Abstract
Therapeutic options for various lung diseases, especially lung cancer, continue to expand with the development of novel therapeutic strategies. RNA interference (RNAi)-based approaches provide a promising modality for the treatment of lung diseases. One of the greatest challenges in RNAi-based therapy continues to be the method for delivering the therapeutic small interfering RNAs (siRNAs) and microRNAs (miRNAs) to the target cells. The advance of pulmonary delivery systems into the clinic illustrates the notion that RNAi will be a valuable modality for the treatment of lung diseases. Currently, the development of miRNA-based therapies for lung cancer is rapidly advancing with the aid of new RNAi technologies. Given the important role of miRNAs in lung carcinogenesis, increasing effort is being dedicated to the research and development of miRNA-based therapies, including the restoration of tumor suppressive miRNA function and the inhibition of oncogenic miRNAs. In this chapter, we discuss the advantages of a pulmonary drug delivery system and the strategies for miRNA-based treatment of lung cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Lung cancer is the leading cause of cancer mortality worldwide. Lung cancer can be classified into two main subtypes: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). Numerous differences are found between these two subtypes, including histological type, biological behavior, prevalence, prognosis and response to therapy. NSCLC accounts for more than 80 % of all lung cancer cases. Only a small percentage of patients with NSCLC present with early stage disease. In this circumstance, surgery remains the best therapeutic option for these patients. Approximately 70 % of all newly diagnosed patients present with locally advanced or metastatic disease and require systemic chemotherapy (Ramalingam et al. 2011). However, the commonly administered chemotherapeutics provide little benefit for patients with advanced stage disease and has reached a plateau in efficacy with a median survival of 8–10 months. The poor prognosis is due to late stage disease presentation, tumor heterogeneity within histological subtypes, and our relatively limited understanding of tumor biology. Furthermore, the high frequency of drug resistance is a key contributor to the poor survival rates of lung cancer patients; improvements in survival rely on continued elucidation of the molecular mechanisms underlying lung cancer tumorigenesis and drug response. Acquiring knowledge through genomic medicine raises the possibility of unraveling the remaining mysteries of lung cancer oncogenesis and opens the door to molecular classification and risk stratification based on gene expression profiles and microRNA (miRNA) signatures.
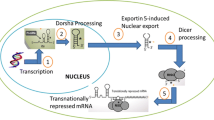
MiRNAs are short (19–23 nucleotides in length) non-coding RNAs found in multiple organisms that regulate gene expression primarily by decreasing the levels of their target mRNAs, through binding to specific target sites in the 3′ untranslated regions (3′UTRs) of these mRNAs (Winter et al. 2009). In the human genome, transcripts of approximately 60 % of all mRNAs are estimated to be targeted by miRNAs. Accumulating evidence shows that miRNAs are grossly dysregulated in human cancers, including NSCLC, and may serve as oncogenes or tumor suppressors (Croce 2009; Babashah and Soleimani 2011). Recent studies have not only shown that miRNAs are useful in lung cancer diagnosis but that specific miRNA profiles may also predict prognosis, drug response and disease recurrence (Yanaihara et al. 2006; Yu et al. 2008). These findings suggest that miRNAs are a promising technology for therapeutic development. In fact, given the significant role of miRNAs in multiple pathways governing lung carcinogenesis, increasing efforts are dedicated to the research and development of miRNA-based therapies, including the restoration of tumor suppressive miRNA function and the inhibition of oncogenic miRNAs (Bader et al. 2010).
The critical problems impeding the development of RNAi-based therapeutics are effective delivery to target sites, therapeutic potency, and elimination of off-target effects (Boudreau et al. 2009). The success of miRNA-based therapeutic delivery is also dependent upon uncovering a delivery route that yields efficient outcomes, is convenient, and promotes patient compliance. For this reason, direct administration of miRNA-based therapeutics to target organs is a promising approach to overcome the problems of systemic administration. Pulmonary delivery offers a new method for the treatment of various lung diseases (Fujita et al. 2013). We believe that delivery of miRNA-based therapeutics using this approach will potentially be useful in clinical practice. Here, we provide an overview of miRNAs as therapeutic targets in lung cancer and discuss the promise and limitations of pulmonary delivery strategies for miRNA-based therapeutics.
2 Role of MicroRNAs in Lung Cancer
Lung cancer biology has traditionally focused on genomic and epigenomic deregulation of protein-coding genes to identify oncogenes and tumor suppressors that are useful as diagnostic and therapeutic targets. Recently, miRNAs were also shown to up-regulate target gene expression by either directly binding to the target mRNA or indirectly repressing nonsense-mediated RNA decay (Vasudevan et al. 2007; Bruno et al. 2011). MiRNAs play an essential role in various cellular processes, such as development, proliferation and apoptosis, to ensure the cellular homeostasis of human cells. Alterations in miRNA expression are increasingly noted in relation to pathophysiological changes in cancer cells, thereby making miRNAs one of the most currently analyzed molecule types in cancer research. Numerous miRNAs are dysregulated in lung cancers, and a single miRNA can have multiple targets that are involved in different oncogenic pathways. A large body of evidence reveals that the aberrant expression of miRNAs in cancer patients can be taken advantage of innumerous ways, such as for potential use as diagnostic, clinicopathological, and/or prognostic markers and as promising therapeutic targets in lung cancer. Aberrant miRNA expression profiles provide additional insight into the clinical application of miRNA-directed therapies in lung cancer (Leidinger et al. 2011). Here, we focus on reviewing the known roles of miRNAs as regulators of cancer cell survival, drug sensitivity and tumorigenesis. These miRNAs hold great potential as targets in the treatment of lung cancer.
2.1 MiRNAs Function as Oncogenes in Lung Cancer
Many oncogenes important in controlling lung cancer tumorigenesis are targets of miRNAs. The miRNAs found in the miR-17-92 cluster (miR-17, miR-18a, miR-19a, miR-19b-1, miR-20a, miR-92-1) are oncogenic miRNAs (oncomiRs) that reside in the amplified chromosomal region 13q31.3 (He et al. 2005). These miRNAs cooperate with c-Myc to accelerate tumor development and promote tumor angiogenesis (Dews et al. 2006). It has been reported that the miR-17-92 cluster is over-expressed in SCLC (Hayashita et al. 2005). Moreover, Ebi et al. reported that miR-17-92 over-expression is associated with retinoblastoma (RB) inactivation (Ebi et al. 2009). Collectively, these results suggest that this miRNA cluster may be a potential therapeutic target in lung cancer.
The miR-21 gene is located on chromosome 17 and was one of the first miRNAs characterized as oncogenic, with its oncogenic function established in various types of cancers (Chan et al. 2005). MiR-21 has been suggested to be an independent negative prognostic factor for the overall survival of NSCLC patients (Markou et al. 2008). MiR-21 targets tumor suppressor genes such as programmed cell death 4 (PDCD4) and phosphatase and tensin homolog deleted from chromosome 10 (PTEN) (Lu et al. 2008; Zhang et al. 2010). Furthermore, miR-21 expression is up-regulated by epidermal growth factor receptor (EGFR) signaling in lung cancer. Antisense miR-21-enhanced EGFR tyrosine kinase inhibitors induce apoptosis of lung cancer cells (Seike et al. 2009). The critical function of miR-21 in regulating lung cancer tumorigenesis makes it a promising target for developing miRNA-based therapeutics and diagnostic tools. However, because miR-21 is also dysregulated in various type of cancer, it appears to be a general oncomiR without tissue specificity (Volinia et al. 2006).
MiR-31 is another miRNA with oncogenic properties in lung cancer. The host gene encoding miR-31 is located on chromosome 9. Liu et al. showed that miR-31 functions as an oncomiR by directly repressing large tumor suppressor 2 (LATS2) and Protein phosphatase 2, regulatory subunit B, Alpha isoform (PPP2R2A) and that knockdown of miR-31 represses lung cancer cell clonal growth and in vivo tumorigenicity (Liu et al. 2010).
2.2 MiRNAs Function as Tumor Suppressors in Lung Cancer
Among the numerous miRNAs that function as tumor suppressors, the let-7 family is one of the most studied. Let-7 was first identified in C. elegans as a regulator of the timing of cell fate determination (Reinhart et al. 2000). In humans, the let-7 family is a cluster of miRNAs whose encoding genes map to various chromosomal regions that are frequently deleted in lung cancer (Calin et al. 2004). Johnson et al. (2007) showed that let-7 over-expression in the A549 cell line inhibits cell growth and reduces cell-cycle progression. In mouse models of lung cancer, over-expression of let-7g reduces tumor growth (Kumar et al. 2008), and let-7a inhibits tumor growth via suppression of v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) and c-Myc (He et al. 2010). Furthermore, reduced let-7 gene expression in NSCLC patients correlates with poor prognosis (Yanaihara et al. 2006; Takamizawa et al. 2004). The 3′ UTR of members of the RAS GTPase family such as v-Ha-ras Harvey rat sarcoma viral oncogene homolog (HRAS), KRAS and neuroblastoma RAS viral oncogene homolog (NRAS) contains multiple putative let-7 binding sites. It has also been revealed that let-7 miRNAs negatively regulate multiple oncogenes, including MYC (Kumar et al. 2007), high mobility group AT-hook 2 (HMGA2) (Lee and Dutta 2007), B-cell leukemia/lymphoma 2 (BCL-2) (Xiong et al. 2011) and cell cycle proto-oncogenes such as cell division cycle 25A (CDC25A), cyclin-dependent kinase 6 (CDK6) and cyclin D2 (Johnson et al. 2007). These data show that let-7 miRNAs act as key tumor suppressors in regulating cell survival and proliferation in lung cancers.
The miR-34 family is another important group of miRNAs that function as tumor suppressors in many types of cancers (Hermeking 2010; Wong et al. 2011). The miR-34a gene is located on chromosome 1p36.22, and miR-34b/c are expressed from a polycistronic transcript encoded on chromosome 11q23.1. These genes are in chromosomal regions associated with fragile sites of the genome that are frequently altered in cancer (Calin et al. 2004). Structurally, miR-34 family members possess p53-binding sites, reflecting their function as tumor suppressors downstream of the p53 pathway. MiR-34a and miR-34b/c were found to be directly regulated by p53 to control apoptosis and cell cycle arrest in cancer cell lines, including lung cancer (Raver-Shapira et al. 2007; Wiggins et al. 2010). Subsequent studies demonstrated that the apoptotic function of miR-34a is mediated by the direct down-regulation of the expression of BCL-2 and sirtuin 1 (SIRT1) (Yamakuchi et al. 2008; Bommer et al. 2007). In addition, AXL (Mudduluru et al. 2011) and SNAIL1 (Kim et al. 2011) were identified as miR-34 direct targets in lung cancer cells; it is plausible that miR-34 expression inhibits lung cancer cell invasion and migration via repression of these genes. In various solid and hematological malignancies, including lung cancer, miR-34 antagonizes processes necessary for basic cancer cell viability as well as cancer stemness, metastasis and chemoresistance (Bader 2012). In the future, the utility of miR-34-directed therapeutics in the treatment of lung cancer will expand dramatically.
The anti-tumor activity of miR-143 and miR-145 in lung cancer is also well characterized. They are co-transcribed from a bicistronic gene cluster on chromosome 5 (Xin et al. 2009). MiR-143/145 have been identified as tumor suppressor in various types of cancer, including lung cancer. The restoration of miR-145 has been shown to inhibit cell growth in mouse and human lung cancer cells (Liu et al. 2009; Cho et al. 2009). It has also been reported that c-MYC, EGFR and nucleoside diphosphate linked moiety X-type motif 1 (NUDT1) are direct targets of miR-145 that regulate cell proliferation in lung cancer (Chen et al. 2010; Cho et al. 2011). Furthermore, miR-145 has also been shown to inhibit lung adenocarcinoma stem-like cell proliferation by targeting octamer-binding transcription factor 4 (OCT4) (Feng et al. 2011). Similarly, the expression of miR-143 was down-regulated in human lung tumor samples compared with normal tissues (Gao et al. 2010; Vosa et al. 2013).
Finally, miR-192 also might serve as a promising therapeutic target for lung cancer treatment. Retinoblastoma 1 (RB1) is a direct target of miR-192, and over-expression of miR-192 results in decreased expression of RB1 mRNA and protein. Caspase-7 and poly ADP-ribose polymerase (PARP) protein were activated by miR-192 over-expression, suggesting that miR-192 induces cell apoptosis through the caspase pathway. In addition, the analysis of miRNA expression in clinical samples has revealed that miR-192 is significantly down-regulated in lung cancer tissues compared with adjacent, normal lung tissues (Feng et al. 2011).
3 MicroRNA-Based Therapies for Lung Cancer
The development of miRNA-based therapeutics represents a new strategy in cancer treatment and is growing rapidly with the help of new RNAi technologies. Compared to siRNA-based therapies, which are already in clinical trials, miRNAs are less toxic and have the potential to target multiple genes. As presented above, miRNAs are generally classified as oncomiRs or tumor suppressors, with different therapeutic approaches developed for each class. Generally, the up-regulation of miRNA expression is achieved through administration of synthetic miRNA mimics or miRNA-expressing vectors. The down-regulation of miRNA expression is achieved through administration of antisense nucleotides, often chemically modified to ensure stability and specificity. Although each approach shares similarities with other therapies, each is sufficiently distinct such that miRNA-inhibitory and replacement approaches should be viewed as separate therapeutic modalities. In view of cancer as a heterogenic disease that cannot be successfully treated via single gene targeting, miRNA-based strategies may hold the key to therapeutic success. Table 17.1 shows a summary of miRNA-based therapeutic strategies for in vivo models of lung cancer.
3.1 MiRNA Inhibitor-Based Therapeutics
To reduce endogenous miRNA levels, anti-miRs are typically employed. Targeting miRNAs for suppression through the use of anti-miRs is possibly the best-studied modality to date. This approach is conceptually similar to other inhibitory therapeutics that target a single gene product, such as small molecule inhibitors and siRNAs. Various methods have been employed to render anti-miR constructs more stable in vivo and ensure adequate tissue availability and specificity (Krutzfeldt et al. 2005). Constructs can be modified with a cholesterol-conjugated 2′-O-methyl group to inhibit degradation and hence improve stability. Locked nucleic acid (LNA) is an additional method of antisense oligonucleotide modification whereby the 2′ oxygen and 4′ carbon of the nucleotide is bridged with methylene to form a cyclic structure. LNA is more resistant to endogenous nucleases, less toxic, and possess a stronger affinity for the target nucleotide (Elmen et al. 2008; Wahlestedt et al. 2000). Relative to studies on miRNA mimics, studies with antisense oligonucleotides have demonstrated greater efficacy using naked oligonucleotides. Furthermore, the LNA-anti-miR compound was well tolerated in both mice and primates, as no acute or subchronic toxicities in the treated animals were detected (Elmen et al. 2008). Recent data from the first Phase IIa study in patients with chronic HCV infection treated with the LNA-modified anti-miR-122 revealed that this compound was well tolerated and provided continuing viral suppression (Janssen et al. 2013). With regard to lung cancer, anti-miR-150 delivered to lung tumor xenografts in mice caused tumor growth inhibition (Li et al. 2012). Although there are few reports using LNA-anti-miR therapeutics in lung cancer mouse models, their inhibition of miRNA function is an important and widely used approach. Currently, miRNA sponges are a novel approach to miRNA inhibition, and this technology works with multiple complementary 3′-UTR mRNA sites of a specific miRNA (Ebert et al. 2007). MiRNA sponges specifically inhibit miRNAs with a complementary heptameric seed; thus, a single sponge can inhibit an entire miRNA seed family. In fact, the development of lung metastasis in a murine breast cancer model was significantly reduced via inhibition of the MYC driven miR-9 using a miRNA sponge (Ma et al. 2010). Furthermore, the use of miRNA sponges to inhibit miR-31 in a breast cancer model resulted in a significant induction of lung metastasis (Valastyan et al. 2009). Of potential concern is the possibility that the antagonist might also non-specifically bind to other RNAs, resulting in unwanted side effects. Therefore, adequate assessment of the functional effects of miRNA inhibition is of key importance for miRNA inhibitor-based loss-of-function studies and development of miRNA therapeutics. The high potency and metabolic stability of chemically modified anti-miRs highlights the utility of anti-miRs in the development of novel RNAi therapeutic modalities based on lung cancer associated miRNAs.
3.2 MiRNA Mimic-Based Therapeutics
Tumor suppressor miRNAs are responsible for down-regulating oncogenes and are primarily expressed in cancer (Croce 2009). In this context, miRNA replacement strategies have been developed to restore normal cellular expression levels via administration of tumor suppressor miRNA mimics (Bader et al. 2010). MiRNA mimics are synthetic RNA duplexes designed to imitate the endogenous functions of miRNAs. In addition, miRNAs may be unstable as a result of rapid degradation by endogenous nucleases or rapid elimination through renal and hepatic metabolism and extraction upon systemic administration (Bader et al. 2011). Local administration of RNAi-based therapeutics to the target cells is a promising approach to overcome the problems of systemic administration (see next section for details). Similarly, chemical modifications at specific positions or formulations with delivery vectors have been shown to improve stability. Lipid-based and polymer-based nanoparticles reduce the negative electrical charge of RNA nucleotides to promote cell uptake (Wu et al. 2011). Another strategy for efficient delivery of miRNA-based therapeutics is the use of viral vectors (Bonci et al. 2008). Indeed, adenoviral (Esquela-Kerscher et al. 2008) or lentiviral vectors (Kumar et al. 2008) can be used to transfer miRNAs to lung cancer cells. Successful delivery of miRNA-based therapeutics requires patient compliance with the intended delivery route and efficient delivery vectors. This approach has attracted much interest as it provides a novel opportunity to exploit tumor suppressors. The concept of miRNA replacement therapy is best exemplified by let-7 miRNA. Intranasal administration of a let-7 mimic into mouse models of lung cancer significantly reduced tumor growth, suggesting that miRNA replacement therapy is indeed promising (Trang et al. 2010). Based on these successful results, a clinical trial in non-small cell lung cancer using a let-7 based therapy will begin in the near future. As an additional example of the value of miRNA replacement strategies, miR-34a-based cancer therapies have powerful potential for clinical use. Both local and systemic delivery of a synthetic miR-34a mimic resulted in accumulation of miR-34a in the tumor tissue and inhibition of lung tumor growth. MiRNA therapeutics will initiate clinical trials of miR-34a mimics in 2013, making these mimics some of the first miRNA mimics to reach the clinic. Thus, the pharmacological delivery of miRNA mimics effectively inhibits tumor growth by targeting multiple genes. However, it is necessary to pay attention to any potential toxicities in normal tissues, given that therapeutic delivery of miRNA mimics can lead to an accumulation of exogenous miRNAs in normal cells. It will be important to investigate miRNA mimic-induced effects in normal cells and carefully assess the resultant toxicity before using such therapies in clinical practice.
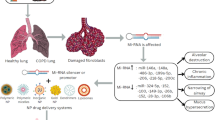
4 Pulmonary Delivery of RNAi-Based Therapeutics
Despite the promise of miRNAs in cancer therapy, there are still hurdles to clear before clinical use, including safety, stability and successful delivery of therapeutic miRNAs to the appropriate tissue and into the appropriate cells. In general, the delivery of miRNAs can be achieved through systemic administration (via intravenous injection) or local administration (via a direct route). Conceptually, systemic delivery is an attractive option because it provides a simple route for miRNA administration to all tissues via the blood stream (Liu et al. 2007). Indeed, there have been some successful reports using systemic delivery of miRNAs in lung cancer models. Nevertheless, this approach has more in vivo barriers to overcome, in addition to nuclease degradation. The delivery barriers are (i) renal clearance of molecules (<50 kDa), (ii) uptake by phagocytic immune cells, (iii) failure of molecules >5 nm in diameter to cross the capillary endothelium, (iv) limited passage through the extra-cellular matrix (polysaccharides and fibrous proteins), (v) inefficient endocytosis by target tumor cells, and (vi) inefficient endosomal release (Bader et al. 2011). Chemical modification and formulation with delivery vectors have been shown to improve stability and delivery to target tumor cells, but these alterations may attenuate the suppressive activity of oligonucleotides (Chernolovskaya and Zenkova 2010). In addition, systemic delivery of miRNAs may induce adverse events similar to those reported for other oligonucleotide-based therapies, such as aggregation and complement activation, liver toxicity and stimulation of the immune response (Kleinman et al. 2008). For these reasons, local administration of miRNAs to the target cancer cells is a promising approach to overcome the problems of systemic administration. Translation of locally administered modalities to the clinical setting is dependent upon the development of an efficient delivery system that is able to improve the pharmacokinetic and biodistribution properties of miRNAs. Thus far, locally administered modalities are available for ocular, transdermal, rectal and pulmonary delivery.
Dozens of RNAi-based therapeutics are being assessed in preclinical and clinical trials, and these studies provide further opportunities for successful results (Davidson and McCray 2011). Many of these studies are conducted using local administration to specific tissues. The lung is anatomically accessible to therapeutic drugs via the pulmonary route. Accessibility is a key requirement for successful RNAi-based in vivo and clinical studies, and this anatomical characteristic offers several important benefits over systemic delivery, including the use of lower doses of miRNAs, the reduction of undesirable systemic side effects, and improved miRNA stability due to reduced nuclease activity in the airways compared to serum. The local approach could potentially enhance the retention of RNAi-based therapeutics in the lungs. Because the delivery of siRNAs to the lungs is well studied using different routes and delivery strategies (Lam et al. 2012), many technologies developed for siRNAs may also be applicable to miRNAs. In most of the pulmonary RNAi-based therapy studies in vivo, agents were delivered intratracheally or intranasally. This approach has allowed remarkable progress in miRNA modulation in preclinical cancer models, bringing us closer to delivering on the promise of miRNAs as cancer therapeutics.
5 Strategies for Pulmonary Delivery of MicroRNA-Based Therapeutics
Pulmonary delivery approaches are very attractive because they tend to be non-invasive, locally restricted, and administered by the patient. With regard to siRNA-based therapeutics, Phase II clinical trials are underway for the treatment of respiratory syncytial virus (RSV) infection using an intranasal application of naked, chemically modified siRNA molecules that target viral gene products (DeVincenzo et al. 2008, 2010). To date, two successful studies of pulmonary delivery of miRNA-based therapeutics for lung cancer mouse models have been reported (Kumar et al. 2008; Esquela-Kerscher et al. 2008). These studies show that pulmonary delivery of miRNA from the let-7 family reduces lung tumor formation in an orthotopic lung cancer mouse model without systemic side effects (Table 17.1). These data suggest that intranasal or intratracheal administration of miRNAs may be a potent strategy for treating lung cancer. Although there are no reports of pulmonary delivery of miRNA-inhibitors in lung cancer at present, we predict that this delivery strategy will become a valuable resource for implementing miRNA-based therapies in vivo and in humans.
We believe that pulmonary delivery of miRNAs has two primary advantages over systemic delivery for clinical use. First, several sophisticated inhalation devices for lung diseases are already in clinical use. Inhaled therapeutics are used routinely to treat a variety of pulmonary conditions, including asthma, chronic obstructive pulmonary disease (COPD) and cystic fibrosis. Metered-dose inhalers (MDIs) and dry powder inhalers (DPIs) are the most common modes of inhaled delivery. The use of DPIs for the in vivo delivery of therapeutic macromolecules such as insulin (Mastrandrea and Quattrin 2006) and low-molecular-weight heparin (Bai et al. 2010) has yielded promising results. Currently, the use of spray-drying as a technique for engineering dry powder formulations of siRNA nanoparticles, which might allow the pulmonary delivery of biologically active siRNAs directly to the lung tissue, has been demonstrated (Jensen et al. 2010, 2012). Although a suitable carrier is also needed to protect miRNAs from degradation given the shear force and increased temperature of the drying process, these delivery technologies could open new avenues for pulmonary delivery of miRNAs and improve patient outcome. To make miRNA-based therapy practical in the treatment of lung cancer, we believe that the administration of inhaled miRNAs by DPIs is the best of choice of delivery strategy. Second, pulmonary delivery also offers the clinical benefit of a lower miRNA dose. The cost related to the development and application of a particular RNAi therapeutic delivery technology is undoubtedly an important factor (Dykxhoorn et al. 2006). Local administration is likely to be a more cost-efficient strategy for miRNA delivery in vivo and in the clinic than systemic administration. Furthermore, the advantage of pulmonary delivery is that it ensures high delivery efficiency with minimal drug loss. For this reason, pulmonary delivery of miRNAs has great potential for clinical use. However, the limitations of pulmonary delivery of miRNA-based therapeutics are important to consider. First, the pharmacokinetics of inhaled miRNAs in in vivo models and humans are estimated inaccurately. It is also unknown whether miRNA-based therapeutics delivered via the intrapulmonary route could also be delivered to other organs, such as the liver and kidneys. To prevent systemic side effects, the precise pharmacokinetics of miRNAs after intrapulmonary administration should be measured. Second, we also must pay attention to the pulmonary inflammatory and toxicological responses caused by the delivery vehicle. In fact, there are some reports that RNAi-based therapeutics with polyethyleneimine (PEI) frequently cause inflammatory responses in the lungs (Beyerle et al. 2011). It has been reported that naked RNAi-therapeutic delivery possesses advantages over other delivery vectors, such as reduced toxicity and reduced inflammatory responses, as well as simple formulation (Heidel et al. 2004). However, the advantage of naked RNAi-therapeutics over delivery vectors in the treatment of lung diseases is controversial (Nielsen et al. 2010; Akinc et al. 2008). Therefore, we need to develop safer delivery technology for practical use in in vivo mouse models and humans.
6 Conclusions
During the past decade, miRNAs have quickly advanced from discovery to therapeutic development programs. This rapid progress reflects the importance of miRNA biology in cancer, leaving little doubt about the therapeutic potential of miRNAs in cancer treatment. Given the encouraging results of the profiled studies and preclinical testing, miRNAs are being integrated into human clinical trials. The first miRNA-targeted drug LNA-anti-miR-122 is successfully undergoing Phase II trials (Janssen et al. 2013). Accordingly, several companies are currently developing miRNA mimics or inhibitors for the treatment of cancer. The main focus in bringing miRNAs to cancer cells is the capacity of pharmacological drug delivery. The success of miRNA-based therapeutic delivery requires efficiency, convenience, and patient compliance using the delivery route. In this chapter, we showed that pulmonary delivery of miRNA-based therapeutics holds powerful potential for lung cancer treatment (Fig. 17.1). A realistic therapeutic intervention, such as inhalation, would enhance drug delivery to the site of action and decrease systemic exposure, thereby reducing off-target effects. In the future, combining miRNA-based therapeutics with chemotherapy may potentiate the cancer treatment efficacy. Therefore, continued investigation on all fronts will be of equal importance to the eventual clinical application of miRNAs.
References
Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N et al (2008) A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol 26(5):561–569
Babashah S, Soleimani M (2011) The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. Eur J Cancer 47(8):1127–1137
Bader AG (2012) miR-34 – a microRNA replacement therapy is headed to the clinic. Front Genet 3:120
Bader AG, Brown D, Winkler M (2010) The promise of microRNA replacement therapy. Cancer Res 70(18):7027–7030
Bader AG, Brown D, Stoudemire J, Lammers P (2011) Developing therapeutic microRNAs for cancer. Gene Ther 18(12):1121–1126
Bai S, Gupta V, Ahsan F (2010) Inhalable lactose-based dry powder formulations of low molecular weight heparin. J Aerosol Med Pulm Drug Deliv 23(2):97–104
Beyerle A, Braun A, Merkel O, Koch F, Kissel T, Stoeger T (2011) Comparative in vivo study of poly(ethylene imine)/siRNA complexes for pulmonary delivery in mice. J Control Release 151(1):51–56
Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE et al (2007) p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 17(15):1298–1307
Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L et al (2008) The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 14(11):1271–1277
Boudreau RL, Martins I, Davidson BL (2009) Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol Ther 17(1):169–175
Bruno IG, Karam R, Huang L, Bhardwaj A, Lou CH, Shum EY et al (2011) Identification of a microRNA that activates gene expression by repressing nonsense-mediated RNA decay. Mol Cell 42(4):500–510
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S et al (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 101(9):2999–3004
Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65(14):6029–6033
Chen Z, Zeng H, Guo Y, Liu P, Pan H, Deng A et al (2010) miRNA-145 inhibits non-small cell lung cancer cell proliferation by targeting c-Myc. J Exp Clin Cancer Res 29:151
Chernolovskaya EL, Zenkova MA (2010) Chemical modification of siRNA. Curr Opin Mol Ther 12(2):158–167
Chiou GY, Cherng JY, Hsu HS, Wang ML, Tsai CM, Lu KH et al (2012) Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial-mesenchymal transdifferentiation and cancer stem-like properties and in lung adenocarcinoma. J Control Release 159(2):240–250
Cho WC, Chow AS, Au JS (2009) Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur J Cancer 45(12):2197–2206
Cho WC, Chow AS, Au JS (2011) MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol 8(1):125–131
Croce CM (2009) Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 10(10):704–714
Davidson BL, McCray PB Jr (2011) Current prospects for RNA interference-based therapies. Nat Rev Genet 12(5):329–340
DeVincenzo J, Cehelsky JE, Alvarez R, Elbashir S, Harborth J, Toudjarska I et al (2008) Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV). Antiviral Res 77(3):225–231
DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E et al (2010) A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A 107(19):8800–8805
Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E et al (2006) Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet 38(9):1060–1065
Dykxhoorn DM, Palliser D, Lieberman J (2006) The silent treatment: siRNAs as small molecule drugs. Gene Ther 13(6):541–552
Ebert MS, Neilson JR, Sharp PA (2007) MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4(9):721–726
Ebi H, Sato T, Sugito N, Hosono Y, Yatabe Y, Matsuyama Y et al (2009) Counterbalance between RB inactivation and miR-17-92 overexpression in reactive oxygen species and DNA damage induction in lung cancers. Oncogene 28(38):3371–3379
Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S et al (2008) LNA-mediated microRNA silencing in non-human primates. Nature 452(7189):896–899
Esquela-Kerscher A, Trang P, Wiggins JF, Patrawala L, Cheng A, Ford L et al (2008) The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle 7(6):759–764
Feng S, Cong S, Zhang X, Bao X, Wang W, Li H et al (2011) MicroRNA-192 targeting retinoblastoma 1 inhibits cell proliferation and induces cell apoptosis in lung cancer cells. Nucleic Acids Res 39(15):6669–6678
Fujita Y, Takeshita F, Kuwano K, Ochiya T (2013) RNAi therapeutic platforms for lung diseases. Pharmaceuticals 6(2):223–250
Gao W, Yu Y, Cao H, Shen H, Li X, Pan S et al (2010) Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed Pharmacother 64(6):399–408
Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S et al (2005) A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res 65(21):9628–9632
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S et al (2005) A microRNA polycistron as a potential human oncogene. Nature 435(7043):828–833
He XY, Chen JX, Zhang Z, Li CL, Peng QL, Peng HM (2010) The let-7a microRNA protects from growth of lung carcinoma by suppression of k-Ras and c-Myc in nude mice. J Cancer Res Clin Oncol 136(7):1023–1028
Heidel JD, Hu S, Liu XF, Triche TJ, Davis ME (2004) Lack of interferon response in animals to naked siRNAs. Nat Biotechnol 22(12):1579–1582
Hermeking H (2010) The miR-34 family in cancer and apoptosis. Cell Death Differ 17(2):193–199
Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K et al (2013) Treatment of HCV infection by targeting microRNA. N Engl J Med 368(18):1685–1694
Jensen DM, Cun D, Maltesen MJ, Frokjaer S, Nielsen HM, Foged C (2010) Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J Control Release 142(1):138–145
Jensen DK, Jensen LB, Koocheki S, Bengtson L, Cun D, Nielsen HM et al (2012) Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J Control Release 157(1):141–148
Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D et al (2007) The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res 67(16):7713–7722
Kim NH, Kim HS, Li XY, Lee I, Choi HS, Kang SE et al (2011) A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol 195(3):417–433
Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ et al (2008) Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452(7187):591–597
Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M et al (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438(7068):685–689
Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39(5):673–677
Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA et al (2008) Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci U S A 105(10):3903–3908
Lam JK, Liang W, Chan HK (2012) Pulmonary delivery of therapeutic siRNA. Adv Drug Deliv Rev 64(1):1–15
Lee YS, Dutta A (2007) The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev 21(9):1025–1030
Leidinger P, Keller A, Meese E (2011) MicroRNAs – important molecules in lung cancer research. Front Genet 2:104
Li YJ, Zhang YX, Wang PY, Chi YL, Zhang C, Ma Y et al (2012) Regression of A549 lung cancer tumors by anti-miR-150 vector. Oncol Rep 27(1):129–134
Liu N, Ding H, Vanderheyden JL, Zhu Z, Zhang Y (2007) Radiolabeling small RNA with technetium-99m for visualizing cellular delivery and mouse biodistribution. Nucl Med Biol 34(4):399–404
Liu X, Sempere LF, Galimberti F, Freemantle SJ, Black C, Dragnev KH et al (2009) Uncovering growth-suppressive microRNAs in lung cancer. Clin Cancer Res 15(4):1177–1183
Liu X, Sempere LF, Ouyang H, Memoli VA, Andrew AS, Luo Y et al (2010) MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest 120(4):1298–1309
Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH et al (2008) MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 27(31):4373–4379
Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D et al (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12(3):247–256
Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Georgoulias V, Lianidou ES (2008) Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem 54(10):1696–1704
Mastrandrea LD, Quattrin T (2006) Clinical evaluation of inhaled insulin. Adv Drug Deliv Rev 58(9–10):1061–1075
Mudduluru G, Ceppi P, Kumarswamy R, Scagliotti GV, Papotti M, Allgayer H (2011) Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene 30(25):2888–2899
Nielsen EJ, Nielsen JM, Becker D, Karlas A, Prakash H, Glud SZ et al (2010) Pulmonary gene silencing in transgenic EGFP mice using aerosolised chitosan/siRNA nanoparticles. Pharm Res 27(12):2520–2527
Rai K, Takigawa N, Ito S, Kashihara H, Ichihara E, Yasuda T et al (2011) Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptor tyrosine kinase inhibitor-resistance in lung cancer cells. Mol Cancer Ther 10(9):1720–1727
Ramalingam SS, Owonikoko TK, Khuri FR (2011) Lung cancer: new biological insights and recent therapeutic advances. CA Cancer J Clin 61(2):91–112
Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N et al (2007) Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26(5):731–743
Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE et al (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403(6772):901–906
Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I et al (2009) MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci U S A 106(29):12085–12090
Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H et al (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64(11):3753–3756
Trang P, Medina PP, Wiggins JF, Ruffino L, Kelnar K, Omotola M et al (2010) Regression of murine lung tumors by the let-7 microRNA. Oncogene 29(11):1580–1587
Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D et al (2011) Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther 19(6):1116–1122
Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC et al (2009) A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 137(6):1032–1046
Vasudevan S, Tong Y, Steitz JA (2007) Switching from repression to activation: microRNAs can up-regulate translation. Science 318(5858):1931–1934
Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F et al (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103(7):2257–2261
Vosa U, Vooder T, Kolde R, Vilo J, Metspalu A, Annilo T (2013) Meta-analysis of microRNA expression in lung cancer. Int J Cancer 132(12):2884–2893
Wahlestedt C, Salmi P, Good L, Kela J, Johnsson T, Hokfelt T et al (2000) Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc Natl Acad Sci U S A 97(10):5633–5638
Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D et al (2010) Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res 70(14):5923–5930
Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11(3):228–234
Wong MY, Yu Y, Walsh WR, Yang JL (2011) microRNA-34 family and treatment of cancers with mutant or wild-type p53 (Review). Int J Oncol 38(5):1189–1195
Wu Y, Crawford M, Yu B, Mao Y, Nana-Sinkam SP, Lee LJ (2011) MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol Pharm 8(4):1381–1389
Wu Y, Crawford M, Mao Y, Lee RJ, Davis IC, Elton TS et al (2013) Therapeutic delivery of microRNA-29b by cationic lipoplexes for lung cancer. Mol Ther Nucleic Acids 2:e84
Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF et al (2009) MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 23(18):2166–2178
Xiong S, Zheng Y, Jiang P, Liu R, Liu X, Chu Y (2011) MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int J Biol Sci 7(6):805–814
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 105(36):13421–13426
Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M et al (2006) Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9(3):189–198
Yu SL, Chen HY, Chang GC, Chen CY, Chen HW, Singh S et al (2008) MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell 13(1):48–57
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH (2010) MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta 411(11–12):846–852
Acknowledgements
This work was supported in part by a grant-in-aid for the Third-Term Comprehensive 10-Year Strategy for Cancer Control of Japan; Project for Development of Innovative Research on Cancer Therapeutics (P-Direct); Scientific Research on Priority Areas Cancer, Scientific Research on Innovative Areas (“functional machinery for non-coding RNAs”) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; the National Cancer Center Research and Development Fund (23-A-2, 23-A-7, 23-C-6,); the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NiBio), the Project for Development of Innovative Research on Cancer Therapeutics; and the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program)” initiated by the Council for Science and Technology Policy (CSTP).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Fujita, Y., Kuwano, K., Ochiya, T. (2014). Challenges and Strategies for Pulmonary Delivery of MicroRNA-Based Therapeutics. In: Babashah, S. (eds) MicroRNAs: Key Regulators of Oncogenesis. Springer, Cham. https://doi.org/10.1007/978-3-319-03725-7_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-03725-7_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03724-0
Online ISBN: 978-3-319-03725-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)