Abstract
Virtually all of the major treatment options for migraine can be categorized as either acute or preventive in nature. Acute treatments are those used on an as-needed basis and designed to either abort or reduce the severity of a current headache attack. Preventive treatments, by comparison, are typically used on a daily basis and intended to reduce the likelihood of future headache episodes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Virtually all of the major treatment options for migraine can be categorized as either acute or preventive in nature. Acute treatments are those used on an as-needed basis and designed to either abort or reduce the severity of a current headache attack. Preventive treatments, by comparison, are typically used on a daily basis and intended to reduce the likelihood of future headache episodes.
4.2 Acute Medications
Acute migraine pharmacotherapy targets a current migraine attack with the aim of returning the patient to normal functioning as soon as possible. Acute treatment can be divided into migraine-specific and nonspecific agents. The migraine-specific agents are those with efficacy for migraine but not other forms of chronic pain. Specific acute migraine agents include the various triptans, ergotamine, and dihydroergotamine (DHE). Nonspecific agents include nonsteroidal anti-inflammatory drugs (NSAIDs), combination analgesics, opioid analgesics, corticosteroids, and antiemetics.
4.2.1 Basic Principles of Acute Pharmacotherapy
Virtually all migraine patients should be offered acute pharmacotherapy, including those with very infrequent attacks. These treatments are most effective when used in the developing stages of the migraine attack (within less than an hour of headache onset), versus delaying treatment once pain and other symptoms become severe. Unlike the preventive agents, in which the dosage is started low and gradually increased as needed, acute medications are given more aggressively; these agents are administered at a high effective dose and lowered if there are side effects or safety concerns. In clinical trials of acute migraine pharmacotherapies, the primary outcome of interest usually is the percentage of patients who are pain-free after 2 h. The principles of acute migraine pharmacotherapy include:
-
Virtually all patients should be offered acute treatment.
-
Overall treatment goal is freedom from pain and returning to functioning within 2 h.
-
Use a high effective dose; decrease dose if there are side effects or safety concerns.
-
Acute treatments work best if taken within 30–40 min of headache onset.
-
Stratify treatment based on the illness severity/disability.
-
Limit use of acute medications to no more than 2 days/week.
-
Non-oral routes are indicated for patients with vomiting/significant nausea or gastroparesis.
-
Avoid prescribing opioids/barbiturates except as a last resort.
-
Provide the patient clear instructions on how and when to use the medication.
-
At follow-up sessions, query the patient about how she used the medication (normalizing nonadherence helps elicit honest answers).
Acute treatment should be individualized with consideration of the patient’s prior drug response, comorbid medical conditions, concurrent medications, and potential for medication overuse. Patients should be cautioned about the role of overusing any acute headache medications (even over-the-counter [OTC] simple analgesics), or combinations of different acute agents, in making headache more frequent over time. Not only does frequent use of acute agents increase headache activity, but it also makes the patient less responsive to triptans. Use of acute agents should be limited to no more than 2 days per week, on average, although up to 3 days per week may be allowed for simple analgesics.
Acute treatment is most effective when stratified by the individual’s migraine severity, which may be operationalized as the patient’s level of headache-related disability on the Migraine Disability Assessment (MIDAS) questionnaire or Headache Impact Test (HIT-6). Stratified care as a function of illness severity is more efficacious in reducing pain and disability than stepped (escalating) care either across or within attacks, as stepped care can delay optimal treatment [1]. Patients with mild/infrequent disability are appropriate candidates for nonspecific acute treatment, while migraine-specific agents are indicated for those with moderate or severe disability. Triptans represent the standard of care; there is also strong evidence for DHE as an acute treatment if triptans fail [2]. Antiemetics are useful as supplementary treatment when vomiting or severe nausea is present. Parenteral routes are recommended for patients with vomiting/significant nausea or to confer more rapid symptom relief among those with allodynia.
Improper adherence to acute medications is a leading cause of failed acute therapy, much of which stems from poor patient–provider communication. Educate the patient about the rationale for selecting a given agent and provide clear instructions regarding limits on frequency of use and appropriate timing of taking the medication. If a patient reports inadequate response to an acute agent, before altering the treatment plan, she should be queried as to whether she used the medication properly. A common reason for poor response is waiting too late in the headache episode to administer the medication, at which point triptans have limited efficacy because central sensitization has already manifested within the attack. For this reason, patients should be discouraged from waiting to determine if a developing headache will progress to a migraine. Instead, they should be instructed to take the acute agent when pain is mild.
The goal for acute treatment is freedom from pain and a return to functioning within 2 h. “Rescue” or backup medications are used in the event that acute treatment fails. Rescue agents may not completely eliminate pain, but they provide some relief and can help avoid an emergency hospital visit. Injectable sumatriptan and DHE, as well as parenteral NSAIDs, are common rescue medications; dopamine antagonists are used if vomiting or severe nausea remains. However, neither triptans nor ergots should be used as recue medications for each other, as they interact. If the patient responds to the initial triptan but headache recurs within 24 h, a second dose is often effective in treating recurrence. Table 4.1 denotes the proven efficacious acute medications for migraine as reported in a recent evidence-based assessment by the American Headache Society [2].
4.2.2 Triptans
Triptans, selective serotonin (5HT1B/1D) agonists, are the only class of drug specifically developed to treat migraine, and their selectivity in targeting migraine pathophysiology is superior to other agents. Sumatriptan was the first to be developed and was released under the brand name Imitrex in the early 1990s. Subsequently numerous other triptans were developed by other pharmaceutical companies: naratriptan, zolmitriptan, rizatriptan, almotriptan, eletriptan, and frovatriptan. Sumatriptan is also available in a combination formulation with naproxen, which confers greater rates of sustained 24 h pain freedom than sumatriptan alone but is not yet available in generic form and thus costly for many patients. (As a result many providers instead prescribe both generic sumatriptan and naproxen to be taken simultaneously, for patients with long-lasting attacks.) The triptans presumably are efficacious as a result of their action in constricting cerebral blood vessels and reducing nociception in trigeminovascular pain pathways.
Speed of onset and route of administration are usually the most important determinants in selecting a triptan. All triptans are available in tablet form; sumatriptan has the widest range of available formulations. Non-oral preparations (injection, nasal spray, transdermal patch, suppository) should be the first choice for patients with severe nausea or any vomiting and considered for patients with poor gastric motility (i.e., gastroparesis). These parenteral routes of administration confer more rapid onset as they do not have to first pass through the stomach. Intranasal acute treatments should be done with the head upright, and the patient should not attempt to sniff the medicine down the nose or tip the head back, as this reduces efficacy.
Though the triptans share more commonalities than differences, a recent meta-analysis quantified the relative efficacy of the various triptan oral tablet formulations across 74 randomized trials [3]. The primary endpoints were pain-free response at 2 h and sustained pain-free response at 24 h, expressed in odds ratios (ORs) of triptan versus placebo. All triptans outperformed placebo and most were 2–4 times more likely to yield pain-free responses at the endpoints (Fig. 4.1) [3]. As compared to one another, at the 2 h time point, eletriptan 40 mg was superior to sumatriptan 50 mg, almotriptan 2.5 mg, zolmitriptan 12.5 mg, and naratriptan 2.5 mg (ORs 1.5–2.9 for pain freedom vs. the other triptans). Rizatriptan and zolmitriptan had the next best efficacy at 2 h. Eletriptan 40 mg was also superior to sumatriptan 50 mg, rizatriptan 10 mg, and naratriptan 2.5 mg on pain freedom at 24 h (ORs ranged from 1.6 to 1.8) [3].
Forest plot of the primary multiple treatment comparison meta-analysis results, triptans versus placebo. (a) Pain-free response at 2 h; (b) 24 h sustained pain-free response (Reproduced with permission from Thorlund et al. [3] ©SAGE)
Thus, although eletriptan is most likely to yield a pain-free response at either time point, all triptans are superior to placebo, and rizatriptan and zolmitriptan also produced comparatively favorable pain-free responses [3]. Frovatriptan and naratriptan have somewhat lower efficacy but are longer-lasting (particularly frovatriptan, which has a 26 h half-life) and thus often used for menstrual migraine prevention or attacks typically lasting >24 h. As this review only assessed oral formulations, comparisons with non-oral routes of administration cannot be made, though another recent meta-analysis concluded that alternative routes of administration (injection, orally disintegrating tablet) compare favorably to standard oral tablets [4].
The majority of patients experience pain relief 2 h after a standard dose of triptan [4]. A patient who does not respond to one triptan usually should be tried on at least one other triptan, or using different route of administration, before resorting to another class of acute medication, as the large majority of migraine patients will respond to at least one triptan. As mentioned earlier, patients with a brief or incomplete response to sumatriptan can take naproxen simultaneously with sumatriptan, which often promotes a more long-lasting or complete response.
Triptans are relatively safe, but because of their vasoactive properties, they are contraindicated in patients with cardiovascular disease and related conditions (e.g., obesity, uncontrolled hypertension, severe liver disease). Possibility of serotonin syndrome must be considered among patients taking a triptan and another serotonin agonist, although the risk of serotonin syndrome from taking a triptan and a newer antidepressant (SSRI or SNRI) appears incredibly low, in large part because these agents act on different serotonin receptor subtypes [5].
4.2.3 Ergotamine Derivatives
Ergotamine tartrate and DHE are migraine-specific agents that are serotonin agonists with vasoconstrictive effects. DHE has poor oral bioavailability but has strong evidence for acute migraine treatment in intranasal form. Rhinitis is a common side effect of intranasal DHE. DHE injection and ergotamine/caffeine tablets have moderate efficacy for migraine [2]. Efficacy for ergot alkaloids is thus not as strong as for the triptans [4] and side effects are more significant; ergotamine derivatives are generally used in patients with severe migraines that have not responded to adequate triptan therapy.
Nausea and vomiting are common side effects, particularly of intravenous DHE, which can be reduced by intramuscular injection instead or by adding an antiemetic. Contraindications include vascular conditions (e.g., heart disease, coronary artery vasospasm, uncontrolled hypertension) as well as renal or hepatic failure, pregnancy, and in patients using potent CYP3A4 enzyme inhibitors (e.g., protease inhibitors, macrolide antibiotics such as erythromycin). Life-threatening peripheral ischemia is associated with use of ergot alkaloids and CYP3A4 inhibitors. Ergotamine, DHE, and triptans should not be taken within 24 h of one another.
4.2.4 Nonsteroidal Anti-inflammatory Drugs and Combination Analgesics
For infrequent migraine attacks that are not severe or that evolve slowly, NSAIDs and combinations represent a reasonable first-line treatment. The most common side effects of NSAIDs are gastrointestinal in nature (bloating, dyspepsia, ulcers, gastrointestinal bleeding), which can often be relieved by taking with food. In some patients NSAIDs raise blood pressure, and they can interfere with the efficacy of antihypertensive drugs.
The most commonly used OTC combination analgesic is acetaminophen–aspirin–caffeine (500 mg/500 mg/130 mg). Caffeine potentiates the analgesic effects of the other compounds, promotes gastric motility, and has vasoconstrictive effects. In addition to elevating risk for medication overuse headache (MOH), frequent use of caffeine-containing combination analgesics can cause caffeine withdrawal headaches, tachycardia, and insomnia.
4.2.5 Opioid Analgesics and Butalbital
Both the American Academy of Neurology and American Headache Society recommend against use of opioids or butalbital for treating migraine except among patients who have not responded to other interventions [6, 7]. These medications are not as effective for migraine or migraine disability as the migraine-specific agents and portend the greatest risks for both dependency/abuse and progression to chronic headache (CM) or MOH [8].
Common side effects include sedation, cognitive slowing, dizziness, nausea, and constipation. Patients who claim that opioids do not produce these side effects and are the only medication that reduces their migraine are likely showing tolerance from overuse or demonstrating drug-seeking behavior. Patients with any history of substance use problems should not be prescribed opioids nor should patients on an MAOI antidepressant.
4.2.6 Acetaminophen
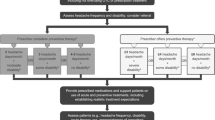
The evidence for acetaminophen is not as strong as that for the NSAIDs or combination analgesics, and thus, acetaminophen is not recommended as a first-line agent. A summary of migraine treatments in nonemergency settings is seen in Fig. 4.2.
Migraine treatment algorithm in nonemergency settings. DHE dihydroergotamine, NSAID nonsteroidal anti-inflammatory drugs. *SNOOP Systemic disease or symptoms, Neurological signs or symptoms, Onset is sudden, Onset in a patient over 50 years of age, Pattern change in headache presentation; **For vomiting and severe nausea, consider adding an antiemetic or using parenteral administration
4.2.7 Acute Treatment of Migraine in Emergency Settings
Most patients who present with migraine in emergency medical settings have been experiencing severe pain for a prolonged period and have already tried an abortive medication. (If not, an appropriate abortive medication is usually indicated.) Some suffer from status migrainosus, in which a debilitating migraine attack lasts longer than 72 h. Because the severity and duration of the attack are atypical, secondary causes (including medication overuse) must be ruled out in cases of status migrainosus. Other priorities of ER treatment are treating nausea, dehydration, and residual pain.
Often migraine patients in the emergency setting present with frequent vomiting or are otherwise already dehydrated. Initiating liberal intravenous fluid replacement is indicated, both to combat dehydration and to provide some protection for administration of subsequent agents [9]. Off-label parenteral administration of dopamine antagonists with antiemetic properties, such as chlorpromazine, prochlorperazine, and metoclopramide, are useful for rapid reduction of acute migraine with vomiting or significant nausea. In cases of status migrainosus or when further treatment is needed, DHE and subcutaneous sumatriptan are efficacious treatment options, followed by parenteral NSAIDs [9]. Patients unresponsive to this combination may require hospitalization to undergo repeated intravenous DHE.
Opioids should not be used as first-line agents even in most emergency settings, as these agents are less efficacious as and interfere with other acute medications, have significant side effects, and portend increased risk for repeated emergency hospital visits and development of MOH. Strikingly, many physicians in emergency hospital settings continue to dispense opioids to migraineurs on a routine basis [10]. Patients presenting to emergency hospital settings should be provided a referral to a neurologist with expertise in headache for regular continued care and detailed evaluation of their headache problem.
4.3 Preventive Medications
Preventive agents are those taken regularly, usually daily, to prevent future headache attacks. The most well-established medications for migraine prevention are tricyclic antidepressants, beta-blockers, and antiepileptics, each of which was developed to treat a condition other than migraine. Discovery of their utility in migraine was largely serendipitous, but numerous clinical trials have confirmed their efficacy for migraine prevention.
4.3.1 Basic Principles of Preventive Pharmacotherapy
Preventive pharmacotherapy is indicated for migraine sufferers with 6 or more headache days per month or who have at least 3 headache days per month with significant functional impairment (rule of thumb: one migraine/week merits consideration for preventive therapy). Preventive treatment may be considered in patients who fall shy of these thresholds but are not indicated for those with ≤3 headache days/month without impairment or those with only one headache/day per month regardless of impairment [11]. These recommendations come from the American Migraine Prevalence and Prevention (AMPP) Advisory Group and were developed for epidemiologic research, and thus clinical considerations may dictate alteration in some cases.
All patients requiring preventive treatment should also be provided acute treatment, usually with triptans if appropriate, with clear limits on frequency of their use. Those requiring preventive treatment should be queried carefully about their current use of any acute agents (including OTC medications), as ongoing medication overuse will limit their responsiveness to preventive pharmacotherapy and likely require supervised withdrawal from the offending medication before efficacy with a preventive agent can be obtained. Preventive pharmacotherapies are not a recognized cause of MOH.
The primary determinants of selecting a preventive medication are based on efficacy for migraine, potential side effects and drug interactions, and comorbid medical conditions. The chosen preventive drug should be started slowly and titrated up to the lowest efficacious dose (“start low, go slow”). Most preventive agents require an adequate trial of 2–3 months to assess treatment response, during which time the patient can monitor headache activity and medication use in a headache diary. Full effect may not be evident for as long as 6 months. Patients should be advised not to stop taking the medication if their headaches begin to improve, as this is a common cause of relapse. If the patient has a sustained strong response, considering discontinuing or tapering the medication may be appropriate. The principles of preventive migraine pharmacotherapy include:
-
Treatment goal are reduction in attack frequency (≥50 %) and disability.
-
Indicated for those with 3 or more headache days/month with impairment (or 6 or more days without impairment).
-
Start dosage low and increase slowly if warranted (“start low, go slow”).
-
Treatment response may not be evident for several months.
-
Caution the patient not to stop taking the medication if headaches improve.
-
Instruct patient to use a headache diary to monitor efficacy.
-
Consider tapering/discontinuing preventive medication if migraine is well controlled after 6 months.
-
Separate agent will often be required to treat comorbid conditions.
In preventive trials, the main outcome of interest is proportion of patients with ≥50 % reduction in frequency of headache attacks. In clinical settings, reductions in headache frequency and headache-related disability are primary outcomes, though reductions in headache severity, headache duration, and decreased usage of or increased responsiveness to acute medications are also of importance.
Although ideally one drug could be used to treat both migraine and a particular comorbid medical condition (e.g., depression, anxiety), in practice this is often unsuccessful. The dosage required for treating migraine is often much different than that needed to adequately treat the comorbidity. In cases of comorbid conditions, often multiple agents are required, each selected based on its efficacy for the individual condition and with attention to potential drug interactions.
Table 4.2 denotes the efficacious preventive treatments for episodic migraine (EM) in adults, as reported in the preventive treatment guidelines developed jointly by the American Academy of Neurology and the American Headache Society [12, 13].
4.3.2 Tricyclics
Amitriptyline in particular has a long history of use for migraine prophylaxis and was until recently considered a first-line agent. Amitriptyline is efficacious for migraine prevention but was downgraded to a second-line treatment in the most recent preventive treatment guidelines, in light of the fact that existing studies on amitriptyline were marked by high rates of dropouts (≥20 %). Adverse anticholinergic and antihistaminic effects, including sedation and weight gain, can limit tolerability, but amitriptyline may be particularly useful for migraineurs who have difficulty sleeping so long as it is given at bedtime. The dosage required for treating migraine is far less than that used for depression, and at those higher doses, side effects become particularly pronounced. Tricyclics can also lower seizure threshold and may not be appropriate for elderly patients or those with significant suicidal ideation given its toxicity in overdose. Comparison studies with the established anticonvulsants suggest that amitriptyline has similar efficacy.
4.3.3 Beta-Blockers
Propranolol is well established for migraine prevention, and recently metoprolol was also judged to be efficacious. Common side effects include drowsiness, sleep problems, weight gain, and fatigue.
4.3.4 Antiepileptics
Topiramate, sodium valproate, and divalproex sodium are each well established for prophylaxis of EM. Topiramate also has efficacy for CM as established in multiple clinical trials, including patients with CM who also have medication overuse. Cognitive impairment is a common adverse effect, as are sleepiness, nausea, and paresthesia. Unlike valproate and divalproex, which often cause weight gain, topiramate is associated with weight loss. These agents are contraindicated in patients with hepatic dysfunction and kidney stones or who are pregnant or planning to become pregnant; antiepileptics can worsen comorbid depression in some patients. Periodic monitoring of blood levels is recommended given risks of liver damage and pancreatitis and to pregnant women [12].
4.3.5 Nonprescription Preventive Medications
The extract of the butterbur plant (Petasites) has evidence of efficacy for EM from multiple trials [13]; longer studies are needed to assess its long-term effects. Gastrointestinal side effects are not uncommon. Careful preparation is required to remove toxic alkaloids in butterbur extract, and thus hepatic toxicity is a major safety concern as butterbur preparations are not actively regulated by any governing agency. Many NSAIDs effectively prevent migraine, but the effects are rather modest; the more well-established preventive medications are better treatment options for most patients.
4.3.6 Prevention of Menstrually Related Migraine
Though they are commonly used as acute agents, administration of particular triptans (e.g., frovatriptan, naratriptan, or zolmitriptan) prior to and during menstruation is efficacious for preventing menstrually related migraine attacks. The triptan is usually taken for 5–6 days, beginning 2 days prior to menses; most trials have used twice-daily dosing. Because menstrual migraine is triggered by drops in estrogen, continuous low-dose estrogen contraception is another preventive treatment option.
4.3.7 Botulinum Toxin for Prevention of Chronic Migraine
Botulinum toxin A has approval by the Food and Drug Administration (FDA) in the United States, Canada, and United Kingdom for prevention of chronic, but not episodic, migraine. Botulinum in small doses promotes muscular relaxation via acetylcholine inhibition and also has analgesic properties. The utility of botulinum for migraine was discovered when patients receiving injections for cosmetic purposes later reported reductions in headache. Botulinum has demonstrated superiority to placebo injections in multiple randomized clinical trials, although a meta-analysis concluded outcomes were rather modest (2.3 fewer headache days/month vs. placebo injection) and not superior to established preventive medications for EM (e.g., amitriptyline, antiepileptics) [14]. Many patients have difficulty obtaining botulinum treatment because it is expensive and typically requires insurance pre-authorization as well as repeated dosing over time.
The injection protocol was established in a series of trials from the Phase III REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT) program. Dosage is 155 units divided equally among 31 fixed head/neck sites (5 units/site): frontalis, corrugator, procerus, occipitalis, temporalis, trapezius, and cervical paraspinal muscle groups. A detailed description of the PREEMPT injection paradigm is seen in Table 4.3 [15].
Because effects taper over time, injections are often repeated in 3+ month intervals and may be gradually spaced further apart to see if continued injections are needed. Side effects are generally mild and temporary and include neck pain, eyelid drooping, and facial muscle paralysis/spasm. Botulinum is contraindicated in children and adolescents, pregnant women, and those with an infection at one or more injection sites. Extreme caution should be used when considering botulinum for patients with neuromuscular diseases or taking drugs that affect neuromuscular transmission.
4.3.8 Calcitonin Gene-Related Peptide Agents
Calcitonin gene-related peptide (CGRP) is a neuropeptide that causes vasodilation and neurogenic inflammation when released at trigeminal nuclei, and its role in migraine pathophysiology has been recognized for many years [16]. Despite promising early results as an acute treatment, efforts to develop the CGRP antagonist telcagepant as a migraine preventive were abandoned after 2.5 % of patients taking telcagepant developed liver aminotransferase elevations orders of magnitude above normal [17]. Development of other CGRP antagonists has stalled somewhat out of concerns about liver damage or limited oral bioavailability, though these drugs appear to have more favorable cardiovascular effect profiles than the triptans as they appear less likely to cause vasoconstrictor-related adverse effects [16].
The newest wave of CGRP efforts centers on developing monoclonal antibodies to CGRP and its receptors, the long half-lives of which allow monthly intravenous instead of daily dosing. These agents thus have potential as migraine preventives with less risk of hepatic toxicity. Recent phase II trials among patients with EM show that these agents reduce migraine frequency approximately 1 day per month more than placebo; common adverse events include pain, erythema, and respiratory tract infections [18, 19]. Despite promising early trials, larger phase III trials are needed to assess longer-term effects and safety over time. CGRP represents the most-researched target for migraine drug development at present, and there is optimism among some headache specialists that these drugs may become the next major breakthrough in migraine since the triptans, though none are yet available commercially.
4.3.9 Ineffective Preventive Medications
The selective serotonin reuptake inhibitors (SSRIs) are not efficacious for migraine prevention [20], and only one serotonin/norepinephrine reuptake inhibitor (SNRI), venlafaxine, has moderate efficacy based on small trials [12]; it is not preferred to the more established preventive agents but may be a useful option for certain patients. Lamotrigine is not effective and clomipramine is probably ineffective.
Despite early positive reports, gabapentin (at any dose) is not effective for migraine prophylaxis, as concluded by a recent Cochrane review, which included negative findings on gabapentin that previously may have been suppressed or misrepresented [21].
4.4 Other Medical Therapies
4.4.1 Electrical and Magnetic Neuromodulation
The field of headache is witnessing a growth of interest in neuromodulation. Cefaly® (Herstal, Belgium), a supraorbital transcutaneous electric nerve stimulation (TENS) device, is the first TENS unit to receive FDA approval for migraine prevention. The device requires a prescription and is worn attached to the forehead via a band containing self-adhesive electrodes. A mild electric current is used to stimulate the trigeminal nerve, and the unit is to be used for 20 min daily. Common side effects include discomfort wearing the device, sedation/sleepiness, and headache after treatment. Most existing studies are small but have shown favorable results in comparison to sham stimulation, although the study methodologies and outcomes are generally not as strong as those with the well-established preventive pharmacotherapies.
Interest is also growing in transcranial magnetic stimulation (TMS) for both acute and preventive treatment of migraine. One sham-controlled trial demonstrated efficacy for acute treatment of migraine with aura [22]. TMS appears safe and may be a useful alternative to more traditional pharmacotherapies or established behavioral interventions [23], but more large controlled trials are needed to establish its efficacy.
4.4.2 Interventional Procedures
Many headache practitioners report positive results with peripheral nerve blocks (commonly of the greater occipital nerve) and trigger point injections for high-frequency headache disorders (CM, chronic tension-type headache) and occipital neuralgia. Both procedures are typically conducted with a local anesthetic (commonly lidocaine or bupivacaine), and corticosteroids are sometimes combined with anesthetic in peripheral nerve blocks. Pain, numbness, and light-headedness are common adverse effects, but these procedures generally appear to be rather safe and well tolerated. Despite their frequent use in clinical settings, there are no accepted guidelines for dosing, injection sites, or injection schedules, and few well-controlled prospective trials have examined the efficacy of these procedures [24].
Owing in large part to their efficacy in cluster headache, interventional procedures targeting the occipital nerves or sphenopalatine ganglion (SPG) are a current source of much current migraine research. Occipital nerve stimulation via implantable pulse generators has some evidence of efficacy for CM from multiple controlled trials, but the effects on headache are somewhat modest when compared to sham stimulation (2.6 fewer headache days/month), owing to large placebo response rates [25]. Common adverse effects are persistent implant site pain, infection, and lead migration, the latter of which not uncommonly requires surgical intervention. Similarly, small trials of SPG nerve blocks or electric stimulation have shown some promise, but a need for larger trials precludes recommendation as a routine intervention. Several small open-label studies have produced mixed findings for the efficacy of vagus nerve stimulation, and like most of the other “emerging therapies” reviewed in this section, large controlled trials are lacking.
4.4.3 Surgical Treatment
Surgical treatments for migraine currently lack evidence of efficacy [26]. In part, this limited efficacy stems from the large variety of surgical sites targeted and procedures employed, as well as limited data on the long-term effects of these invasive procedures. As such, the American Headache Society explicitly recommends against surgical attempts to “deactivate” migraine trigger sites for any form of migraine [7]. Conflicting results have emerged regarding a potential link between patent foramen ovale (PFO) and migraine, and the evidence that PFO closure improves migraine is weak [27].
References
Lipton RB, Stewart WF, Stone AM, Láinez MJA, Sawyer JPC. Stratified care versus step care strategies for migraine. The Disability in Strategies of Care Study: a randomized trial. JAMA. 2000;284:2599–605.
Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3–20.
Thorlund K, Mills EJ, Wing P, Ramos E, Chatterjee A, Druyts E, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258–67.
Cameron C, Kelly S, Hsieh SC, Murphy M, Chen L, Kotb A, et al. Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache. 2015;55:221–35.
Evans RW, Tepper SJ, Shapiro RE, Sun-Edelstein C, Tietjen GE. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50:1089–99.
Langer-Gould AM, Anderson WE, Cohen AB, Eccher MA, Iverson DJ, Potrebic SB, et al. The American Academy of Neurology’s top five choosing wisely recommendations. Neurology. 2013;81:1004–11.
Loder E, Weizenbaum E, Frishberg B, Silberstein S. Choosing wisely in headache medicine: The American Headache Society’s list of five things physicians and patients should question. Headache. 2013;53:1651–9.
Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157–68.
Gelfand AA, Goadsby PJ. A neurologist’s guide to acute migraine therapy in the emergency room. Neurohospitalist. 2012;2:51–9.
Friedman BW, West J, Vinson DR, Minen MT, Restivo A, Gallagher EJ. Current management of migraine in US emergency departments: an analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301–9.
Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF; for the American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–9.
Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman C. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–45.
Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman C. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–45.
Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults. JAMA. 2012;307:1736–45.
Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50:1406–18.
Karsan N, Goadsby PJ. CGRP mechanism antagonists and migraine management. Curr Neurol Neurosci Rep. 2015;15:25.
Ho TW, Conner KM, Zhang Y, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83:958–66.
Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100–7.
Dodick DW, Goadsby PJ, Spierings ELH, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13:885–92.
Banzi R, Cusi C, Randazzo C, Sterzi R, Tedesco D, Moja L. Selective serotonin re-uptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults (Review). Cochrane Database Syst Rev. 2015;(4):CD002919.
Mulleners WM, McCrory DC, Linde M. Antiepileptics in migraine prophylaxis: an updated Cochrane review. Cephalalgia. 2015;35:51–62.
Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373–80.
Dodick DW, Schembri CT, Helmuth M, Aurora SK. Transcranial magnetic stimulation for migraine: a safety review. Headache. 2010;50:1153–63.
Ashkenazi A, Blumenfeld A, Napchan U, Narouze S, Grosberg B, Nett R, et al. Peripheral nerve blocks and trigger point injections in headache management: a systematic review and suggestions for future research. Headache. 2010;50:943–52.
Chen YF, Bramley G, Unwin G, Hanu-Cernat D, Dretzke J, Moore D, et al. Occipital nerve stimulation for chronic migraine: a systematic review and meta-analysis. PLoS One. 2015;10:e0116786.
Diener HC, Bingel U. Surgical treatments for migraine: time to fight against the knife. Cephalalgia. 2015;35:465–8.
Dowson A, Mullen MJ, Peatfield R, Muir K, Khan AA, Wells C, et al. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. 2008;117:1397–404.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Smitherman, T.A. (2016). Pharmacotherapy and Other Medical Treatments. In: Clinician's Manual on Migraine. Adis, Cham. https://doi.org/10.1007/978-3-319-02777-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-02777-7_4
Published:
Publisher Name: Adis, Cham
Print ISBN: 978-3-319-02776-0
Online ISBN: 978-3-319-02777-7
eBook Packages: MedicineMedicine (R0)