, R.A. Kirian, M.M. Seibert, P.A. Montanez, C. Kenney, R. Herbst, P. Hart, J. Pines, G. Haller, S.M. Gruner, H.T. Philipp, M.W. Tate, M. Hromalik, L.J. Koerner, N. van Bakel, J. Morse, W. Ghonsalves, D. Arnlund, M.J. Bogan, C. Caleman, R. Fromme, C.Y. Hampton, M.S. Hunter, L.C. Johansson, G. Katona, C. Kupitz, M. Liang, A.V. Martin, K. Nass, L. Redecke, F. Stellato, N. Timneanu, D. Wang, N.A. Zatsepin, D. Schafer, J. Defever, R. Neutze, P. Fromme, J.C. Spence, H.N. Chapman, I. Schlichting, High-Resolution Protein Structure Determination by Serial Femtosecond Crystallography. Science 337, 362–364 (2012)
M. Caffrey, C. Porter, Crystallizing membrane proteins for structure determination using lipidic mesophases (2010). http://www.jove.com/video/1712/ crystallizing-membrane-proteins-for-structure-determination-using
L.M. Chavas, Y. Yamada, M. Hiraki, N. Igarashi, N. Matsugaki, S. Wakatsuki, UV LED lighting for automated crystal centring. J Synchrotron Radiat. 18, 11–15 (2011)
H.N. Chapman, A. Barty, M.J. Bogan, S. Boutet, M. Frank, S.P. Hau-Riege, S. Marchesini, B.W. Woods, S.C. Bajt, W.H. Benner, R.A. London, E. Plönjes, M. Kuhlmann, R. Treusch, S. Düsterer, T. Tschentscher, J.R. Schneider, E. Spiller, T. Möller, C. Bostedt, M. Hoener, D.A. Shapiro, K.O. Hodgson, D. van der Spoel, F. Burmeister, M. Bergh, C. Caleman, G. Huldt, M.M. Seibert, Maia FRNC, R.W. Lee, A. Szöke, N. Timneanu, J. Hajdu, Femtosecond diffractive imaging with a soft-X-ray free-electron laser. Nat. Phys. 2, 839–843 (2006)
H.N. Chapman, P. Fromme, A. Barty, T.A. White, R.A. Kirian, A. Aquila, M.S. Hunter, J. Schulz, D.P. DePonte, U. Weierstall, R.B. Doak, F.R. Maia, A.V. Martin, I. Schlichting, L. Lomb, N. Coppola, R.L. Shoeman, S.W. Epp, R. Hartmann, D. Rolles, A. Rudenko, L. Foucar, N. Kimmel, G. Weidenspointner, P. Holl, M. Liang, M. Barthelmess, C. Caleman, S. Boutet, M.J. Bogan, J. Krzywinski, C. Bostedt, S. Bajt, L. Gumprecht, B. Rudek, B. Erk, C. Schmidt, A. Hömke, C. Reich, D. Pietschner, L. Strüder, G. Hauser, H. Gorke, J. Ullrich, S. Herrmann, G. Schaller, F. Schopper, H. Soltau, K.U. Kühnel, M. Messerschmidt, J.D. Bozek, S.P. Hau-Riege, M. Frank, C.Y. Hampton, R.G. Sierra, D. Starodub, G.J. Williams, J. Hajdu, N. Timneanu, M.M. Seibert, J. Andreasson, A. Rocker, O. Jönsson, M. Svenda, S. Stern, K. Nass, R. Andritschke, C.D. Schröter, F. Krasniqi, M. Bott, K.E. Schmidt, X. Wang, I. Grotjohann, J.M. Holton, T.R. Barends, R. Neutze, S. Marchesini, R. Fromme, S. Schorb, D. Rupp, M. Adolph, T. Gorkhover, I. Andersson, H. Hirsemann, G. Potdevin, H. Graafsma, B. Nilsson, J.C. Spence, Femtosecond X-ray protein nanocrystallography. Nature 470, 73–77 (2011)
A.E. Cohen, S.M. Soltis, A. Gonz´alez, L. Aguila, R. Alonso-Mori, C.O. Barnes, E.L. Baxter, W. Brehmer, A.S. Brewster, A.T. Brunger, G. Calero, J.F. Chang, M. Chollet, P. Ehrensberger, T.L. Eriksson, Y. Feng, J. Hattne, B. Hedman, M. Hollenbeck, J.M. Holton, S. Keable, B.K. Kobilka, E.G. Kovaleva, A.C. Kruse, H.T. Lemke, G. Lin, A.Y. Lyubimov, A. Manglik, I.I. Mathews, S.E. McPhillips, S. Nelson, J.W. Peters, N.K. Sauter, C.A. Smith, J. Song, H.P. Stevenson, Y. Tsai, M. Uervirojnangkoorn, V. Vinetsky, S. Wakatsuki, W.I. Weis, O.A. Zadvornyy, O.B. Zeldin, D. Zhu, K.O. Hodgson, Goniometer-based femtosecond crystallography with X-ray free electron lasers. Proc. Natl. Acad. Sci. U. S. A. 111, 17122–17127 (2014)
J.P. Colletier, A. Royant, A. Specht, B. Sanson, F. Nachon, P. Masson, G. Zaccai, J.L. Sussman, M. Goeldner, I. Silman, D. Bourgeois, M. Weik, Use of a ’caged’ analogue to study the traffic of choline within acetylcholinesterase by kinetic crystallography. Acta Crystallogr. D Biol. Crystallogr. 63, 1115–1128 (2007)
C.E. Conrad, S. Basu, D. James, D. Wang, A. Schaffer, S. Roy-Chowdhury, N.A. Zatsepin, A. Aquila, J. Coe, C. Gati, M.S. Hunter, J.E. Koglin, C. Kupitz, G. Nelson, G. Subramanian, T.A. White, Y. Zhao, J. Zook, S. Boutet, V. Cherezov, J.C. Spence, R. Fromme, U. Weierstall, P. Fromme, A novel inert crystal delivery medium for serial femtosecond crystallography. IUCrJ. 2, 421–430 (2015)
G. David, J. P´erez, Combined sampler robot and high-performance liquid chromatography: a fully automated system for biological small-angle X-ray scattering experiments at the Synchrotron SOLEIL SWING beamline. J. Appl. Crystallogr. 42, 892–900 (2009)
M.C. Deller, B. Rupp, Approaches to automated protein crystal harvesting. Acta Crystallogr. F Struct. Biol. Commun. 70, 133–155 (2014)
M.D. de Jonge, S. Vogt, Hard X-ray fluorescence tomography–an emerging tool for structural visualization. Curr. Opin. Struct. Biol. 20, 606–614 (2010)
D. Deng, P.C. Sun, C.Y. Yan, M. Ke, X. Jiang, L. Xiong, W. Ren, K. Hirata, M. Yamamoto, S. Fan, N. Yan, Molecular basis of ligand recognition and transport by glucose transporters. Nature (2015). doi:10.1038/nature14655. [Epub ahead of print]
D.P. DePonte, U. Weierstall, K. Schmidt, J. Warner, D. Starodub, J.C.H. Spence, R.B. Doak, Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D-Appl. Phys. 41, 195505 (2008). doi:10.1088/0022-3727/41/19/195505
S. Desbois, S.A. Seabrook, J. Newman, Some practical guidelines for UV imaging in the protein crystallization laboratory. Acta Crystallogr. Sect. F Struct. Biol Crystallogr. Commun. 69, 201–208 (2013)
O. Duss, M. Yulikov, G. Jeschke, F.H. Allain, EPR-aided approach for solution structure determination of large RNAs or protein-RNA complexes. Nat. Commun. 5, 3669 (2014)
G. Evans, D. Axford, D. Waterman, R.L. Owen, Macromolecular microcrystallography. Crystallogr. Rev. 17, 105–142 (2011)
M. Frank, D.B. Carlson, M.S. Hunter, G.J. Williams, M. Messerschmidt, N.A. Zatsepin, A. Barty, W.H. Benner, K. Chu, A.T. Graf, S.P. Hau-Riege, R.A. Kirian, C. Padeste, T. Pardini, B. Pedrini, B. Segelke, M.M. Seibert, J.C. Spence, C.J. Tsai, S.M. Lane, X.D. Li, G. Schertler, S. Boutet, M. Coleman, J.E. Evans, Femtosecond X-ray diffraction from two-dimensional protein crystals. IUCrJ. 1, 95–100 (2014)
M.A. Graewert, D. Franke, C.M. Jeffries, C.E. Blanchet, D. Ruskule, K. Kuhle, A. Flieger, B. Schäfer, B. Tartsch, R. Meijers, D.I. Svergun, Automated pipeline for purification, biophysical and x-ray analysis of biomacromolecular solutions. Sci. Rep. 5, 10734 (2015)
L. Galli, Phasing using high intensity free-electron laser radiation. Doctoral Dissertation, University of Hamburg, 2014
L. Galli, S.K. Son, T.A. White, R. Santra, H.N. Chapman, M.H. Nanao, Towards RIP using free-electron laser SFX data. J. Synchrotron Radiat. 22, 249–255 (2015)
C. Gati, G. Bourenkov, M. Klinge, D. Rehders, F. Stellato, D. Oberthür, O. Yefanov, B.P. Sommer, S. Mogk, M. Duszenko, C. Betzel, T.R. Schneider, H.N. Chapman, L. Redecke, Serial crystallography on in vivo grown microcrystals using synchrotron radiation. IUCrJ. 1, 87–94 (2014)
H.M. Ginn, A.S. Brewster, J. Hattne, G. Evans, A. Wagner, J.M. Grimes, N.K. Sauter, G. Sutton, D.I. Stuart, A revised partiality model and post-refinement algorithm for X-ray free-electron laser data. Acta Crystallogr. D Biol. Crystallogr. 71(Pt 6), 1400–1410 (2015)
H. Hara, T. Tanaka, T. Tanabe, X.-M. Mar´echal, S. Okada, H. Kitamura, In-vacuum undulators of SPring-8. J. Synchrotron Radiat. 5, 403–405 (1998)
J. Hattne, N. Echols, R. Tran, J. Kern, R.J. Gildea, A.S. Brewster, R. Alonso-Mori, C. Glöckner, J. Hellmich, H. Laksmono, R.G. Sierra, B. Lassalle-Kaiser, A. Lampe, G. Han, S. Gul, D. DiFiore, D. Milathianaki, A.R. Fry, A. Miahnahri, W.E. White, D.W. Schafer, M.M. Seibert, J.E. Koglin, D. Sokaras, T.C. Weng, J. Sellberg, M.J. Latimer, P. Glatzel, P.H. Zwart, R.W. Grosse-Kunstleve, M.J. Bogan, M. Messerschmidt, G.J. Williams, S. Boutet, J. Messinger, A. Zouni, J. Yano, U. Bergmann, V.K. Yachandra, P.D. Adams, N.K. Sauter, Accurate macromolecular structures using minimal measurements from X-ray free-electron lasers. Nat. Methods 11, 545–548 (2014)
R. Henderson, Cryo-protection of protein crystals against radiation damage in electron and x-ray radiation. Proc. R. Soc. Lond. 241, 6–8 (1990)
K. Hirata, Y. Kawano, G. Ueno, K. Hashimoto, H. Murakami, K. Hasegawa, T. Hikima, T. Kumasaka, M. Yamamoto, Achievement of protein micro-crystallography at SPring-8 beamline BL32XU. J. Phys. Conf. Ser. 425, 012002 (2013)
K. Hirata, K. Shinzawa-Itoh, N. Yano, S. Takemura, K. Kato, M. Hatanaka, K. Muramoto, T. Kawahara, T. Tsukihara, E. Yamashita, K. Tono, G. Ueno, T. Hikima, H. Murakami, Y. Inubushi, M. Yabashi, T. Ishikawa, M. Yamamoto, T. Ogura, H. Sugimoto, J.R. Shen, S. Yoshikawa, H. Ago, Determination of damage-free crystal structure of an X-ray-sensitive protein using an XFEL. Nat. Methods 11, 734–736 (2014)
K.C. Holmes, D. Popp, W. Gebhard, W. Kabsch, Atomic model of the actin filament. Nature 347, 44–49 (1990)
M.S. Hunter, B. Segelke, M. Messerschmidt, G.J. Williams, N.A. Zatsepin, A. Barty, W.H. Benner, D.B. Carlson, M. Coleman, A. Graf, S.P. Hau-Riege, T. Pardini, M.M. Seibert, J. Evans, S. Boutet, M. Frank, Fixed-target protein serial microcrystallography with an x-ray free electron laser. Sci Rep. 4, 6026 (2014)
G. Jeschke, DEER distance measurements on proteins. Ann. Rev. Phys. Chem. 63, 419–446 (2012)
W. Kabsch, Processing of X-ray snapshots from crystals in random orientations. Acta Crystallogr. D Biol. Crystallogr. 70, 2204–2216 (2014)
Z. Kam, Determination of macromolecular structure in solution by spatial correlation of scattering fluctuations. Macromolecules 10, 927–934 (1977)
Z. Kam, M.H.J. Koch, J. Bordas, Fluctuation x-ray scattering from biological particles in frozen solution by using synchrotron radiation. PNAS 78, 3559–3562 (1981)
Y. Kang, X.E. Zhou, X. Gao, Y. He, W. Liu, A. Ishchenko, A. Barty, T.A. White, O. Yefanov, G.W. Han, Q. Xu, P.W. de Waal, J. Ke, M.H. Tan, C. Zhang, A. Moeller, G.M. West, B.D. Pascal, N. Van Eps, L.N. Caro, S.A. Vishnivetskiy, R.J. Lee, K.M. Suino-Powell, X. Gu, K. Pal, J. Ma, X. Zhi, S. Boutet, G.J. Williams, M. Messerschmidt, C. Gati, N.A. Zatsepin, D. Wang, D. James, S. Basu, S. Roy-Chowdhury, C.E. Conrad, J. Coe, H. Liu, S. Lisova, C. Kupitz, I. Grotjohann, R. Fromme, Y. Jiang, M. Tan, H. Yang, J. Li, M. Wang, Z. Zheng, D. Li, N. Howe, Y. Zhao, J. Standfuss, K. Diederichs, Y. Dong, C.S. Potter, B. Carragher, M. Caffrey, H. Jiang, H.N. Chapman, J.C. Spence, P. Fromme, U. Weierstall, O.P. Ernst, V. Katritch, V.V. Gurevich, P.R. Griffin, W.L. Hubbell, R.C. Stevens, V. Cherezov, K. Melcher, H.E. Xu, Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567 (2015)
J. Kern, R. Alonso-Mori, J. Hellmich, R. Tran, J. Hattne, H. Laksmono, C. Glöckner, N. Echols, R.G. Sierra, J. Sellberg, B. Lassalle-Kaiser, R.J. Gildea, P. Glatzel, R.W. Grosse-Kunstleve, M.J. Latimer, T.A. McQueen, D. DiFiore, A.R. Fry, M. Messerschmidt, A. Miahnahri, D.W. Schafer, M.M. Seibert, D. Sokaras, T.C. Weng, P.H. Zwart, W.E. White, P.D. Adams, M.J. Bogan, S. Boutet, G.J. Williams, J. Messinger, N.K. Sauter, A. Zouni, U. Bergmann, J. Yano, V.K. Yachandra, Room temperature femtosecond X-ray diffraction of photosystem II microcrystals. Proc. Natl. Acad. Sci. U. S. A. 109, 9721–9726 (2012)
A. le Maire, M. Gelin, S. Pochet, F. Hoh, M. Pirocchi, J.F. Guichou, J.L. Ferrer, G. Labesse, In-plate protein crystallization, in situ ligand soaking and X-ray diffraction. Acta Crystallogr. D Biol. Crystallogr. 67, 747–755 (2011)
M. Levantino, G. Schiro, H.T. Lemke, G. Cottone, J.M. Glownia, D. Zhu, M. Chollet, H. Ihee, A. Cupanem, M. Cammarata, Ultrafast myoglobin structural dynamics observed with an X-ray free-electron laser. Nat. Commun. 6, 6772 (2015)
Q. Liu, T. Dahmane, Z. Zhang, Z. Assur, J. Brasch, L. Shapiro, F. Mancia, W.A. Hendrickson, Structures from anomalous diffraction of native biological macromolecules. Science 336, 1033–1037 (2012)
H. Liu, B.K. Poon, D.K. Saldin, J.C. Spence, P.H. Zwart, Three-dimensional single-particle imaging using angular correlations from X-ray laser data. Acta Crystallogr. A 69, 365–373 (2013)
W. Liu, D. Wacker, C. Gati, G.W. Han, D. James, D. Wang, G. Nelson, U. Weierstall, V. Katritch, A. Barty, N.A. Zatsepin, D. Li, M. Messerschmidt, S. Boutet, G.J. Williams, J.E. Koglin, M.M. Seibert, C. Wang, S.T. Shah, S. Basu, R. Fromme, C. Kupitz, K.N. Rendek, I. Grotjohann, P. Fromme, R.A. Kirian, K.R. Beyerlein, T.A. White, H.N. Chapman, M. Caffrey, J.C. Spence, R.C. Stevens, V. Cherezov, Serial femtosecond Crystallography of G protein–coupled receptors. Science 342, 1521–1524 (2013)
Q. Liu, Y. Guo, Y. Chang, Z. Cai, Z. Assur, F. Mancia, M.I. Greene, W.A. Hendrickson, Multi-crystal native SAD analysis at 6 keV. Acta Crystallogr. D Biol. Crystallogr. 70, 2544–2557 (2014)
N.T. Loh, V. Elser, Reconstruction algorithm for single-particle diffraction imaging experiments. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 80, 026705 (2009)
A.A. Lutman, F.J. Decker, J. Arthur, M. Chollet, Y. Feng, J. Hastings, Z. Huang, H. Lemke, H.D. Nuhn, A. Marinelli, J.L. Turner, S. Wakatsuki, J. Welch, D. Zhu, Demonstration of single-crystal self-seeded two-color x-ray free-electron lasers. Phys Rev Lett. 113, 254801 (2014)
A.Y. Lyubimov, T.D. Murray, A. Koehl, I.E. Araci, M. Uervirojnangkoorn, O.B. Zeldin, A.E. Cohen, S.M. Soltis, E.L. Baxter, A.S. Brewster, N.K. Sauter, A.T. Brunger, J.M. Berger, Capture and X-ray diffraction studies of protein microcrystals in a microfluidic trap array. Acta Crystallogr. D Biol. Crystallogr. 71, 928–940 (2015)
J.T. Madden, E.L. DeWalt, G.J. Simpson, Two-photon excited UV fluorescence for protein crystal detection. Acta Crystallogr. D Biol. Crystallogr. 67, 839–846 (2011)
J.T. Madden, S.J. Toth, C.M. Dettmar, J.A. Newman, R.A. Oglesbee, H.G. Hedderich, R.M. Everly, M. Becker, J.A. Ronau, S.K. Buchanan, V. Cherezov, M.E. Morrow, S. Xu, D. Ferguson, O. Makarov, C. Das, R. Fischetti, G.J. Simpson, Integrated nonlinear optical imaging microscope for on-axis crystal detection and centering at a synchrotron beamline. J Synchrotron Radiat. 20, 531–540 (2013)
E. Malmerberg, C.A. Kerfeld, P.H. Zwart, Operational properties of fluctuation X-ray scattering data. IUCrJ. 2, 309–316 (2015)
A. Marinelli, D. Ratner, A.A. Lutman, J. Turner, J. Welch, F.J. Decker, H. Loos, C. Behrens, S. Gilevich, A.A. Miahnahri, S. Vetter, T.J. Maxwell, Y. Ding, R. Coffee, S. Wakatsuki, Z. Huang, High-intensity double-pulse X-ray free-electron laser. Nat. Commun. 6, 6369 (2015)
A. Martel, P. Liu, T.M. Weiss, M. Niebuhr, H. Tsuruta, An integrated high-throughput data acquisition system for biological solution X-ray scattering studies. J. Synchrotron Radiat. 19, 431–434 (2012)
D. Mendez, T.J. Lane, J. Sung, J. Sellberg, C. Levard, H. Watkins, A.E. Cohen, M. Soltis, S. Sutton, J. Spudich, V. Pande, D. Ratner, S. Doniach, Observation of correlated X-ray scattering at atomic resolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130315 (2014)
M. Møller, S.S. Nielsen, S. Ramachandran, Y. Li, G. Tria, W. Streicher, M.V. Petoukhov, R.A. Cerione, R.E. Gillilan, B. Vestergaard, Small angle X-ray scattering studies of mitochondrial glutaminase C reveal extended flexible regions, and link oligomeric state with enzyme activity. PLoS One 8, e74783 (2013)
J.W. Murray, E. Garman, R. Ravelli, X-ray absorption by macromolecular crystals: the effects of wavelength and crystal composition on absorbed dose. J. Appl. Crystallogr. 37, 513–522 (2004)
P. Nogly, D. James, D. Wang, T.A. White, N. Zatsepin, A. Shilova, G. Nelson, H. Liu, L. Johansson, M. Heymann, K. Jaeger, M. Metz, C. Wickstrand, W. Wu, P. Båth, P. Berntsen, D. Oberthuer, V. Panneels, V. Cherezov, H. Chapman, G. Schertler, R. Neutze, J. Spence, I. Moraes, M. Burghammer, J. Standfuss, U. Weierstall, Lipidic cubic phase serial millisecond crystallography using synchrotron radiation. IUCrJ. 2(Pt 2), 168–176 (2015)
R.L. Owen, E. Rudi˜no-Pi˜nera, E.F. Garman, Experimental determination of the radiation dose limit for cryocooled protein crystals. Proc. Natl. Acad. Sci. U. S. A. 103, 4912–4917 (2006)
R.L. Owen, D. Axford, J.E. Nettleship, R.J. Owens, J.I. Robinson, A.W. Morgan, A.S. Dor´e, G. Lebon, C.G. Tate, E.E. Fry, J. Ren, D.I. Stuart, G. Evans, Outrunning free radicals in room-temperature macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 68, 810–818 (2012)
R.L. Owen, N. Paterson, D. Axford, J. Aishima, C. Schulze-Briese, J. Ren, E.E. Fry, D.I. Stuart, G. Evans, Exploiting fast detectors to enter a new dimension in room-temperature crystallography. Acta Crystallogr. D Biol. Crystallogr. 70, 1248–1256 (2014)
A. Perrakis, F. Cipriani, J.C. Castagna, L. Claustre, M. Burghammer, C. Riekel, S. Cusack, Protein microcrystals and the design of a microdiffractometer: current experience and plans at EMBL and ESRF/ID13. Acta Crystallogr. D Biol. Crystallogr. 55, 1765–1770 (1999)
A. Round, F. Felisaz, L. Fodinger, A. Gobbo, J. Huet, C. Villard, C.E. Blanchet, P. Pernot, S. McSweeney, M. Roessle, D.I. Svergun, F. Cipriani, BioSAXS Sample Changer: a robotic sample changer for rapid and reliable high-throughput X-ray solution scattering experiments. Acta Crystallogr. D Biol. Crystallogr. 71, 67–75 (2015)
M.V. Petoukhov, D.I. Svergun, Ambiguity assessment of small-angle scattering curves from monodisperse systems. Acta Crystallogr. D 71, 1051–1058 (2015)
G. Rosenbaum, K.C. Holmes, J. Witz, Synchrotron radiation as a source for X-ray diffraction. Nature 230, 434–437 (1971)
C.G. Roessler, A. Kuczewski, R. Stearns, R. Ellson, J. Olechno, A.M. Orville, M. Allaire, A.S. Soares, A. H´eroux, Acoustic methods for high-throughput protein crystal mounting at next-generation macromolecular crystallographic beamlines. J. Synchrotron Radiat. 20, 805–808 (2013)
T. Saio, K. Ogura, M. Yokochi, Y. Kobashigawa, F. Inagaki, Two-point anchoring of a lanthanide-binding peptide to a target protein enhances the paramagnetic anisotropic effect. J. Biomol. NMR 44, 157–166 (2009)
T. Saio, X. Guan, P. Rossi, A. Economou, C.G. Kalodimos, Structural basis for protein antiaggregation activity of the trigger factor chaperone. Science 344, 1250494 (2014)
D.K. Saldin, H.C. Poon, M.J. Bogan, S. Marchesini, D.A. Shapiro, R.A. Kirian, U. Weierstall, J.C. Spence, New light on disordered ensembles: ab initio structure determination of one particle from scattering fluctuations of many copies. Phys. Rev. Lett. 106, 115501 (2011)
N.K. Sauter, J. Hattne, R.W. Grosse-Kunstleve, N. Echols, New Python-based methods for data processing. Acta Crystallogr. D Biol. Crystallogr. 69, 1274–1282 (2013)
N.K. Sauter, J. Hattne, A.S. Brewster, N. Echols, P.H. Zwart, P.D. Adams, Improved crystal orientation and physical properties from single-shot XFEL stills. Acta Crystallogr. D Biol. Crystallogr. 70, 3299–3309 (2014)
M.R. Sawaya, S. Sambashivan, R. Nelson, M.I. Ivanova, S.A. Sievers, M.I. Apostol, M.J. Thompson, M. Balbirnie, J.J. Wiltzius, H.T. McFarlane, A.Ø. Madsen, C. Riekel, D. Eisenberg, Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447, 453–457 (2007)
M. Schmidt, Mix and inject: reaction initiation by diffusion for time-resolved macromolecular crystallography. Adv. Condens. Matter Phys. 2013, Article ID 167276, 10 pages (2013). http://dx.doi.org/10.1155/2013/167276
R.G. Sierra, H. Laksmono, J. Kern, R. Tran, J. Hattne, R. Alonso-Mori, B. Lassalle-Kaiser, C. Glöckner, J. Hellmich, D.W. Schafer, N. Echols, R.J. Gildea, R.W. Grosse-Kunstleve, J. Sellberg, T.A. McQueen, A.R. Fry, M.M. Messerschmidt, A. Miahnahri, M.M. Seibert, C.Y. Hampton, D. Starodub, N.D. Loh, D. Sokaras, T.C. Weng, P.H. Zwart, P. Glatzel, D. Milathianaki, W.E. White, P.D. Adams, G.J. Williams, S. Boutet, A. Zouni, J. Messinger, N.K. Sauter, U. Bergmann, J. Yano, V.K. Yachandra, M.J. Bogan, Nanoflow electrospinning serial femtosecond crystallography. Acta Crystallogr. D68, 1584–1587 (2012)
M. Skou, S. Skou, T.G. Jensen, B. Vestergaard, R.E. Gillilan, In situ microfluidic dialysis for biological small-angle X-ray scattering. J. Appl. Crystallogr. 47, 1355–1366 (2014)
J.L. Smith, R.F. Fischetti, M. Yamamoto, Micro-crystallography comes of age. Curr. Opin. Struct. Biol. 22, 602–612 (2012)
A.S. Soares, M.A. Engel, R. Stearns, S. Datwani, J. Olechno, R. Ellson, J.M. Skinner, M. Allaire, A.M. Orville, Acoustically mounted microcrystals yield high-resolution X-ray structures. Biochemistry 50, 4399–4401 (2011)
S.K. Son, H.N. Chapman, R. Santra, Multiwavelength anomalous diffraction at high x-ray intensity. Phys. Rev. Lett. 107, 218102 (2011)
H.P. Stevenson, A.M. Makhov, M. Calero, A.L. Edwards, O.B. Zeldin, I.I. Mathews, G. Lin, C.O. Barnes, H. Santamaria, T.M. Ross, S.M. Soltis, C. Khosla, V. Nagarajan, J.F. Conway, A.E. Cohen, G. Calero, Use of transmission electron microscopy to identify nanocrystals of challenging protein targets. Proc. Natl. Acad. Sci. U. S. A. 111, 8470–8475 (2014a)
H.P. Stevenson, D.P. DePonte, A.M. Makhov, J.F. Conway, O.B. Zeldin, S. Boutet, G. Calero, A.E. Cohen, Transmission electron microscopy as a tool for nanocrystal characterization pre- and post-injector. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 369, 20130322 (2014b)
M. Suga, F. Akita, K. Hirata, G. Ueno, H. Murakami, Y. Nakajima, T. Shimizu, K. Yamashita, M. Yamamoto, H. Ago, J.R. Shen, Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517, 99–103 (2015)
M. Sugahara, E. Mizohata, E. Nango, M. Suzuki, T. Tanaka, T. Masuda, R. Tanaka, T. Shimamura, Y. Tanaka, C. Suno, K. Ihara, D. Pan, K. Kakinouchi, S. Sugiyama, M. Murata, T. Inoue, K. Tono, C. Song, J. Park, T. Kameshima, T. Hatsui, Y. Joti, M. Yabashi, S. Iwata, Grease matrix as a versatile carrier of proteins for serial crystallography. Nat. Methods 12, 61–63 (2015)
J. Tenboer, S. Basu, N. Zatsepin, K. Pande, D. Milathianaki, M. Frank, M. Hunter, S. Boutet, G.J. Williams, J.E. Koglin, D. Oberthuer, M. Heymann, C. Kupitz, C. Conrad, J. Coe, S. Roy-Chowdhury, U. Weierstall, D. James, D. Wang, T. Grant, A. Barty, O. Yefanov, J. Scales, C. Gati, C. Seuring, V. Srajer, R. Henning, P. Schwander, R. Fromme, A. Ourmazd, K. Moffat, J.J. Van Thor, J.C. Spence, P. Fromme, H.N. Chapman, M. Schmidt, Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science 346, 1242–1246 (2014)
T. Terai, E. Maki, S. Sugiyama, Y. Takahashi, H. Matsumura, Y. Mori, T. Nagano, Rational development of caged-biotin protein-labeling agents and some applications in live cells. Chem. Biol. 18, 1261–1272 (2011)
K. Tono, E. Nango, M. Sugahara, C. Song, J. Park, T. Tanaka, R. Tanaka, Y. Joti, T. Kameshima, S. Ono, T. Hatsui, E. Mizohata, M. Suzuki, T. Shimamura, Y. Tanaka, S. Iwata, M. Yabashi, Diverse application platform for hard X-ray diffraction in SACLA (DAPHNIS): application to serial protein crystallography using an X-ray free-electron laser. J Synchrotron Radiat. 22, 532–537 (2015)
M. Uervirojnangkoorn, O.B. Zeldin, A.Y. Lyubimov, J. Hattne, A.S. Brewster, N.K. Sauter, A.T. Brunger, W.I. Weis, Enabling X-ray free electron laser crystallography for challenging biological systems from a limited number of crystals. Elife. 4 (2015). doi:10.7554/eLife.05421
T. Ursby, M. Weik, E. Fioravanti, M. Delarue, M. Goeldner, D. Bourgeois, Cryophotolysis of caged compounds: a technique for trapping intermediate states in protein crystals. Acta Crystallogr. D Biol. Crystallogr. 58, 607–614 (2002)
N. Van Eps, A.M. Preininger, N. Alexander, A.I. Kaya, S. Meier, J. Meiler, H.E. Hamm, W.L. Hubbell, Interaction of a G protein with an activated receptor opens the interdomain interface in the alpha subunit. Proc. Natl. Acad. Sci. U. S. A. 108, 9420–9424 (2011)
R.D. Wampler, D.J. Kissick, C.J. Dehen, E.J. Gualtieri, J.L. Grey, H.F. Wang, D.H. Thompson, J.X. Cheng, G.J. Simpson, Selective detection of protein crystals by second harmonic microscopy. J. Am. Chem. Soc. 130, 14076–14077 (2008)
W. Wan, H. Wille, J. Stöhr, A. Kendall, W. Bian, M. McDonald, S. Tiggelaar, J.C. Watts, S.B. Prusiner, G. Stubbs, Structural studies of truncated forms of the prion protein PrP. Biophys. J. 108, 1548–1554 (2015)
N. Watanabe, H. Murai, I. Tanaka, Semi-automatic protein crystallization system that allows in situ observation of X-ray diffraction from crystals in the drop. Acta Crystallogr. D Biol. Crystallogr. 58, 1527–1530 (2002)
U. Weierstall, D. James, C. Wang, T.A. White, D. Wang, W. Liu, J.C. Spence, R. Bruce Doak, G. Nelson, P. Fromme, R. Fromme, I. Grotjohann, C. Kupitz, N.A. Zatsepin, H. Liu, S. Basu, D. Wacker, G.W. Han, V. Katritch, S. Boutet, M. Messerschmidt, G.J. Williams, J.E. Koglin, M. Marvin Seibert, M. Klinker, C. Gati, R.L. Shoeman, A. Barty, H.N. Chapman, R.A. Kirian, K.R. Beyerlein, R.C. Stevens, D. Li, S.T. Shah, N. Howe, M. Caffrey, V. Cherezov, Nat. Commun. 5, 3309 (2014)
M.S. Weiss, G. Mander, R. Hedderich, K. Diederichs, U. Ermler, E. Warkentin, Determination of a novel structure by a combination of long-wavelength sulfur phasing and radiation-damage-induced phasing. Acta Crystallogr. D Biol. Crystallogr. 60, 686–695 (2004)
T.A. White, R.A. Kirian, A.V. Martin, A. Aquila, K. Nass, A. Barty, H.N. Chapman, CrystFEL: a software suite for snapshot serial crystallography J. Appl. Crystallogr. 45, 335–341 (2012)
T.A. White, A. Barty, F. Stellato, J.M. Holton, R.A. Kirian, N.A. Zatsepin, H.N. Chapman, Crystallographic data processing for free-electron laser sources. Acta Crystallogr. D Biol. Crystallogr. 69, 1231–1240 (2013)
B. Xiao, S.J. Smerdon, D.H. Jones, G.G. Dodson, Y. Soneji, A. Aitken, S.J. Gamblin, Structure of a 14-3-3 protein and implications for coordination of multiple signalling pathways. Nature 376, 188–191 (1995)
H. Zhang, H. Unal, C. Gati, G.W. Han, W. Liu, N.A. Zatsepin, D. James, D. Wang, G. Nelson, U. Weierstall, M.R. Sawaya, Q. Xu, M. Messerschmidt, G.J. Williams, S. Boutet, O.M. Yefanov, T.A. White, C. Wang, A. Ishchenko, K.C. Tirupula, R. Desnoyer, J. Coe, C.E. Conrad, P. Fromme, R.C. Stevens, V. Katritch, S.S. Karnik, V. Cherezov, Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell 161, 833–844 (2015)
Q. Zhou, Y. Lai, T. Bacaj, M. Zhao, A.Y. Lyubimov, M. Uervirojnangkoorn, O.B. Zeldin, A.S. Brewster, N.K. Sauter, A.E. Cohen, S.M. Soltis, R. Alonso-Mori, M. Chollet, H.T. Lemke, R.A. Pfuetzner, U.B. Choi, W.I. Weis, J. Diao, T.C. Sudhof, A.T. Brunger, Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature 525, 62–67 (2015)
Acknowledgements
The author is deeply indebted to Profs. Jochen Schneider and Jerome Hastings for their encouragements throughout the process, is grateful for fruitful discussions with the colleagues at SLAC and Stanford University, in particular to Marc Deller, Sebastien Boutet, Han Dao, Fatemeh Jabbarpour and Varun Bhadkamkar for their help with editing of the manuscript, and NIH-NIGMS, DOE BES and BER, SLAC and Stanford University for funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this entry
Cite this entry
Wakatsuki, S. (2015). Structural Biology Applications of Synchrotron Radiation and X-Ray Free-Electron Lasers. In: Jaeschke, E., Khan, S., Schneider, J., Hastings, J. (eds) Synchrotron Light Sources and Free-Electron Lasers. Springer, Cham. https://doi.org/10.1007/978-3-319-04507-8_44-1
Download citation
DOI: https://doi.org/10.1007/978-3-319-04507-8_44-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Online ISBN: 978-3-319-04507-8
eBook Packages: Springer Reference Physics and AstronomyReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics
Publish with us
Chapter history
-
Latest
The SACLA X-Ray Free-Electron Laser Based on Normal-Conducting C-Band Technology- Published:
- 25 January 2016
DOI: https://doi.org/10.1007/978-3-319-04507-8_9-2
-
Characterization of the Time Structure of Free-Electron Laser Radiation
- Published:
- 23 January 2016
DOI: https://doi.org/10.1007/978-3-319-04507-8_49-2
-
Structural Biology Applications of Synchrotron Radiation and X-Ray Free-Electron Lasers
- Published:
- 16 September 2015
DOI: https://doi.org/10.1007/978-3-319-04507-8_44-1
-
Original
The SACLA X-Ray FEL Based on Normal-Conducting C-Band Technology- Published:
- 09 May 2015
DOI: https://doi.org/10.1007/978-3-319-04507-8_9-1