Abstract
The prescription of perioperative fluids has been a persistent controversy among anesthesiologists, surgeons and intensivists. Interestingly, disagreements within each specialty as to the appropriative types and amounts of fluids required are just as intense as those seen among specialties. The challenge of navigating these waters is demanding because the safe harbor of optimal fluid administration is bounded by hypovolemia and end-organ hypoperfusion, resulting from inadequate fluid resuscitation, and the negative effects of edema formation on respiration and wound healing, resulting from excessive fluid administration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pulmonary Artery Catheter
- Fluid Responsiveness
- Pulse Pressure Variation
- Transpulmonary Thermodilution
- Cardiac Output Measurement
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The prescription of perioperative fluids has been a persistent controversy among anesthesiologists, surgeons and intensivists. Interestingly, disagreements within each specialty as to the appropriative types and amounts of fluids required are just as intense as those seen among specialties. The challenge of navigating these waters is demanding because the safe harbor of optimal fluid administration is bounded by hypovolemia and end-organ hypoperfusion, resulting from inadequate fluid resuscitation, and the negative effects of edema formation on respiration and wound healing, resulting from excessive fluid administration.
Different strategies of fluid management have been implemented in the perioperative setting. The terms of ‘liberal’ or ‘restrictive’ fluid administration were used to define algorithms that used greater or lesser amounts of fluid for maintenance and substitution for losses caused by bleeding, preoperative fasting, and perspiration. With technological progress and the possibility of measuring hemodynamic variables, even with non-invasive technologies, a third fluid administration concept has become established in the perioperative setting – goal-directed fluid management. This therapy concept has been repeatedly shown to significantly improve both short-term and long-term outcomes. Goal-directed therapy is centered on the optimization of individually needed cardiac output and, thus, oxygen delivery (DO2) by incremental fluid administration. The disadvantages of this concept are, in addition to the further invasiveness, the additional costs of these monitoring devices.
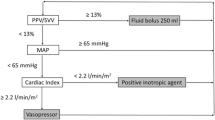
In clinical routine, intravascular measured pressures, e. g., central venous pressure (CVP) or mean arterial pressure (MAP), are often used to quantify the patient’s volume status. Although these parameters are all important components of hemodynamic assessment, none has been shown to be a good predictor of the response of cardiac output to fluid administration [1]. The same applies to the more reliable preload parameters, such as left ventricular end-diastolic area (LVEDA) and global end-diastolic volume (GEDV). However, these static parameters are limited to predicting an increase (responders) or lack of increase (non-responders) in stroke volume (SV) and, thus, cardiac output in response to fluid loading. The inadequacy of commonly used hemodynamic parameters as predictors of the response to fluid stems from the fact that this response depends not only on the preload status, but also on the contractile state of the heart. In this context, dynamic variables like SV variation (SVV) and pulse pressure variation (PPV) are able to measure the change in cardiac output in response to a change in preload due to fluid administration [2].
However, in addition to the physiological variables mentioned above, this chapter aims to provide an update on perioperative hemodynamic monitoring and a brief overview of the different fluid administration concepts in the perioperative setting.
Monitoring Technology
Cardiac Output
Pulmonary artery catheter
The pulmonary artery catheter (PAC) is the classical invasive method for hemodynamic monitoring. It was the gold standard for goal-directed fluid management for many years. None of the other devices used for hemodynamic measurement has raised more controversy than the PAC.
The measurement of cardiac output follows the indicator transpulmonary thermodilution principle. After injection of a defined volume of a cold solution into the PAC’s proximal lumen, the cooling of blood in the pulmonary artery is quantified via the PAC’s distal catheter containing a thermistor. The variation in temperature over time is illustrated in an indicator dilution curve. The area under the curve (AUC) is inversely proportional to the cardiac output that can be calculated with the Stewart-Hamilton equation. The measured time/temperature curve is displayed on the cardiac output monitor. The smaller the decrease in temperature (the greater the cardiac output) the smaller is the AUC that is displayed. Modern catheters are fitted with a heating filament which intermittently heats and measures the thermodilution curve providing serial cardiac output measurement and making assessment of continuous cardiac output available. In this context the term ‘continuous’ should be considered carefully, because although there is a continuous serial heart rate (HR) triggered discharge of heat boluses, changes in cardiac output are detected with a clinically relevant mean delay of 8–10 minutes. Because of this interval, goal-directed volume therapy can be delayed and this technique may not be as useful and accurate as online measurements of stroke volume by other technology.
A further development of the volumetric thermodilution PAC is electrocardiogram (EKG)-triggered computation of the right ventricular end-diastolic volume (RVEDV) and from this, the right ventricular ejection fraction (RVEF, %), using modern specialized monitors (e. g., Vigilance CEDV, Edwards Lifescience, Irvine, CA) and the following formula:
The RVEDV index (RVEDVI) connects the RVEDV with the body surface area and serves as an estimation of the RV, but limited LV, preload and enables conclusions to be drawn about a patient’s circulatory status.
Measurement of the pulmonary artery occlusion pressure (POAP) enables estimation of the LV end-diastolic pressure (LVEP) and, thus, the LV preload. Limiting factors for interpretation of LV preload using the POAP are: Incorrect positioning of the PAC tip, mitral valve defects and reduced LV compliance, e. g., due to LV insufficiency. Therefore, when using POAP for hemodynamic therapy, several measurements should be compared over time. Furthermore, the POAP should not be used to determine SV responsiveness to fluid loading, because there is no evidence that this parameter enables any conclusions to be drawn regarding the increases in SV from fluid loading. For estimation of the above mentioned variables, further parameters like MAP, CVP and mixed venous oxygen saturation (SvO2) are required. The advantages and disadvantages of the PAC are shown in Table 1.
Concerning the international literature, there is still controversy regarding the PAC. Because of higher costs and the availability of less invasive technology for determination of stroke volume, PAC use for hemodynamic monitoring has decreased in the USA. Furthermore, a recently published survey of leading physicians from 80 intensive care units (ICUs) in Germany treating patients after cardiothoracic surgery, showed that although availability of the PAC is 100 %, usage of PACs in patients had decreased to 48 % compared to data from 2005 in which 58 % of these patients were managed using a PAC [3]. Although a study by Tuman et al. [4] showed no benefit in terms of duration of ICU stay, mortality and incidence of postoperative myocardial ischemia using a PAC after coronary bypass surgery compared to clinical management using CVP, another study by Pölönen et al. showed a significantly shorter “median hospital stay” if hemodynamic therapy was targeted to reach SvO2 > 70 % and serum lactate levels ≤ 2.0 mmol/l [5]. Only a few studies have investigated perioperative PAC usage in patients not undergoing cardiac surgery. One of the most comprehensive analyses done in this patient cohort was a study by Sandham et al. using PAC for patients classified 3 and 4 by the American Society of Anesthesiologists (ASA). This study showed no benefit on duration of hospital stay or mortality rate after six months [6]. Jules-Elysee et al., who investigated the use of a PAC-compared to a CVP-based protocol for patients undergoing bilateral knee replacement, reported similar results [7].
It is presumed that the benefit of a PAC depends on its use for applying goal-directed hemodynamic therapy, implementing supranormal oxygen supply and stroke volume optimization, and not just as an instrument of observation. This hypothesis is reflected by the fact that all major retrospective or observational studies that have been performed showed no benefit of PAC use. In contrast, in a meta-analysis by Hamilton et al. [8], the PAC was the only technology that showed a benefit on morbidity and mortality if used in a goal-directed treatment algorithm.
In conclusion, there is need for further investigation to identify patient groups who can gain from using a PAC during the perioperative period.
Transpulmonal thermodilution technology
Transpulmonary thermodilution is a method to estimate intrathoracic and global end-diastolic volumes and is commercially available as a bedside monitoring device (PiCCO, Pulsion Medical Systems, Munich, Germany; EV1000, Volume View, Edwards Life Sciences). Placement of a central venous catheter and a modified arterial catheter equipped with both temperature and pressure sensors are required. The tip of the arterial catheter is positioned in a central artery through access from the femoral, brachial, axillary and radial arteries. The monitor offers recording of SV, cardiac index (CI), static volume parameters such as GEDV index (GEDVI) and extravascular lung water index (EVLWI), and dynamic volume parameters, such as SVV and PVV.
For cardiac output measurement, in vivo calibration has to be performed. A defined volume of a cold solution is injected into the central venous catheter. The blood temperature modulation is recorded via the thermistor placed at the tip of the arterial catheter. The recorded thermodilution curve is used to compute the cardiac output using the Stewart-Hamilton equation. Formerly, the volumetric parameter, the intrathoracic blood volume (ITBV), was calculated as the product of cardiac output and the mean transit time of a dye indicator. For clinical use, the previous, cumbersome method of transpulmonary double-indicator (cold and dye) dilution has been replaced by single-indicator thermodilution. The literature shows that the volumetric parameters, GEDV and ITBV, are superior to the CVP and POAP for assessing cardiac preload [9, 10].
The ability to estimate preload and volume reliably from these parameters has been studied by several authors. Transpulmonary thermodilution cardiac output shows good correlation with pulmonary arterial thermodilution cardiac output. In contrast to the many studies that have shown high validity and reliability of the transpulmonary thermodilution system during the perioperative period, especially following cardiac surgery [11, 12], only a few studies have investigated the use of transpulmonary thermodilution systems for intraoperative goal-directed volume management. Hence, transpulmonary thermodilution has been established in the intensive care setting, but not in the perioperative setting. This observation can be explained by the relative invasiveness of the transpulmonary thermodilution system, leading to less use of this method for intraoperative goal-directed volume management. Additionally, as mentioned above, the lack of clinical trials demonstrating an advantage of intraoperative volume therapy could explain these findings.
Uncalibrated and auto-calibrated pulse contour/pulse wave technology
Because of the invasiveness of cardiac output measurements via the PAC or transpulmonary thermodilution technology, non-invasive systems have been developed to make intraoperative goal-directed fluid management more available and less invasive. One of these non-invasive systems is uncalibrated pulse-contour analysis. This technique is a further development of the original algorithm of pulse contour analysis described by Wesseling in which the relationship between arterial blood pressure and arterial blood flow that is determined by the vascular resistance is characterized. The cardiac output is calculated from the AUC of the arterial waveform. For uncalibrated pulse contour analysis, there are several devices available (e. g., Vigileo, Edwards Lifesciences LLC, Irvine, CA, USA; Pulsioflex, Pulsion Medical Germany). In some monitors, the cardiac output is calibrated using internal databases and adjusting for vascular resistance and compliance by demographic data; other devices use auto-calibration. In these latter devices, the calibration coefficient that adjusts for individual characteristics of vascular resistance and arterial compliance is auto-re-calculated every 10 minutes on the basis of demographic data and the arterial waveform analysis.
Clinical trials concerning the reliability and validity of uncalibrated pulse contour technology compared to established system, such as PAC thermodilution and transpulmonary thermodilution technology, show contrasting results. In one trial, a high bias and wide range of limits of agreement were found in cardiac output measurement using arterial waveform analysis [13]. Other studies confirmed these findings [14]. However, other studies showed no significant differences when comparing calibrated and uncalibrated pulse contour measurements [15]. These findings lead to the conclusion that a final assessment of this technology cannot be performed at this point in time. Nevertheless, although the validity of cardiac output determination compared to calibrated methods seems to be inferior, goal-directed volume administration using this monitoring technology appears to result in clinical benefits with decreased morbidity.
Bioimpedance and reactance technology
Another non-invasive procedure for cardiac output measurement is impedance cardiography. This technique is performed by attaching four electrodes on each side of the patient’s neck, and on the left and right sides of the chest. Microelectric currents that flow through the patient’s chest cavity are registered through these electrodes, and changes in impedance caused by the changes in thoracic aortic volume and blood flow are measured. Volumetric and static variables, such as SV, cardiac output, systemic vascular resistance (SVR) and thoracic fluid content can be observed by this technique [16]. Owing to limitations associated with its use, such as the various surgical manipulations undertaken, acute changes in patient fluid status and frequent electrocautery, impedance cardiography is interference-prone and, therefore, not widely used in the perioperative setting at the present stage of technical development [17].
Non-invasive cardiac output measurement devices
In addition to the above mentioned tools for non-invasive cardiac output measurements, there are considerable efforts being taken for the development of other non-invasive devices to make cardiac output determination easier and more available without having the disadvantages of invasive technologies. One of these recent devices is the Nexfin technology (BMEye, Edwards Life Sciences, Amsterdam, The Netherlands). This instrument provides a non-invasive estimation of cardiac output in two steps. First, the device enables continuous estimation of the arterial pressure curve using the volume-clamp method. For this purpose, the device includes an inflatable cuff that is wrapped around a finger. Additionally, a second device is included to measure the diameter of the finger’s arteries by photoplethysmography. During measurement, the photoplethysmographic device senses the increase in the diameter of the finger’s arteries at each systole and the cuff inflates immediately to keep the diameter constant and, thus, the cuff pressure reflects the arterial pressure. The continuous measurement allows estimation of the arterial pressure curve. During the second step, the cardiac output is computed from the arterial pressure curve by pulse contour analysis, which is included in the Nexfin device [18].
Since its launch, the Nexfin system has been the subject of many investigations showing divergent results. A large perioperative validation study for measuring arterial pressure showed positive results [19, 20], but contrasting results were reported in critically ill patients. Broch et al. showed that Nexfin was a reliable system for measuring cardiac output during and after cardiac surgery compared to the PiCCO system [21]. Other studies revealed similar results [18]. In contrast to these findings, a study by Fischer et al. showed higher percentage errors when comparing Nexfin with transpulmonary thermodilution. Furthermore, rapid changes in CI following a fluid challenge were detected less well compared to with the PiCCO system so that prediction of fluid responsiveness was reduced [22]. Similar results were shown for cardiac output by Monnet et al. in critically ill patients treated on the ICU [23].
As the Nexfin is a relatively new advice, further investigation is needed to substantiate its reliability and variability in the perioperative setting, including for surgeries other than cardiac. The Nexfin device is prone to error in cases of diminished peripheral perfusion, which might occur in critically ill patients. However, goal-directed volume therapy in high and intermediate risk surgery may prove to be an interesting indication for non-invasive monitoring. Another encouraging approach is the notion of external calibration to improve accuracy [22].
Venous and Tissue Saturation
Mixed venous versus central venous saturation
Advanced hemodynamic monitoring with determination of cardiac output and venous saturation measurements is widely used in the perioperative setting especially in cardiac surgical patients. SvO2 and central venous oxygen saturation (ScvO2) are different physiological variables. Both are parameters used to indicate the global ratio of oxygen supply and demand as well as tissue oxygenation. Consequently, it is possible to gather information on the adequacy of actual cardiac output in relation to demand from these parameters. SvO2 measures the venous saturation in the pulmonary artery and thus a PAC is needed. In contrast, ScvO2 is measured mostly in the venous blood of the superior vena cava, which makes this parameter easily available. In healthy subjects, oxygen saturation in the inferior vena cava, which contains blood from the upper and lower body, is higher than in the superior vena cava, which contains blood from the upper body only. In contrast to the situation described above, in clinical practice there is almost no mean difference between SvO2 and ScvO2 in a given patient population. It has been suggested that the difference between SvO2 and ScvO2 is not constant, but may be affected by conditions such as anesthesia and redistribution of blood; for example, following systemic inflammatory response syndrome (SIRS) or shock. ScvO2 can exceed SvO2 in critically ill patients. This difference between SvO2 and ScvO2 may be caused by increased cerebral blood flow owing to the vasodilating effect of inhalational anesthetics and reduced cerebral oxygen demand in anesthetized patients, both reducing cerebral oxygen extraction, which would lead to higher ScvO2 in the superior vena cava. Increased oxygen extraction in the splanchnic region can also reverse the physiological difference between SvO2 and ScvO2. After hemodynamic deterioration, mesenteric blood flow decreases, resulting in venous de-saturation in the lower body. It, therefore, has to be assumed that the oxygen extraction rate is the major factor in the difference between SvO2 and ScvO2. Considering the above mentioned conditions, ScvO2 and SvO2 can be useful parameters for estimating cardiac output during surgery. It is important to mention that both variables, but especially ScvO2, are limited when trying to exclude general or local hypoperfusion and for a more precise prediction both variables are needed, which narrows their use in the intraoperative setting [24]. Another limitation of the use of these parameters is that with reduced oxygen uptake in the periphery, high values of venous saturation do not exclude microcirculatory hypoperfusion.
Cerebral and tissue saturation
As described above, it is possible to draw indirect conclusions from tissue saturation about cardiac output and implement goal-directed, perioperative volume therapy. For monitoring tissue saturation there are several devices available. Near-infrared spectroscopy should be mentioned in this context. Using this technology, reduced tissue saturation, which can be caused by a discrepancy in oxygen supply and demand, can be measured non-invasively. The benefit of this technology has been shown in several studies, notably in vascular and cardiac surgery, but further investigation has to be performed to prove its clinical utility and its impact on outcome [25, 26].
Dynamic Parameters
Intermittent positive airway pressure during controlled mechanical ventilation in patients with regular heart rhythm results in intermittent variation of biventricular preload. This effect results in intermittent variation of SV and arterial pressure. Pulse contour algorithm-based quantification of SV and arterial pressure parameters and the availability of modern devices (Flotrac, Volume view, Edwards Life Systems; LidCO rapid, LIDCO, London, UK; and PiCCO2, Pulsioflex, Pulsion Medical Systems, Munich, Germany) have introduced these dynamic parameters into clinical practice. High variation values can be indicative of hypovolemia and are used to monitor volume therapy by their assessment of volume responsiveness.
Systolic pressure variation
Systolic pressure variation (SPV) represents the difference between the maximum and minimum value of systolic arterial pressure during one mechanical breath. The SPV is composed of an early inspiratory increase in the systolic blood pressure, which reflects the inspiratory augmentation of the LV SV, and a later decrease in systolic blood pressure, which reflects the decreased SV due to the decrease in venous return. It has been shown experimentally and clinically that SPV reflects fluid responsiveness very well in a variety of different surgeries. Moreover, SPV can be easily and accurately estimated from visual examination of the arterial waveform tracing.
Pulse pressure variation
PPV also mirrors changes in pulse pressure induced by ventilation. It is calculated as the difference between maximum and minimum pulse pressure values during mechanical ventilation divided by their mean. PPV is an indicator of the position on the Frank-Starling curve and can predict the deleterious hemodynamic effects of fluid depletion. In the perioperative setting, patients who have reached the plateau of the Frank-Starling curve can be identified as patients in whom PPV is low. The clinical and intraoperative goal of maximizing SV by volume loading can, therefore, be achieved easily by minimizing PPV. These findings have been confirmed in various studies in cardiac as well bowel or other general surgery [27–29].
Stroke volume variation
SVV is the difference between the maximum and minimum SV during one mechanical breath divided by mean SV. Due to pulse contour measuring technology for cardiac output it is possible to provide continuous metering of SVV. High SVV is indicative of hypovolemia and differentiates responders from non-responders. The use of SVV for goal-directed fluid management has been investigated in various studies in the perioperative setting including cardiac and abdominal surgery as well as liver transplantation [2].
Pleth variability index
Variations in pulse oximeter waveform amplitude caused by respiration have been shown to be related to PPV. This measure is sensitive to changes in ventricular preload and is a good predictor of fluid responsiveness. The pleth variability index (PVI) provides automatic and continuous monitoring of respiratory variations in the pulse oximeter waveform amplitude. PVI was shown to be able to predict fluid responsiveness during cardiac and colorectal surgery [30, 31]. The limitation of these findings is that the majority of these studies were conducted in patients with a stable hemodynamic condition. A study by Monnet et al. showed that PVI was less reliable than PPV and SVV for predicting fluid responsiveness in patients receiving norepinephrine [32]. This technology is prone to error in cases of diminished peripheral perfusion, which may occur in unstable and critically ill patients. Due to conflicting findings in recent studies the advantage of PVI is still unclear.
Echocardiography and Doppler Technology
Esophageal Doppler monitoring
Esophageal Doppler monitoring is an easy to use, accurate and minimally invasive method for SV optimization. The CardioQ-System (Deltex Medical, Chichester, West Sussex, UK) is a device that utilizes a normogram incorporating age, weight and height and calculates descending aortic blood flow velocity directly based on the Doppler equation. The monitor displays a waveform of the velocity plotted against time. Because flow time depends on the heart rate, it is usually corrected automatically. The resulting corrected flow time (FTc) represents the systolic ejection time adjusted to one cardiac cycle per second. Further correction allows estimation of the SVR. SVR is inversely proportional to FTc, i. e., the higher the SVR, the shorter the FTc, and increased SVR is often associated with hypovolemia. Since FTc is an indicator of cardiac afterload, in hypovolemia, volume administration will lead to an increase in SV and FTc, because of increased cardiac preload. This effect can be interpreted as volume responsiveness and facilitates goal-directed volume therapy. In addition to being able to measure FTc, it is possible to draw inferences about LV inotropy, because myocardial contractility correlates with measured peak velocity.
In addition to the validity and reliability of esophageal Doppler monitoring compared to PAC-derived variables, several studies have demonstrated the benefit of this technique regarding postoperative complication rates and lengths of hospital stay [33]. It should be noted that interobserver differences because of lack of experience in using esophageal Doppler monitoring are limiting factors in the use of these systems [34, 35].
Transesophageal echocardiography
In current clinical practice, the LVEDA estimated by transesophageal echocardiography (TEE) is the preferred echocardiographic parameter for the assessment of preload. The simplicity of measurement and its reliability in reflecting the ventricle’s loading status have led LVEDA to become the most popular choice in the intraoperative setting, especially in cardiac surgery. LVEDA is commonly measured in the transgastric midpapillary short-axis view. It has been shown that the LVEDA measured by TEE correlates quite well with ventricular volumes measured by nuclear medicine methods. The volumes identified by echocardiography give more detailed information about the volume status than parameters measured by PAC. In clinical routine, this parameter is mostly ‘eyeballed’. Several studies have compared the end-diastolic area as an indicator for cardiac preload with conventional monitoring procedures. LVEDA was shown to be a sensitive method for detecting changes in preload after volume administration [36]. Moreover, ITBV and LVEDA were shown to be equivalent indices of cardiac preload [37]. Additionally, LVEDA is superior to static hemodynamic parameters, such as CVP or PAOP, in assessment of cardiac surgery patients’ fluid responsiveness after fluid administration [38]. Furthermore, it is possible to measure the superior vena cava collapse index by TEE in mechanically ventilated patients. This index can predict a patient’s fluid responsiveness because of a volume and ventilation-dependent collapse of the superior vena cava. The reliability of this index was shown by Viellard-Baron et al. in septic patients [39].
The main limitation of echocardiography is its relatively limited availability, high cost of the devices and the challenges in insuring adequate staff training. Diagnosis and treatment of acute hemodynamic instability are the main domains of echocardiography and it should be performed by trained experts in these conditions. Furthermore, echocardiographic parameters can be used to assess volume status and for goal-directed hemodynamic optimization if adequately trained staff and technology are available.
Optimization Concepts
Patient Selection
The health system’s resources are limited. Therefore, it is clear that expensive and invasive, less-invasive and even non-invasive additional monitoring devices cannot be used in every patient for perioperative goal-directed fluid therapy. For this reason, high-risk patients who could benefit from this specialized therapy need to be identified preoperatively. Using the ASA classification, it is possible to assess a patient’s preoperative physical condition; perioperative cardiac risk and intervention-based risk are not included in this classification. Nevertheless, the patient’s risk depends not only on his/her preoperative health condition but also on intraoperative- and surgery-related factors as proposed by Shoemaker et al. (Table 2) [40]. Therefore, for identification of high-risk patients, a combination of variables to assess the patients risk due to the actual health status (e. g., ASA score, cardiac risk score) and the surgical risk (e. g., Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity [POSSUM] Score) are therefore needed. Most classifications, such as the POSSUM score, are not currently used in clinical practice but only for research purposes. Nevertheless, meta-analyses have shown positive effects of goal-directed fluid therapy and hemodynamic management in high-risk patients (using different definitions of risk) in the perioperative setting [8, 41–43].
Intraoperative Versus Postoperative Optimization
The main objective of goal-directed fluid management is to maintain tissue perfusion and assure organ function. Optimization of cardiac output, resulting in the optimization of oxygen supply and demand as well as tissue oxygenation, should be performed at an early stage prior to appearance of organ dysfunction. It is ineffective or even harmful when performed later. The oxygen debt that can result during surgery leads to a higher incidence of complications, such as infections, organ failure and, as a final consequence, death. Various studies and meta-analyses showed the benefit of intraoperative goal-directed fluid management [44, 45]. A review by Dalfino et al. [46] showed that goal-directed fluid therapy is an effective tool for reducing the incidence of infectious complications, and, more specifically, that goal-directed therapy significantly decreases the rate of surgical site infections, pneumonia and urinary tract infections. During surgery, goal-directed fluid therapy, by preserving or increasing cardiac output, may protect patients against severe gut ischemia-reperfusion injury and thus decrease the incidence of postoperative infections [46]. These findings were reported in a meta-analysis, which showed that goal-directed fluid therapy decreased the incidence of postoperative gastrointestinal dysfunction by maintaining an adequate systemic oxygenation in patients undergoing major surgery [47]. In addition to the benefits of intraoperative goal-directed fluid therapy, the benefit of this strategy during the immediate postoperative period included reductions in complications and duration of hospital stay [48]. Nevertheless, from a physiological point of view, it appears obvious that the patient will benefit from goal-directed fluid therapy that starts earlier in order to prevent intraoperative hypoperfusion, so that it should start intraoperatively.
Fluid Optimization Concepts
Apart from the benefits of goal-directed fluid therapy during major surgery discussed earlier, there are divergent opinions concerning whether liberal or restrictive fluid management leads to better outcome. The disadvantages of goal-directed fluid therapy are seen in excessive volume administration and consequently interstitial space overload, which might influence patient outcome. In a randomized, observer-blinded, multicenter trial, Brandstrup et al. showed that a restricted perioperative intravenous fluid regimen aimed at unchanged body weight reduced complications after elective colorectal resection [49]. Other studies in patients undergoing different kinds of surgery confirmed these findings. Nevertheless, it is difficult to make an objective decision as to whether goal-directed fluid therapy or restrictive fluid therapy is superior, because there are no studies comparing goal-directed fluid therapy with a standardized restrictive fluid therapy. Furthermore, the object of ‘restrictive’ fluid therapy is not clearly defined and is associated with different amounts of fluid administration in the current literature.
In addition to the amount of volume administration, the type of fluid used should not be disregarded. Whereas use of large amounts of crystalloids can lead to interstitial overload, as described above, or to iatrogenic hyperchloremic acidosis when using normal saline, a balanced use of different kinds of solution may help to prevent the disadvantages of liberal fluid therapy. Furthermore, the role of transfusion should not be ignored; especially in bleeding surgical patients, early fluid resuscitation with blood products seems to be advantageous [50].
Conclusions
Because of the increased numbers of high-risk patients undergoing surgery, the perioperative challenge to the anesthesiologist concerning monitoring and fluid management has increased and the benefits of goal-directed fluid therapy have become more evident. Technological advances in hemodynamic monitoring encourage the anesthesiologist to use extensive monitoring for this group of patients. However, further development of non-invasive monitoring devices will help customize goal-directed fluid therapy for a greater group of patients, to provide standardized fluid therapies in the perioperative setting. Nevertheless, there is still a lack of randomized controlled studies comparing the different concepts of fluid-management. Further trials are needed to study the benefits in lower risk patients and the long-term effects of perioperative, standardized fluid-management. Interesting research questions for the future will deal with the ‘right’ fluid for goal-directed volume optimization – crystalloids or colloids – and its effects on transfusion rates and coagulopathy, as well as implementation of a universal scoring system for patient characterization.
References
Marik PE, Cavallazzi R (2013) Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med 41:1774–1781
Perel A, Habicher M, Sander M (2013) Bench-to-bedside review: Functional hemodynamics during surgery – should it be used for all high-risk cases? Crit Care 17:203
Kastrup M, Carl M, Spies C et al (2013) Clinical impact of the publication of S3 guidelines for intensive care in cardiac surgery patients in Germany: results from a postal survey. Acta Anaesthesiol Scand 57:206–213
Tuman KJ, McCarthy RJ, Spiess BD et al (1989) Effect of pulmonary artery catheterization on outcome in patients undergoing coronary artery surgery. Anesthesiology 70:199–206
Pölönen P, Ruokonen E, Hippeläinen M et al (2000) A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 90:1052–1059
Sandham JD, Hull RD, Brant RF et al (2003) A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 348:5–14
Jules-Elysee KM, YaDeau JT, Urban MK (2009) Pulmonary artery versus central venous catheter monitoring in the outcome of patients undergoing bilateral total knee replacement. HSS J 5:27–30
Hamilton MA, Cecconi M, Rhodes A (2011) A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg 112:1392–1402
Sakka SG, Bredle DL, Reinhart K, Meier-Hellmann A (1999) Comparison between intrathoracic blood volume and cardiac filling pressures in the early phase of hemodynamic instability of patients with sepsis or septic shock. J Crit Care 14:78–83
Goedje O, Seebauer T, Peyerl M et al (2000) Hemodynamic monitoring by double-indicator dilution technique in patients after orthotopic heart transplantation. Chest 118:775–781
Button D, Weibel L, Reuthebuch O et al (2007) Clinical evaluation of the FloTrac/Vigileo system and two established continuous cardiac output monitoring devices in patients undergoing cardiac surgery. Br J Anaesth 99:329–336
Sander M, von Heymann C, Foer A et al (2005) Pulse contour analysis after normothermic cardiopulmonary bypass in cardiac surgery patients. Crit Care 9:R729–R734
Sander M, Spies CD, Grubitzsch H et al (2006) Comparison of uncalibrated arterial waveform analysis in cardiac surgery patients with thermodilution cardiac output measurements. Crit Care 10:R164
Østergaard M, Nielsen J, Nygaard E (2009) Pulse contour cardiac output: an evaluation of the FloTrac method. Eur J Anaesthesiol 26:484–489
Hofer CK, Senn A, Weibel L, Zollinger A (2008) Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Crit Care 12:R82
Woltjer HH, Bogaard HJ, de Vries PM (1997) The technique of impedance cardiography. Eur Heart J 18:1396–1403
Perrino AC, Lippman A, Ariyan C et al (1994) Intraoperative cardiac output monitoring: comparison of impedance cardiography and thermodilution. J Cardiothorac Vasc Anesth 8:24–29
Bogert LWJ, Wesseling KH, Schraa O et al (2010) Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia 65:1119–1125
Hofhuizen CM, Lemson J, Hemelaar AEA et al (2010) Continuous non-invasive finger arterial pressure monitoring reflects intra-arterial pressure changes in children undergoing cardiac surgery. Br J Anaesth 105:493–500
Maggi R, Viscardi V, Furukawa T, Brignole M (2010) Non-invasive continuous blood pressure monitoring of tachycardic episodes during interventional electrophysiology. Europace 12:1616–1622
Broch O, Renner J, Gruenewald M et al (2012) A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia 67:377–383
Fischer M-O, Coucoravas J, Truong J et al (2013) Assessment of changes in cardiac index and fluid responsiveness: a comparison of Nexfin and transpulmonary thermodilution. Acta Anaesthesiol Scand 57:704–712
Monnet X, Picard F, Lidzborski E et al (2012) The estimation of cardiac output by the Nexfin device is of poor reliability for tracking the effects of a fluid challenge. Crit Care 16:R212
Sander M, Spies CD, Foer A et al (2007) Agreement of central venous saturation and mixed venous saturation in cardiac surgery patients. Intensive Care Med 33:1719–1725
Pennekamp CWA, Bots ML, Kappelle LJ et al (2009) The value of near-infrared spectroscopy measured cerebral oximetry during carotid endarterectomy in perioperative stroke prevention. A review. Eur J Vasc Endovasc Surg 38:539–545
Taillefer M-C, Denault AY (2005) Cerebral near-infrared spectroscopy in adult heart surgery: systematic review of its clinical efficacy. Can J Anaesth 52:79–87
Auler JO, Galas F, Hajjar L et al (2008) Online monitoring of pulse pressure variation to guide fluid therapy after cardiac surgery. Anesth Analg 106:1201–1206
Gan TJ, Soppitt A, Maroof M et al (2002) Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 97:820–826
Sander M, Spies CD, Berger K et al (2007) Prediction of volume response under open-chest conditions during coronary artery bypass surgery. Crit Care 11:R121
Hood JA, Wilson RJT (2011) Pleth variability index to predict fluid responsiveness in colorectal surgery. Anesth Analg 113:1058–1063
Wyffels PAH, Durnez P-J, Helderweirt J et al (2007) Ventilation-induced plethysmographic variations predict fluid responsiveness in ventilated postoperative cardiac surgery patients. Anesth Analg 105:448–452
Monnet X, Guérin L, Jozwiak M et al (2013) Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth 110:207–213
Walsh SR, Tang T, Bass S, Gaunt ME (2008) Doppler-guided intra-operative fluid management during major abdominal surgery: systematic review and meta-analysis. Int J Clin Pract 62:466–470
Roeck M, Jakob SM, Boehlen T et al (2003) Change in stroke volume in response to fluid challenge: assessment using esophageal Doppler. Intensive Care Med 29:1729–1735
Lefrant JY, Bruelle P, Aya AG et al (1998) Training is required to improve the reliability of esophageal Doppler to measure cardiac output in critically ill patients. Intensive Care Med 24:347–352
Tousignant CP, Walsh F, Mazer CD (2000) The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg 90:351–355
Buhre W, Buhre K, Kazmaier S et al (2001) Assessment of cardiac preload by indicator dilution and transoesophageal echocardiography. Eur J Anaesthesiol 18:662–667
Wiesenack C, Fiegl C, Keyser A et al (2005) Continuously assessed right ventricular end-diastolic volume as a marker of cardiac preload and fluid responsiveness in mechanically ventilated cardiac surgical patients. Crit Care 9:R226–R233
Vieillard-Baron A, Chergui K, Rabiller A et al (2004) Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 30:1734–1739
Shoemaker WC, Appel PL, Kram HB et al (1988) Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 94:1176–1186
Gurgel ST, do Nascimento P (2011) Maintaining tissue perfusion in high-risk surgical patients: a systematic review of randomized clinical trials. Anesth Analg 112:1384–1391
Kern JW, Shoemaker WC (2002) Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med 30:1686–1692
Rhodes A, Cecconi M, Hamilton M et al (2010) Goal-directed therapy in high-risk surgical patients: a 15-year follow-up study. Intensive Care Med 36:1327–1332
Grocott MPW, Dushianthan A, Hamilton MA et al (2013) Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a Cochrane Systematic Review. Br J Anaesth 111:535–548
Aya HD, Cecconi M, Hamilton M, Rhodes A (2013) Goal-directed therapy in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth 110:510–517
Dalfino L, Giglio MT, Puntillo F et al (2011) Haemodynamic goal-directed therapy and postoperative infections: earlier is better. A systematic review and meta-analysis. Crit Care 15:R154
Giglio MT, Marucci M, Testini M, Brienza N (2009) Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth 103:637–646
Pearse R, Dawson D, Fawcett J et al (2005) Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care 9:R687–R693
Brandstrup B, Tønnesen H, Beier-Holgersen R et al (2003) Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 238:641–648
Fouche Y, Sikorski R, Dutton RP (2010) Changing paradigms in surgical resuscitation. Crit Care Med 38:S411–S420
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mezger, V., Habicher, M., Sander, M. (2014). Update on Perioperative Hemodynamic Monitoring and Goal-directed Optimization Concepts. In: Vincent, JL. (eds) Annual Update in Intensive Care and Emergency Medicine 2014. Annual Update in Intensive Care and Emergency Medicine, vol 2014. Springer, Cham. https://doi.org/10.1007/978-3-319-03746-2_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-03746-2_24
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03745-5
Online ISBN: 978-3-319-03746-2
eBook Packages: MedicineMedicine (R0)