Abstract
This chapter focuses in a theoretical and experimental study of food dehydration with particular reference to drying of fruits and vegetables. Topics related to fundamentals, theory and effect of drying and modeling are presented and discussed. Application has been done to drying of banana fruit. Whole bananas were peeled manually and dried in an oven at temperatures ranging from 40 to 70 °C. Drying, heating and shrinkage lumped models were proposed and fitted to experimental data. Non-linear regression analyses were done to verify the consistence of the models to predict the experimental data. Results revealed which air temperature affect significantly moisture removal, heating and dimensions variations rates and quality of banana fruit. The fitted models presented good concordance with experimental data.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Basic Concept in Food
Food is any substance consumed to provide nutritional support for the body. It is ingested and assimilated by an organism to produce energy, stimulate growth, and maintain life.
Food products exist in the solid and liquid phases. They present great variability in composition and physical characteristics. For example, the composition of grains, fruits and vegetables depends on variety, site variables, climate, etc. [1].
Constituents commonly found in foods include moisture, protein, fat, carbohydrate, fiber, and ash [2]. The volatile part of a food item can be termed moisture. Moisture in the form of water molecules is bonded to various parts of the product in varying ways as follows: (a) ionic groups, such as carboxyl and amino acids, and (b) hydrogen groups, such as hydroxyl and amides. In high-moisture foods in which moisture contents are more than 50 % wet basis, unbound free water exists in the interstitial pores and in intercellular spaces [3].
Because water (continuous liquid phase) is the predominant constituent (highest concentration) in most fresh foods, water concentration significantly influences the palatability, digestibility, physical structure, technical handling ability, and thermophysical properties of food materials [2, 4].
For agricultural and horticultural products, water content varies with the cultivar as well as with the stage of development or maturity when harvested, the growing conditions, and amount of moisture lost after harvest [2].
Most of the deteriorative processes that take place in foodstuffs are influenced by the temperature, and concentration and mobility of water inside the food. Perishable food materials are more stable at low than at high moisture content, and low storage temperature [4]. Thus, one of the prime goals of food processing on preservation is to convert perishable foods such as grains, fruits and vegetable in stabilized products, consequently reducing postharvest losses, in order to extend storage self life and to quality enhancement.
Today, several process technologies have been employed on an industrial scale to preserve fresh foods, such as packing, canning, cooling, freezing, heating, and dehydration [5].
A food product is in equilibrium with its surrounding when its internal vapor pressure becomes in equilibrium with the outside vapor pressure. This condition occurs as the wet material has been exposed to a particular air condition for a sufficiently long period of time (steady-state limit condition). The moisture content of the product at this stage is called the equilibrium moisture content [3] and its magnitude is a function of the structure and type of the subject food and of the prevailing drying condition [6]. It has been used as reference to safe storage of the perishable materials.
Thus, there is a wide range of techniques available in the literature for measuring the wet material moisture content. They are divided in direct and indirect methods. In direct methods the following can be cited: material heating, chemical drying, azeotropic distillation, chemical methods, gas chromatography and refractometry. In indirect methods the following can be distinguished: electrical conductivity, capacitance, microwaves, infrared absorption, equilibrium relative humidity and temperature difference [7, 8].
2 Food Dehydration Foundations
2.1 Fundamentals
Food dehydration is one of energy-intensive unit operations that has been used to removal the moisture from solids food as an integral part of food processing. Dehydration can be realized by heating (drying) and osmotic pressure difference (osmotic dehydration).
Dehydration of fresh foods generally involves a series of interdependent unit operations like blanching, pasteurization, preconcentration, and drying, all of which contribute to the overall quality of the final product after processing. Dehydration by drying involves simultaneous transfer of heat, mass and momentum in which heat penetrates into the product and moisture is removed by evaporation into an unsaturated gas phase.
Many perishable food products of great nutritional importance for peoples, such as fruits and vegetables, are dehydrated in the food industry. The loss of nutrients and functional attributes varies with regard to the type, condition and time of dehydration process and the sensitivity of specific food components. During dehydration the product is generally above room temperature but well below sterilization temperature. The added heat and exposure time of the product at elevated temperature affect the nutrient quality of the food products, especially that very sensitive to heating.
The different types of food degradation during dehydration by drying are chemical (enzymatic and non enzymatic browning reaction, lipid oxidation, color loss), physical and structural (rehydration, solubility, texture, and aroma loss) and nutritional (vitamin loss, protein loss, and microbial changes). All changes depend on water activity, temperature and exposure time [3, 6].
The aim of dehydration is to prevent the growth and reproduction of microorganisms causing decay and minimizes many of the moisture-mediated deteriorative reactions. It brings about substantial reduction in weight and volume, minimizing packing, storage, and transportation costs and enables storability of the product under ambient temperature [5]. Thus, following advantages of this thermal process can be cited [3]:
-
a.
Extended storage life;
-
b.
Quality enhancement;
-
c.
Ease of packaging, handling, and transportation;
-
d.
Sanitation (Destruction of insects and the microorganisms);
-
e.
Improved milling, mixing, or segregations.
Most perishable food products, such as, fruits and vegetables contain more than 80 % water and they are, therefore, highly perishable. Water loss and decay account for most of their loss, which are estimated to be more than 30–40 % in the developing countries in the tropics and subtropics due to inadequate handling, transportation, and storage facilities.
Consequently, the need to reduce postharvest losses of these perishable horticultural foods is of paramount importance for developing countries, in order, to increase their availability, especially in the present context when the constraints on food production are continually increasing [5].
Heat and mass transfer plays a very important role in different unit operations of food processing, such as drying, extraction, distillation, and absorption [9]. They involve moisture and heat fluxes in solid foods and interphase heat and moisture transfer in food processing and storage.
Moisture flux within the solid porous food during drying, rehydration, or storage is a complex physical process that depending on the size, shape, and connectivity of the pores may take place via mechanisms molecular diffusion, capillary flow, Knudsen flow, hydrodynamic flow, or surface diffusion. Molecular diffusion is the mass transfer provoked by random movement of molecules. Knudsen diffusion occurs when the mean free path of the diffusing molecules is large in comparison with the diameter of the capillary [9, 10].
Mass diffusion of gases, vapors, and liquids in solids media is more complex process than diffusion in liquids. The porous solids usually have a heterogeneous structure, and they may interact with the diffusing compounds. Consequently, the diffusion velocity of small molecules in these solids is much lower than in liquids, and this may affect the various physical and chemical processes velocities involving mass transfer [9].
Diffusion in gases can be treated in terms of kinetic theory, but an empirical treatment is necessary for diffusion in solids. In this context, mass diffusivity is a physical property of the system (diffusing substance and medium) that represents as quickly the moisture migrates within the porous solid [9].
In isotropic porous solids, diffusion can be expressed in terms of an effective mass diffusivity (De), which is smaller than the molecular diffusivity (DAB), being defined as follows [11]:
where ε is porosity (void fraction) of the solid and τ is the tortuosity, a factor that connects for the long tortuous path through the pores (varying from 1.5 to 5) [9]. Estimation of the effective diffusion coefficient in isotropic macroporous media, has also been proposed by van Brakel and Heertjes [12] as follows:
In this equation \( \delta \) represent the constrictivity, and \( {\text{D}}_{\text{AB}} \) is the vapor diffusivity in air in the absence of porous media [13]. The constrictivity accounts for the fact that the cross section of a pore space segment varies over its length. Many fresh foods present a porous structure, which is developed during the drying process, especially in process at high temperature, when water is removed as a vapor. The porous structure can be characterized by the bulk porosity or void fraction of the material, which can be estimated by using the following equation [14]:
where \( \rho_{b} \) is the bulk density and \( \rho_{p} \) the particle (solid) density.
Effective or apparent moisture diffusivities, reported in the literature, have been estimated, usually from drying or sorption rate experimental data at a specified temperature [9].
The diffusivity of the various compounds in the porous solid depends on the temperature, and the following form of the Arrhenius equation has been applied:
where E is the energy of activation for diffusion, which may vary with the concentration of the diffusant in the solids [9]. Further, it is found in the literature different equations for mass diffusivity as a function of the moisture content and/or temperature [15]. In general comparison between diffusivities reported in the literature is difficult because of the different methods of estimation and the variation of food composition and physical structure. The need for more reliable data of diffusivity is obvious [9].
In porous (granular extruded, puffed) foods, the effective moisture diffusivity increases gradually as the moisture content is reduced to about 0.1, evidently due to the development of porosity [9].
The diffusion of volatile aroma compounds is very important in food processing and storage, and an understanding of the mechanism of mass transfer will help in maintaining the food quality. On the basis of high relative volatility, high losses of volatiles would be expected during evaporation and drying of food products. However, those components may be retained at a relatively high percentage in the dried product because of the presence of soluble and insoluble solids in the food. Retention of aroma compounds depends on the concentration and nature of the food solids. During the fast evaporation in liquids food or wet solids foods, thermodynamic equilibrium is not reached with the very volatile components [9].
Interphase mass transfer plays an important role in different processes such as evaporation, drying, freezing, and storage of foods. During drying of wet porous materials simultaneous heat and mass transfer occurs both inside and in the boundary layer of the drying agent surrounding it. In general, in the drying process a considerable influence is exerted both by the external conditions and by the internal structure of the material to be dried. In this context, hygroscopic porous solids are more likely to exhibit internal resistance to moisture migration for some materials, particularly foodstuffs, because their internal cellular structure and the presence of shell. The relative significance of the internal and external heat and mass transfer process can be expressed by Kirpichev number or Biot number [7]. If the resistance to mass transfer at the surface of the porous solid is significant compared to the resistance within the solid, it must be considered. For this purpose the Biot number of mass transfer has been used. This parameter is defined by the following equation:
where h is the convective mass transfer coefficient at the surface of the solid. By analyzing the Eq. (5), we notice that high Biot number can be obtained by increasing the mass transfer coefficient [9].
Similar equation for Biot number has been applied to heat transfer process by changing diffusion coefficient by thermal conductivity and convective mass transfer coefficient by convective heat transfer coefficient.
The literature contains very few experimental data on mass (or heat) transfer coefficients pertinent to food systems.
Interphase mass (or heat) transfer rates can be increased by increasing the air—velocity and /or temperature. Centrifugal force may increase the mass transfer rate, and a centrifugal fluidized bed has been proposed to accelerate air—drying of fruits and vegetables [9, 16].
2.2 Prediction in Drying
In recent days, with the drying of food materials the following trends are found [10]:
-
(a)
Understanding of the physical and mathematical aspects of the dehydration process;
-
(b)
Reduction of the changes in foodstuffs during dehydration;
-
(c)
Optimization of the drying process.
Modeling of the drying process ultimately has presented great potential to quickly identify the consequences of schedule alterations and provide focus to the solution of drying problems. The modeling of wet porous solids drying includes two inter-related areas [17]: (a) modeling of single-particle drying or collection of particle (thin-layer model) and (b) modeling of deep-bed layer drying (dryer model).
Mathematical modeling and numerical simulation not only allows some rather expensive and repetitive experimentation to be avoided, but perhaps most importantly, can be used to elucidate on the underlying physic associated with the convoluted heat and mass transport phenomena which are generated within the porous media during the drying process [18].
It is very difficult to mathematically describe the transport of water in structured food material. Despite of this situation, several empirical, semi-empirical, and theoretical models have been proposed to describe drying process. Theoretical models take into account only internal resistance while the semi-empirical and empirical models (thin-layer drying models) take into account only external resistance to heat and moisture transfer between wet porous solid and air (internal resistance is negligible). Further, as shrinkage in fruits is an observable phenomenon and may have significant effect on mass diffusivity, it must be taken into account in order to obtain reliable predictions of performance. The changes in the volume of a solid during diffusion can be taken into consideration by using suitable frames of reference based on the dry solids content of the material.
Because the simplifications, the empirical and semi-empirical models cannot give clear and accurate of the fundamentals of the drying process, but they can used to complete mathematical models at the level of the drying equipment or supply information of the average moisture content and temperature at a specified drying time.
In general, the non-steady state mass diffusion in solids can be treated mathematically in manner similar to the heat conduction (Fourier’s law).
The moisture transfer in heterogeneous porous media can be conveniently analyzed by using Fick’s second law applied to homogeneous porous materials, in which the heterogeneity of the material is accounted by using an effective diffusivity.
Moisture diffusivity depends strongly on temperature and, often, very strongly on the moisture content. Further, as reported before, the void fraction affects diffusivity significantly, and the pore structure and distribution do so even more [13].
In foods, mass diffusion is often moisture concentration dependent. Because of this non-linearity the differential equation of diffusion must be solved by numerical or other special methods. On the other hand, when strong interactions between the diffusing component and the porous medium occur, for example, swelling, diffusion does not obey Fick’s law (non-Fickian diffusion). Non-Fickian diffusion or anomalous diffusion may be directly related to the influence of the changing structure on solubility and diffusional mobility, or they may result from the internal stresses exerted by one part of the porous media an another as diffusion proceeds [19].
Exact solution of the non-steady state (transient) diffusion equation for constant mass diffusivity (or thermal diffusivity) and various boundary conditions are available in the literature for the basic shapes such as slab, finite and infinite cylinder, parallelepiped, and sphere [19–23].
The mass diffusivity of various substances in solid media is of special interest to food engineering, because most mass transport operations in the food industry involve solid or semisolid foods. Literature data are very limited, and they vary considerable because of the complex structure of foods and the lack of a standard method for determination of diffusivity [9].
The mechanism of water transport may change from liquid diffusion at high moisture to vapor diffusion, which is much faster. At low moisture (<0.1) the mass diffusivity drops sharply because of the difficulty of removing the strongly sorbed water molecules from solid matrix [9, 24].
Now that we have discussed about moisture diffusion in wet food materials to be dried, the next step is to perform application related to this topic.
3 Application: Drying of Banana Fruit
3.1 Background
Several types of dryers and drying methods, each better suited for a particular situation, are commercially used to remove moisture from a wide variety of fruits and vegetables. Factors on which the selection of a particular dryer/drying method depends include form of raw material and its properties, desired physical form and characteristics of the product, necessary operating conditions, and operation costs. In general, the following quality changes during drying and storage of fruits can be cited [5]:
-
(a)
Loss of vitamins (vitamins A and C);
-
(b)
Loss of natural pigments (carotenoids and chlorophylis);
-
(c)
Discoloration due to enzymatic or nonenzymatic browning;
-
(d)
Oxidative degradation and flavor loss;
-
(e)
Irreversible damage to the texture (shrinkage, slow cooking, and incomplete rehydration);
-
(f)
Loss of water reducing microbiological spoilage.
Fruits and vegetables play an important role in human diet and nutrition as sources of vitamins and minerals. Overall post-harvest losses of fruit and vegetables in developing countries are estimated at about 20–50 % of the production. Fresh fruits are dried after harvesting, in order to reduce the waste and the spoilage, and to extend their shelf life. During the drying of fruits and vegetables occurs big variations of the volume and surface area of the product simultaneously with the loss of moisture and increase of the temperature. Therefore, it is necessary to devote more attention to shrinkage phenomenon, because it affects the drying and heating rates.
Bananas are fruits with aromatic flavor which are naturally sweet. They contain different constituents such as fat, high natural sugars content, protein, potassium and vitamins A, B complex and C. They provide flavor and variety to the human diet, and also serve as an important source of essential vitamins and minerals, although they are not good and economical sources of protein, fat and energy. From the viewpoint of production and consumption banana fruit post-harvested contains high moisture content, and therefore very perishable and susceptible to fast deterioration during transportation and storage. This in turn causes serious economic losses as a result of reduction in weight and quality.
Because the sequential growth of banana cultures and the amount of harvested banana, losses of these fruits has increased and thus generates the necessity of studies for conservation of these fruits [25–27]. Commercially, banana, whole or in slices, is dried from approximately 80 % to less than 20 % final moisture content [28, 29] or down to 14–15 % final moisture content (on dry basis) [30]. Due to the high sugar and water contents, bananas are dried normally in high temperatures and prolonged drying times, which can cause serious adverse changes in the finished products [31].
In order to accurately understand moisture migration phenomenon within biological products, mainly foodstuffs (grains, fruits, vegetables, etc.), and to explain the effects of drying on the quality of the material, the moisture and heat transfer in individual particles should be understood and accurately represented by a mathematical model. Now, this chapter presents both theoretical (drying, heating and shrinkage lumped models) and experimental drying study of banana fruit.
3.2 Experimental Procedure
Farias [32] and Farias et al. [33] conducted several batch drying experiments of peeled whole banana. Ripened banana fruits (Musa species and “prata” variety), purchased from a local super market were used in the investigation, immediately. The whole fruits were hand peeled. Measurements of the thickness, length and diameter were made at different points with a digital paquimeter and initial weight was measured using a digital electronic balance. All bananas used for drying were from the same batch. Drying experiments were performed for peeled whole banana using a laboratory-scale system (oven) in different drying temperatures. The air velocity was set at a constant 1 m/s. Fruit was placed into the oven on a stainless steel mesh tray. Figure 1 shows one the fruits used in the drying experiments. Table 1 shows the experimental information about the fruit and drying air.
Moisture loss was measured by periodically taking out and weighting the banana using a digital electronic balance with 0.1 g precision; surface temperature was measured using an infrared thermosensor. Following, we putting the sample within an oven for continue drying until the next time point. Each time interval for the measurement was 5 min. The drying continued until there was no significant decrease of the fruit moisture with increasing the drying time between consecutive measurements. After this instant of drying the fruit was maintained for 24 h at the same temperature, in order to obtain the moisture in the equilibrium condition. This moisture was used to calculate the value of equilibrium moisture content. Following, the fruit was maintained into the oven for more 24 h at temperature of 70 °C, in order, for obtain dry matter mass.
3.3 Mathematical Treatment
In order to describe the drying behavior of banana, and predict it under different drying conditions, it is necessary to model the drying process. Drying of banana predominantly follows a falling rate profile. Because the high moisture content of fruit post-harvested mass transfer during this period is mainly caused by liquid diffusion. As results, the rate of diffusion is governed by moisture concentration gradient as the driving force. Thus, Fick’s law of diffusion and empirical models can be used to model the drying behavior for this period. At present, there are very few models that represent the batch drying of tropical fruits specially banana. Herein, it was propose models to moisture removal, heating and shrinkage of the banana fruit.
-
(a)
Drying model
Mass diffusion equation as applied to simple geometry, for example, plate, cylinder, sphere and ellipsoid, has exact solution which average value of the moisture content is given for a convergent infinite series as follows [19–23, 34, 35]:
where Na and Kn are dependent of the body shape and specified boundary condition.
In the Eq. (6), the successive terms of the infinite series decrease by increasing the numbers of terms (n). For long drying time, convergence must be faster, and few terms of the series are sufficient to calculate the average value of the moisture content. Thus, considering n = 3, Eq. (6) assumes the form presented in Eq. (7), as follows:
-
(b)
Heating model
The model to predict surface temperature of fruit during drying was based on the works of Azzouz et al. [36], Pérez [37] and Lima et al. [38]. It is given by Eq. (8) following:
-
(c)
Shrinkage model
During drying process, the porous body changes dimensions by heating (dilation) and moisture removal (contraction, well-known as shrinkage). Dilation phenomenon is insignificant as compared with shrinkage, because the volumetric dilation coefficient is smallest that the shrinkage coefficient, mainly for fruits and vegetables. However, when moisture movement from the body to surrounding is stopped, dimension variation due to heating is a very important phenomenon; it provokes large thermal stress within the solid, mainly for more rigid materials (post-drying). A fundamental point in the theory of shrinkage phenomenon is to explain by an equation like both volume and average moisture content are related. According by Lima [38] and Lima et al. [39, 40, 41], the following mathematical relation for linear shrinkage was proposed:
In the Eqs. (7), (8) and (9), the parameters Ai and Ki are obtained by fitting the models to experimental data.
During drying process banana shrinks, modify its shape, decreases the area of heat transfer and increases the superficial roughness. This last characteristic provides an increase of the turbulence level in the boundary layer, thus, favoring transfer of energy between air and fruit. For determination of the volume [42] and surface area [43] of the banana, which was considered as a prolate spheroid it was used the following equations:
3.4 Results Analysis
3.4.1 Drying Experimentation
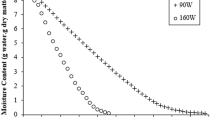
Based on the current experimental drying characteristics data, it was plotted in Figs. 2, 3, 4, 5, 6 and 7 moisture content, surface temperature and volume variations (shrinkage) as a function of the drying time. From the analyses of these figures we can see that moisture content decreases with the time and that the drying and shrinkage rates are higher as higher temperature and lower relative humidity of the drying-air are used.
Experimental dimensionless surface temperature of the banana as a function of drying time at different drying conditions (see Fig. 1)
The effect of drying-air temperature on drying time showed that increase in air temperature resulted in decrease in drying time. Further, it can be seen that drying rate decreased continuously with decreasing moisture content or increasing drying time. An almost linear behavior of the volume and surface area with the moisture content has been verified. Surface temperature of the fruit varied during drying process and reached thermal equilibrium more quickly in the lower drying temperature. Thus, we notice that the drying occurred in falling drying period, for a fixed temperature, in accordance to literature [25, 31, 44–50].
From the analysis of the Fig. 7 it can be seen that temperature has different behavior. This is an indicative that both thermal diffusivity and convective heat transfer coefficient at the surface of fruit are affected by volume and temperature variations.
Table 2 presents the dimensionless length, dimensionless surface area and volume for each experimental test. From the analysis of this table we notice that the shrinkage of the fruit correspond almost to the volume of water removed during drying for each experiments. The, according to Eq. (9), there is almost linearity between the volume variation and moisture content. Shrinkage is more intense in higher air temperature. Similar behavior occurs with surface area. Dimensionless data of the diameter and length are different, so, we can say that the shrinkage velocities are different in both radial and axial directions of the fruit. Thus, it was verified a non-uniform shrinkage. Area/volume relationships remain approximately constant for all samples.
Figure 8 illustrates the fruit at different instants of drying at temperature 70 °C. We can see that the surface hardness, shape variation and loss of color were verified in the fruit post-drying.
3.4.2 Drying Simulations
The constants and coefficients of the thin layer-models as applied to drying at 70°C were computed through non-linear regression analysis. The various statistical parameters corresponding to the models (coefficient of determination and variance) were determined using the Hooke-Jeeves numerical method with convergence criterion 0.0001. All details of the results and statistical parameters are presented in Tables 3, 4 and 5 and Figs. 9, 10 and 11.
Dimensionless surface temperature at the surface of the banana during drying at 70 °C (see Fig. 1)
The statistical parameters were used to determine the consistency of the models for the current experimental conditions. The values for the coefficient of determination (R2) were greater around 0.999 for moisture content, 0.994 for temperature and 0.969 for shrinkage, as a result, the proposed models may be assumed to represent the drying behavior of whole banana.
4 Concluding Remarks
In this chapter transport phenomena (heat conduction, mass diffusion and dimensions variations) in wet porous solid has been explored, with special reference to dehydration by drying. Interest in this class of physical problem is motivated by its importance in many industrial applications (for example, food and chemical processing). Here, our attention is focused on drying of banana fruit drying.
The drying, heating and shrinkage kinetics of banana were analyzed with one drying technique (convection in oven). For unsaturated air condition, the analysis showed that air temperature has greatly affected moisture removal, temperature and dimensions of the fruit. Lumped models (drying, heating and volume variation) available in the literature were fitted for all the drying experimental conditions and the coefficients of the models were established through the non-linear regression analysis. The applicability of the models was examined through the statistical parameters and was found that all the predicted data agreed well with experimental data. Finally, we would like to cite that the proposed models with the new constants can be satisfactorily used to understand the drying characteristics and to aid engineers in optimized design of the banana processing units.
References
Sweat, V.E.: Thermal properties of foods. In: Rao, M.A., Rizvi, S.S.H. (eds.) Engineering properties of foods, pp. 99–138. Marcel Dekker Inc, New York (1995)
ASHRAE: Thermal properties of foods. In: ASHRAE Refrigeration Handbook. American Society of Heating, Refrigeration and Air-Conditioning Engineers, Inc., Atlanta. p. 8.1 (1998)
Sokhansanj, S.: Drying of foodstuffs. In: Mujumdar, A.S. (ed.) Handbook of industrial drying, vol. 1, pp. 589–625. Marcel Dekker Inc., New York (1995)
Wolf, W., Spiess, W.E.L., Jung, G.: Sorption isotherms and water activity of food materials. Elsevier, New York (1985)
Jayaraman, K.S., Das Gupta, D.K.: Drying of fruits and vegetables. In: Mujumdar, A.S. (ed.) Handbook of Industrial drying, vol. 1, pp. 643–690, Marcel Dekker, Inc., New York (1995)
Rizvi, S.S.H.: Thermodynamic properties of foods in dehydration, In: Rao M.A., Rizvi, S.S.H. (eds.) Engineering properties of foods. pp. 223–309. Marcel Dekker., New York (1995)
Strumillo, C., Kudra, T.: Drying: principles, science and design. Gordon and Breach Science Publishers, New York (1986)
Molnár, K.: Experimental techniques in drying. In: Mujumdar, A.S. (ed.) Handbook of Industrial drying, vol. 1, pp. 41–70. Marcel Dekker Inc., New York (1995)
Saravacos, G.D.: Mass transfer properties of foods. In: Rao, M.A., Rizvi, S.S.H. (eds.) Engineering properties of foods, pp. 169–221. Marcel Dekker Inc., New York (1995)
Bruin, S., Luyben, KChAM: Drying of food materials: a review of recent developments. In: Mujumdar, A.S. (ed.) Advances in drying, vol. 1, pp. 155–215. Hemisphere Publishing Corporation, Washington (1980)
Geankoplis, C.J.: Transport processes and unit operations, 2nd edn. Allyn and Bacon, Boston (1983)
van Brakel, J., Heertjes, P.M.: Analysis of diffusion in macroporous media in terms of a porosity, a tortuosity and a constrictivity factor. Int. J. Heat Mass Transfer. 17, 1093–1103 (1974)
Marinos-Kouris, D., Maroulis, Z.B.: Transport properties in the drying of solids. In: In: Mujumdar, A. S. (ed.) Handbook of Industrial drying, vol. 1, pp. 113–159. Marcel Dekker, Inc., New York (1995)
Marousis, S.N., Saravacos, G.D.: Density and porosity in drying starch materials. J. Food Sci. 5, 1367–1372 (1990)
Zogzas, N.P., Maroulis, Z.B., Marinos-Kouris, D.: Moisture diffusivity data compilation in foodstuffs. Drying Technol. 14(10), 2225–2253 (1996)
Lazar, M.E., Farkas, D.F.: The centrifugal fluidized bed. 2. Drying studies on piece-form foods. J. Food Sci. 36(2), 315–319 (1971)
Pang, S., Haslett, A.N.: High–temperature kiln drying of softwood timber: the role of mathematical modeling. In: Turner, I., Mujumdar, A.S. (eds.) Mathematical modeling and numerical technique in drying technology, pp. 179–219. Marcel Dekker Inc., New York (1997)
Turner, I., Perré, P.: A synopsis of the strategies and efficient resolution techniques used for modeling and numerically simulation the drying process. In: Turner, I., Mujumdar, A.S. (eds.) Mathematical modeling and numerical technique in drying technology, pp. 1–81. Marcel Dekker Inc., New York (1997)
Crank, J.: The mathematics of diffusion. Oxford Science Publications, New York (1992)
Carslaw, H.S., Jaeger, J.C.: Conduction of heat in solids. University Press, New York (1959)
Gebhart, B.: Heat conduction and mass diffusion. McGraw-Hill Inc., New York (1993)
Luikov, A.V.: Analytical heat diffusion theory. Academic Press Inc., Ltd, London (1968)
Incropera, F.P., DeWitt, D.P.: Fundamentals of heat and mass transfer. John Wiley & Sons, New York (2002)
Leslie, R.B., Carrillo, P.J., Chung, T.Y., Gilbert, S.G., Hayakawa, K., Marousis, S., Saravacos, G.D., Solberg, M.: Water diffusivity in starch-based systems. In: Levin, H., Slade, L. (eds.) Water relationships in foods, pp. 365–390. Plenum, New York (1991)
Karim, A.M.D., Hawlader, M.N.A.: Drying characteristics of banana: theoretical modeling and experimental validation. J. Food Eng. 70, 35–45 (2005)
Mariani, V.C., Lima, A.G.B., Coelho, L.S.: Apparent thermal diffusivity estimation of the banana during drying using inverse method. J. Food Eng. 85, 569–579 (2008)
Nguyen, M.H., Price, W.E.J.: Air-drying of banana: Influence of experimental parameters, slab thickness, banana maturity and harvesting season. J. Food Eng. 79(1), 200–207 (2007)
Bowrey, R.G., Buckle, K.A., Hamey, I., Pavenayotin, P.: Use of solar energy for banana drying. Food Technol. Aust. 32(6), 290–291 (1980)
Robinson, A.A.: Research design and development of banana dehydration process. Food Engineering. UNSW, Sydney, (1980)
Garcia, R., Leal, F., Rolz, C.: Drying of bananas using microwave and air ovens. Int. J. Food Sci. Technol. 23(2), 73–80 (1988)
Maskan, M.: Microwave/air and microwave finish drying of banana. J. Food Eng. 44, 71–78 (2000)
Farias, R.P.: Drying of banana in oven: thermal and geometric effects. Doctorate thesis. Process engineering, Federal University of Campina Grande. Campina Grande, Brazil (2011)
Farias, R.P., Silva, E.G., Lima, W.M.P.B., Silva, W.P., Lima, A.G.B. : Drying of banana: a theoretical and experimental investigation. Deff. Diff. Forum, 2014
Haji-Sheikh, A., Sparrow, E.M.: Transient heat conduction in a prolate spheroidal solid. Trans. ASME J. Heat Transf. 88(3), 331–333 (1966)
Oliveira, V.A.B., Lima, A.G.B.: Mass diffusion inside prolate spherical solids: An analytical solution. Braz. J. Agro-ind. Prod. 4(1), 41–50 (2002)
Azzouz, S., Guizani, A., Belguith, A.: Experimental analysis of heat and mass transfer during grape air drying. In: Proceedings of the 10th International Drying Symposium (IDS ‘96), vol. B, pp. 881–887. Krakow (1996)
Pérez, V.H.: Study of the behavior of temperature of banana during the drying process. Master thesis, State University of Campinas, Campinas, Brazil (1998) (In portuguese)
Lima, A.G.B.: Diffusion phenomenon in prolate spheroidal solids. Case studies: Drying of banana. Doctorate thesis, State University of Campinas, Campinas, Brazil (1999) (In portuguese)
Lima, A.G.B., Queiroz, M.R., Nebra, S.A.: Simultaneous moisture transport and shrinkage during drying of solids with ellipsoidal configuration. Chem. Eng. J. 86, 85–93 (2002)
Lima, A.G.B., Queiroz, M.R., Nebra, S.A.: Heat and mass transfer model including shrinkage applied to ellipsoidal products: case study for drying of bananas. Develop. Chem. Eng. Miner. Process. 10, 281–304 (2002)
Lima, A.G.B., Farias Neto, S.R., Silva, W.P.: Heat and mass transfer in porous materials with complex geometry: Fundamentals and applications. In: Delgado, J.M.P.Q. (ed.) Heat and mass transfer in porous media. Springer, Berlin (2012)
Provenza, F.: Machine designer. Editora F. Provenza, São Paulo, p. 2.47, (1989) (In Portuguese)
Pólya, G., Szegö, G.: Inequalities for the capacity of a condenser. Am. J. Math. LXVII, 1–32 (1945)
Phoungchandang, S., Woods, J.L.: Moisture diffusion and desorption isotherms for banana. J. Food Sci. 65, 651–657 (2000)
Dandamrongrak, R., Young, G., Mason, R.: Evaluation of pre-treatments for the dehydration of banana and selection of suitable drying models. J. Food Eng. 55, 139–146 (2002)
Queiroz, M.R., Nebra, S.A.: Theoretical and experimental analysis of the drying kinetics of bananas. J. Food Eng. 47, 127–132 (2001)
Demirel, D., Turhan, M.: Air-drying behavior of cavendish and gros Michel banana slices. J. Food Eng. 59, 1–11 (2003)
Kaddumukasa, P., Kyamuhangire, W., Muyonga, J., Muranga, F.I.: The effect of drying methods on the quality of green banana flour. In African Crop Science Conference Proceedings, vol. 7, pp. 1267–1271. Kampala, Uganda (2005)
Talla, A., Puiggali, J.-R., Jomaa, W., Jannot, Y.: Shrinkage and density evolution during drying of tropical fruits: application to banana. J. Food Eng. 64, 103–109 (2004)
Queiroz, M.R.: Theoretical and experimental study of the drying kinetics of banana. Doctorate thesis, State University of Campinas, Campinas, Brazil (1994) (In portuguese)
Acknowledgements
The authors would like to express their thanks to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil), and FINEP (Financiadora de Estudos e Projetos, Brazil) for supporting this work; to the authors of the references in this paper that helped in our understanding of this complex subject, and to the Editors by the opportunity given to present our research in this book.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
de Lima, A.G.B., de Farias, R.P., da Silva, W.P., de Farias Neto, S.R., Farias, F.P.M., de Lima, W.M.P.B. (2014). Convective Drying of Food: Foundation, Modeling and Applications. In: Delgado, J., Barbosa de Lima, A. (eds) Transport Phenomena and Drying of Solids and Particulate Materials. Advanced Structured Materials, vol 48. Springer, Cham. https://doi.org/10.1007/978-3-319-04054-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-04054-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-04053-0
Online ISBN: 978-3-319-04054-7
eBook Packages: EngineeringEngineering (R0)