Abstract
We select a chemical magnetohydrodynamic simulation of molecular cloud formation and evolution as a typical example of a Galactic molecular cloud. Its analysis helps us understand how to interpret temperatures and densities inferred from CO line emission maps. We find that the kinetic temperature is always underestimated if it is inferred only from the excitation temperature, T ex, of the12CO(1–0) emission line. We find also that CO primarily traces material at densities above the mean cloud density. In addition, we show that if one assumes a fixed value for the CO–H2 conversion factor, then one will underestimate the density (and hence the mass) of H2 at low column densities. In this scenario, the total H2 mass of the cloud inferred from the emission map is only 60 % of the true mass.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Molecular Cloud Formation
- Line Emission Maps
- Emission Map
- Excitation Temperature
- Increasing Column Density
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Our simulation considers magnetized turbulent gas in a periodic box, and follows the chemical, thermal and dynamical evolution [1, 2]. It is characterized by a mean number density of 100 cm−3, solar metallicity, volume (20 pc)3, and a turbulent RMS velocity of 5 km/s. We present results after three turbulent crossing times, when the turbulence has reached a statistical steady state. We use the Monte Carlo radiative transfer code RADMC-3DFootnote 1 to calculate the emergent CO line intensity [3]. Our aim here is to quantify the range of temperatures and densities where CO could be a good tracer of the physical conditions of the cloud.
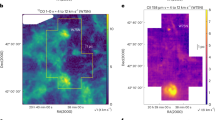
Figure 1b shows the 2D Probability Density Function (PDF) of the excitation temperature (T ex) calculated from the12CO(1–0) population levels. We see that the excitation temperature increases with increasing column density. On the other hand, the mean kinetic temperature at the CO (\(\langle T_{K}\rangle\)) starts with a high value but then decreases with increasing column density. The two values approach each other only at high column densities, N ≳ 1022 cm−2.
(a) True H2 column density. (b) 2D PDF of the projected T ex vs. N, with reference to A V . Cyan line: average of the T ex, white line: CO mass weighted average along the LoS of T K . (c) 2D PDF of the H2 column density estimated from W CO vs. the true H2 column density. Yellow line: one-to-one relation. Red line: best fit to the low density gas. (d) Cumulative mass of H2 – normalized by the total mass of H2. Dotted line: inferred from W CO. Solid line: true H2 mass fraction
In Fig. 1c, we show the 2D PDF of the H2 column density (\(N_{\mathrm{H2,est}}\)), estimated from the integrated emission map (W CO), with a fixed CO–H2 conversion factor \(X_{\mathrm{CO}} = 2 \times 1{0}^{20}\) cm−2 K−1 km−1 s, and the true N H2 (Fig. 1a). The values agree only at high densities, where the gas is optically thick. At low densities, the relationship lies far from the one-to-one relation. The cumulative mass of H2 – normalized by the total mass of H2 – as a function of N is shown in Fig. 1d. We compare the mass estimated from the emission map with true mass. The H2 mass derived from W CO is an underestimate at all densities. In total, the H2 mass estimated from the W CO map, and considering a fixed CO–H2 conversion factor is only ∼ 60 % of the true value.
These results suggest that CO observations alone give a misleading view of the physical properties of molecular clouds. Complementary observations of the lower density gas using tracers such as C or C+, are required.
References
Glover, S. C. O., Federrath, C., Mac Low, M.-M., and Klessen, R. S. MNRAS, 404, 2 (2010)
Glover, S. C. O. and Mac Low, M.-M. MNRAS, 412, 337 (2011)
Shetty, R., Glover, S. C. O., Dullemod, C.-P., and Klessen, R. S. MNRAS, 412, 1686 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this paper
Cite this paper
Molina, F.Z., Glover, S.C.O., Shetty, R., Klessen, R.S. (2014). Can We Trust CO as a Probe of the Densities and Temperatures of Molecular Clouds?. In: Stamatellos, D., Goodwin, S., Ward-Thompson, D. (eds) The Labyrinth of Star Formation. Astrophysics and Space Science Proceedings, vol 36. Springer, Cham. https://doi.org/10.1007/978-3-319-03041-8_57
Download citation
DOI: https://doi.org/10.1007/978-3-319-03041-8_57
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03040-1
Online ISBN: 978-3-319-03041-8
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)