Abstract
Photovoltaic technologies could play a pivotal role in tackling future fossil fuel energy shortages, while significantly reducing our carbon dioxide footprint. Crystalline silicon is pervasively used in single junction solar cells, taking up ~80 % of the photovoltaic market. Semiconductor-based inorganic solar cells deliver relatively high conversion efficiencies at the price of high material and manufacturing costs. A great amount of research has been conducted to develop low-cost photovoltaic solutions by incorporating organic materials. Organic semiconductors are conjugated hydrocarbon-based materials that are advantageous because of their low material and processing costs and a nearly unlimited supply. Their mechanical flexibility and tunable electronic properties are among other attractions that their inorganic counterparts lack. Recently, collaborations in nanotechnology research have combined inorganic with organic semiconductors in a “hybrid” effort to provide high conversion efficiencies at low cost. Successful integration of these two classes of materials requires a profound understanding of the material properties and an exquisite control of the morphology, surface properties, ligands, and passivation techniques to ensure an optimal charge carrier generation across the hybrid device. In this chapter, we provide background information of this novel, emerging field, detailing the various approaches for obtaining inorganic nanostructures and organic polymers, introducing a multitude of methods for combining the two components to achieve the desired morphologies, and emphasizing the importance of surface manipulation. We highlight several studies that have fueled new directions for hybrid solar cell research, including approaches for maximizing efficiencies by controlling the morphologies of the inorganic component, and in situ molecular engineering via electrochemical polymerization of a polymer directly onto the inorganic nanowire surfaces. In the end, we provide some possible future directions for advancing the field, with a focus on flexible, lightweight, semitransparent, and low-cost photovoltaics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The advancements in the design and fabrication of photovoltaics can be categorized into three different generations of solar cells. The first generation of solar cells includes large-area, silicon-based modules estimated to account for 86 % of the total photovoltaic market. However, mainly due to high manufacturing costs, a second generation of cells, thin-film solar cells, has been introduced to address low costs and high flexibility that permit integration on roof tops, solar shingles, etc. The most cutting-edge technology is represented by the third generation of solar cells that completely replaces the standard paradigm of a two-dimensional (2-D) planar p–n junction with new materials and novel three-dimensional (3-D) design approaches. Polymer-based, nanostructure-based, or dye-sensitized solar cells represent some of the innovative alternatives for which research teams are investing a considerable amount of time. Crystalline silicon solar cells have recently achieved laboratory efficiencies of 25 % [1], requiring a honeycomb texturing along with a rear local diffusion of dopants. The adjective “crystalline” refers to the crystal growth phase during the preparation of the cylindrical semiconductor ingots.
After oxygen, silicon (Si) is the most abundant element on earth. Generally, silicon is obtained by the reduction of silicon dioxide with carbon in an electric furnace from 1,500 to 2,000 °C. The silicon used in the semiconductor industry for the fabrication of devices is then further purified to bring impurity levels below the parts-per-billion level. In order to fabricate a silicon solar cell, a large, doped ingot is first sectioned into smaller ingots of proper size. Subsequently, each ingot is sliced and cleaned to yield a series of wafers. Phosphorus is then diffused into the semiconductor to constitute the second side of the photojunction. Lastly, metal grid contacts are realized by screen-printing techniques. The process chain is depicted schematically in Fig. 12.1.
The nonoptimized material usage and the multi-stepped fabrication involved are hindering the achievement of a solar grid parity. A situation of grid parity occurs when a source of alternative power (e.g. solar) becomes cost competitive with standard sources (e.g. fossil fuel-based). In order to minimize the material usage and reduce the overall costs, new radical strategies to further slice the silicon wafers into thin films of semiconductors are being explored. An alternative solution to lower the cost of solar cells is represented by the development of organic photovoltaic devices. One of the most important milestones was the introduction of a C60 fullerene derivative to replace the conventional n-type polymers in organic solar cells [2]. In a typical organic device, donor and acceptor interfaces are intimately mixed together to constitute a bulk heterojunction (Fig. 12.2). Such blends constitute the active absorber, able to harvest the photons and generate free charge carriers that can be collected at the electrodes. The mixture is in liquid form and can be promptly spin-coated on arbitrary substrates. To date, the highest efficiency for this class of solar cells amounts to 10.6 %. Building efficient devices requires a systematic analysis of three different areas: materials design, morphology, and interface engineering. The total thickness for the light-harvesting active layer in solution-processed photovoltaics is only a few hundred nanometers. This allows for low cost and flexible architectures, with processes compatible with roll-to-roll manufacturing for high-volume production. To date, one of the main challenges is to unravel the complex selection of degradation phenomena that occurs in this type of optoelectronic device. Photolytic and photochemical reactions are activated under illumination directly from the polymer, degrading the conversion efficiency over time in ambient atmosphere [3].
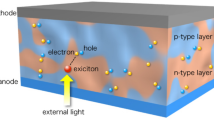
In order to provide the highest efficiency at reasonable prices, hybrid photovoltaic technologies emerged to combine the benefits of organic and inorganic materials. The photovoltaic layer is typically constituted of semiconducting polymers (e.g. P3HT, Fig. 12.3a) intimately mixed with inorganic nanocrystals or other nanostructures. The classical inorganic solar cells mentioned earlier have a higher carrier mobility; whereas organic solar cells benefit from considerably higher optical absorption coefficients. The charge transfer in this new class of photojunctions can be studied in composites of p-type conjugated polymers with n-type inorganic semiconductors, or vice versa [4] (Fig. 12.3b, c).
2 Background on Hybrid Photovoltaics
Organic/inorganic hybrid solar cells are attracting growing attention and becoming a vibrant field of research. All inorganic solar cells have higher efficiencies due to their excellent carrier mobility, but are generally expensive.
The low-cost polymer solar cells, on the other hand, often lacks air stability and satisfactory performance due to inferior transport properties. Combining these two types of materials offers a potential solution to create the next-generation of low-cost, efficient solar cells. Numerous hybrid devices have been demonstrated. However, common processing techniques for polymers, including spin-coating, inkjet printing, or doctor blading (only applicable to planar substrates) result in nonconformal coatings or thickness gradients when applied to three-dimensional structures. Hence, the reported hybrid solar cells are usually fabricated by either intimately mixing n-type inorganic nanostructures such as silicon nanocrystals, cadmium selenide, zinc oxide, lead sulfide, or titanium dioxide into the polymer prior to spin-coating, or infiltrate the polymer solution/dispersion into the 3-D inorganic arrays to form an interdigitated block-like film (Fig. 12.4). Both approaches diminish the purpose of having well-oriented, high mobility, 3-D structured inorganic arrays that possess the unique advantage of directional charge transport and light trapping. Typical power conversion efficiencies for these devices ranges from ~0.04 to 3.2 % [5], demanding further improvement. Aside from the device structure, the flexibly tunable physical properties of the polymeric conductors by modern synthetic chemistry enable the optimization of the energy level alignment for organic and hybrid solar cells. Unfortunately, such desirable properties come at the price of extensive molecular engineering and intensive ad hoc synthesis, and the resulting polymers are often air-sensitive and need to be processed in glove-boxes. These structural and property limitations constitute as the major hurdle in building a practical, highly efficient hybrid solar cell that can be potentially commercialized. Furthermore, coating a well-defined 3-D inorganic semiconductor pattern, such as oriented nanowires, with an organic conductor allows for a high interface area and 3-D features for the overall device, which is difficult to achieve with the all organic counterparts due to the lack of effective patterning techniques. As a result, materials development leading to inorganic and organic semiconductor/conductors with desired properties that can offer optimized morphology for charge transport and light harvesting is crucial and has become an expanding field of investigation.
3 Materials Development
Materials development refers to a research field aimed at prototyping engineered materials with innovative functionalities. Some representative examples include light-weight, resilient carbon-fiber-based fabrics, porous structures for enhanced battery capacity, ultraviolet-resistant coatings based on nanocrystals, and many others. Sun-driven energy generation requires efficient materials that combine ultra-high photon absorption with a carrier generation/extraction close to unity. To do so, inorganic and organic chemists are intensively investigating novel approaches to tailor artificial materials in such a way that the power conversion efficiency is maximized. Inorganic materials developments concentrate on nanostructures (i.e. nanowires, colloidal quantum dots, etc.) that could bring in novel capabilities at the materials level in terms of optimized photon management or carrier transport. Organic materials developments are aimed at engineering polymers with more planar backbones, lower bandgaps, and good solubilities in order to increase their carrier mobility, stability, and solution processability.
3.1 Inorganic Nanomaterials: Top-Down vs. Bottom-Up Approach
Semiconductor nanomaterials represent the main thrust of nanotechnology and nanoscience. Their unique size-scaling properties are of interest to charge transport, energy conversion, fracture strength, and many other fields. Generally, nanomaterials can comprise both classical and quantum behaviors suggesting approaches like “materials-by-design” [6]. The ratio of surface area-to-volume for nanomaterials is much greater than their bulk counterparts; therefore, a majority of the atoms are close to the surface, rendering them more weakly bonded and more reactive. Quantum mechanical effects become important when the structure size is on the same scale as the electron wavelength, and quantum confinement occurs resulting in changes in electronic and optical properties. Currently, semiconductor nanomaterials are still at the research level, holding promise in many applications such as photovoltaics and hydrogen production (renewable energies), nanoscaled transistors, light-emitting diodes, bio-chemical sensors (e.g. track and destroy cancer cells), energy storage (e.g. batteries). Nanosized materials can, in principle, be made using both top-down and bottom-up techniques. The former can be imagined as sculpting from a block of stone. The starting material is gradually modified in shape until the desired structure is achieved. Nanolithography can be considered the most representative example of a top-down fabrication approach for nanomaterials. Semiconductor materials are first patterned by a mask where the exposed portion is etched away. Based on the level of resolution, the etching step can be carried out chemically (etchants), mechanically, or through X-rays or focused ion beams. The limitations of top-down fabrication are related to diffraction effects where the practical limit for deep ultraviolet optical lithography is around 100 nm. To define sub-100 nm features, electron beams can be used down to 15 nm features. The drawback is the very low throughput. On the other hand, bottom-up techniques can be visualized as building a brick house where the single brick is represented by atoms or molecules, placed one by one to constitute the targeted structure. These processes are time-inefficient so self-assembly techniques are employed where atoms arrange themselves as required. Part of the challenge is to be able to fabricate robust structures along with complicated patterns.
3.1.1 Nanocrystals: A Colloidal Synthesis
Monodispersed, nanometer-scale crystals (known as nanocrystals) give rise to novel optical, electronic, magnetic, and structural features by exploiting size-dependent effects. In order to map out size-dependent properties, it is necessary to achieve a uniform synthesis in terms of shape, size, and composition [7] (Fig. 12.5a). When the diameter of such particles is reduced below the carrier wavefunction, the absorption spectrum becomes widely tunable as well as the corresponding bandgap (Fig. 12.5b). The synthesis of colloidal nanocrystals is an elegant interplay between molecular inorganic chemistry and solid-state semiconductor materials chemistry. The adoption of solution-processable synthetic chemistry to engineer small inorganic colloids represents a powerful tool to tune size, shape, and composition in a bottom-up approach [8]. Colloidal nanocrystals can be grown in the form of nanorods, faceted prisms, and spheres (Fig. 12.5c) and composed of metals, oxides, or semiconductors. Alternatively, hydrothermal techniques represent a promising synthetic method because of the low-temperature process and ease of controllability of the particle size. Furthermore, the growth is catalyst-free, environmentally benign, and only requires the use of simple equipment. At the research level, the conversion efficiency record for colloidal quantum dot solar cells is 6 % [9]. In order to surpass efficiencies of 10 %, it is necessary for the hole and electron mobilities to exceed 10−1 cm2 V−1 s−1, with a bandgap as trap-free as possible [9].
(a) After synthesis, the colloidal quantum dots can be deposited onto flexible substrates by spray-coating, reel-to-reel printing, or ink-jet printing. (b) The quantum dot absorption can be tuned as a function of diameter size to better match the solar spectrum energy distribution. (c) A transmission electron microscopy image of a sphere-shaped single-crystalline colloidal quantum dot
Recently, the first tandem nanocrystal-based solar cell has been reported [10] exploiting the size-effect tuning of a single material (lead sulfide). The tandem configuration boosts the open-circuit voltage of the solar cell to above one volt with an overall power conversion efficiency of up to 4.2 %.
3.1.2 Nanowires: Epitaxy vs. Etching
Nanowires can be defined as structures that have a constrained lateral size from a few to several tens of nanometers, longitudinally unconstrained. Epitaxial synthesis of nanowires from gaseous sources was first developed by Wagner and collaborators [11], where crystalline nanowires were grown epitaxially from the surface of catalytic metal clusters. This approach opened up a route to heterogeneous, radial, or super-lattice structures [12] (Fig. 12.6a). Furthermore, the opportunity of incorporating dopants during growth allows for the design of advanced optoelectronic devices including solar cells, light-emitting diodes, and transistors. Controlling the density and position of each nanowire for practical fabrication can be difficult. Patterning the metal catalyst is a method to precisely define dimensions, spacings, and aspect ratios: the corresponding vertical growth will directly stem from the patterned metal catalyst (i.e. Au or Cu). The growth direction can be guided by adopting different crystallographic directions (e.g. 〈110〉 vs. 〈100〉) of the substrate. Top-down nanowires combine wafer-scale semiconductor growth with advanced lithographic processing. Photolithography, electron-beam lithography, nanoimprint lithography have been exploited as patterning methods to define a mask for either wet or dry etching. Subsequently, the unmasked constituent material is isotropically or anisotropically etched to realize the targeted structure. The roughness of the sidewalls limits the selectivity of the process. The etching approach only allows for engineering the design along the longitudinal direction, without accessing radial components (Fig. 12.6b). Despite that, the exact knowledge of doping levels (from the planar growth) represents a powerful tool with respect to bottom-up techniques.
(a) Bottom-up growth of nanowires. The bulk wafer is first patterned (silicon dioxide mask or metal catalyst). Subsequently, gases are flown into a reactor and the growth of nanowires initiates. The final nanowire length can be tuned according to the growth time. (b) Top-down fabrication of nanowires. The bulk wafer is first covered by resist, which is patterned by a lithographic technique (e.g. nanoimprint, electron-beam lithography, and self-assembled beads). The pattern constitutes a hard mask. The wafer is then dry- (or wet-) etched and the nanostructures formed
3.1.3 Nanoporous Structures
Nanoporous structures have received increasing attention due to their unique applications in catalysis reactions, DNA translocation, hybrid solar cells, and as templates for nanostructure self-assembly [13]. Nanoporous structures are comprised of a multitude of nanopores, nanocavities, or nanochannel arrays where the pore size and porosity can be tuned at will at the nanoscale. Anodic aluminum oxide constitutes a prime example of a self-ordered nanopore membrane (Fig. 12.7), exhibiting tunable diameters with high aspect ratio as well as superior thermal and chemical stabilities [14].
Nanoporous TiO2 electrodes have been actively investigated in dye-sensitized hybrid solar cells where each nanopore represents a storage site for the electrolyte solution [15]. High power conversion efficiencies (10–11 %) have been reported with promising solutions to overcome charge recombination, and increase light scattering properties of the film while improving the interfacial energetics between the electrolyte and the nanoporous material. The science of infiltration of a polymer or electrolyte into such porous matrices is still under investigation. Recently, polymers with different molecular weights have been shown to modify the degree of infiltration, suggesting a direct relationship with the size of the polymer. Benzene-based solutions can infiltrate down the network up to several micrometers where the major constraint is the average chain length that needs to be smaller with respect to the diameter of the nanopore [16].
3.2 Organic Polymers: Properties, Doping, Design, and Synthesis
Compared with inorganic materials, the field of organic semiconductors/conductors is nascent. Nevertheless, ever since the discovery of polyacetylene in the 1970s, conducting polymers have garnered tremendous amount of interest in both fundamental science and novel applications, leading to the Nobel Prize in Chemistry awarded to Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa in 2000 for the discovery and development of synthetic metals and semiconductors [17]. Through rigorous research over the past few decades, the chemical and physical properties of conducting polymers are now fairly understood. Their unique aspects of combining the electrical properties of metals and semiconductors coupled with their mechanical flexibility and solution processability has enabled a vibrant field of research that led to the development of flexible supercapacitors, artificial mussels/actuators, sensors, organic field-effect transistors (OFET), organic light emitting diodes (OLED), organic photovoltaic devices (OPV), among many other applications [18]. Common examples of conducting polymers are listed in Fig. 12.8, featuring the chemical structure of polyacetylene (PA), polyparaphenylene (PPP), polyparaphenylene vinylene (PPV), polypyrrole (PPy), poly(2,5-dialkoxy)paraphenylene vinylene (MEH-PPV), polythiophene (PT), poly(3-alkyl)thiophene (P3AT), poly(ethylenedioxythiophene) (PEDOT), and polyaniline (PANI).
3.2.1 Charge Transfer and Doping
In order to understand the conducting polymers’ role in photovoltaic research, it is necessary to have a basic knowledge of their charge transport and doping mechanism. The conjugated backbones of these polymers allow the delocalization of electrons in π-bonds formed by the overlapping p z -orbitals. The electronic structure of a neutral conjugated polymer typically consists of a completely filled valence band, more commonly known as the π-band or the HOMO to chemists, and an empty conduction band (the π*-band or the LUMO). The energy difference between the π and the π* bands is the bandgap of the material, typically ranging from 1 to 2.5 eV, reminiscent of those of semiconductors. In order to induce carrier transport and thus electrical conductivity, a doping process to either inject electrons into the π* band or remove electrons from the π band is necessary, analogous to the doping concept in inorganic materials [19, 20].
There are four general ways to dope a conducting polymer:
-
1.
Chemical doping
-
2.
Electrochemical doping
-
3.
Interfacial doping
-
4.
Photo-excitation doping
3.2.1.1 Chemical Doping
Chemical doping can be achieved by exploring the charge-transfer redox chemistry of the material or its acid-base chemistry if applicable. The polymer can be oxidized into a positively charged moiety, balanced out by the negative charges of the oxidizing agent, creating hole mobility in the conjugated backbone and thus rendering the material p-type. Similarly, reducing the polymer backbone causes it to become negatively charged with electron mobility, thus n-type. A common example is shown in Fig. 12.9. Most conducting polymers are more readily p-type doped. When the doping level is sufficiently high, the polymer becomes metallic.
Polyaniline represents an interesting case of chemical doping [21]. Simply oxidizing it into its conjugated emeraldine base oxidation state does not lead to electrical conductivity as the polymer remains neutral in charge. An acid has to be used to protonate the imine nitrogens in order to induce hole mobility. This process is highly reversible as the acid dopants can be readily removed by neutralizing with a base (dedoping), rendering the polymer poorly conductive again (Fig. 12.10).
3.2.1.2 Electrochemical Doping
Chemical doping is an excellent method to realize metallic behavior by fully doping the backbone. However, intermediate doping levels are difficult to control as partial doping often leads to regions of completely doped polymer, while other regions remain undoped. Electrochemical doping shares a similar underlying mechanism as chemical doping, but offers much better control of doping levels. In a typical setup, three electrodes are immersed in an electrolyte solution: (1) the working electrode in general is a conducting substrate with the polymer of interest attached; (2) the counter electrode, typically a metal wire, coil, sheet, or mesh, balances the charge with that from the working electrode; and (3) the reference electrode, such as a saturated calomel electrode (SCE) or Ag/AgCl electrode, defines 0 V reference voltage in the electrochemical cell. A certain doping level can be readily achieved by applying a fixed voltage in order for the conducting polymer on the working electrode to reach electrochemical equilibrium, allowing homogeneous doping throughout the entire film.
3.2.1.3 Photo-excitation Doping
Semiconducting polymers can also be doped by photo-excitation, which is of great importance for photovoltaic applications. Most of these polymers have high absorption efficiency. Upon light illumination, bound electron-hole pairs, also known as excitons, are generated. In an organic solar cell where a p–n junction interface occurs, excitons diffuse to such an interface and split into electrons and holes due to the potential difference (a minimum of 0.3 V is required). The electrons and holes are then extracted to the cathode and anode of the device, respectively, and are collected [22].
3.2.1.4 Interfacial Doping
Semiconducting polymers can be doped by charge injection at a metal-semiconductor interface as well [23]. Holes can be injected into the filled π-band from the metal electrodes, leading to hole mobility. Similarly, electron mobility can be created by the injection of electrons into the empty π* band. The most widely used application for this type of doping is field-effect transistors (FET). Figure 12.11 shows a typical organic thin-film FET structure.
For instance, in order to achieve hole mobility, a negative gate voltage needs to be applied to cause electron accumulation at the dielectric surface, thus creating holes at the semiconducting polymer/dielectric interface. At the same time, a negative drain voltage is necessary for the movement of the holes across the source and drain electrodes through this interface, leading to hole mobility.
Among the four general types of doping mechanisms, it is necessary to make the distinction that the chemical and electrochemical doping in a sense are permanent, since the dopants are integrated as part of the material and will remain until removed via chemical or electrochemical methods. On the other hand, doping by photo-excitation or charge injection are temporary, as a continuous photon or charge source is needed, and the mobility in the material will vanish once the source is removed.
3.2.2 Molecular Design and Engineering
With the different methods to induce doping and thus carrier mobility, organic conductors pose as interesting alternatives in many applications to their inorganic counterparts that have traditionally dominated the market. In particular, the conducting polymers are attractive because of their high absorption efficiency, flexibility, tunable chemical and electronic structures, and solution processability. In order to tailor these polymers for specific applications, a tremendous amount of effort has been devoted to the design and synthesis of sophisticated polymers with optimized solubility, energy levels, and solid-state packing. These parameters are crucial in the realm of solar cells. The different methods for achieving these properties are discussed below.
3.2.2.1 Methods for Inducing Solubility
It is a priority to make conducting polymers soluble or solution dispersible because this allows the polymers to be processed by methods such as spin-coating, spray coating, roll-to-roll processing, in order to significantly lower the manufacturing cost, and render conducting polymers an attractive alternative to their inorganic counterparts despite the inferior electrical properties. One approach to induce solubility of an otherwise insoluble polymer is by using a chemical dopant that can be dissolved in organic solvents [24]. For example, camphorsulfonic acid, a bulky organic acid, can be used to chemically dope polyaniline (PANI) into the metallic form and induce solubility in many organic solvents. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) is another benchmark material that takes advantage of counter-ion induced solubility, as PSS functions as the dopant, but also assists the dispersion of PEDOT in water [25].
Forming colloidal dispersions is another method for rendering polymers processable [26]. This method has become especially popular since the advent of nanotechnology in the 1990s. Typically, uniformly shaped small particles, such as nanofibers or nanospheres, are produced by either a template-guided or a template-free method, and can often form stable dispersions due to their small size and the inter-particle repulsion, arising from the surface charges.
Within the realm of organic solar cell research, probably the most important and commonly used method for inducing solubility is by covalently attaching solubilizing functional groups to the polymer backbone using organic chemistry. Semiconducting polymers used in p–n junction solar cells typically cannot contain chemical dopants as they need to be doped via photo-excitation when illuminated with light, which eliminates the possibility of counter-ion doping induced solubility. Hence, synthesizing polymers that can be readily dissolved in common organic solvents becomes crucial for the solar cell industry. The most noticeable example is the functionalization of polythiophene, which is insoluble in virtually all organic or aqueous solvents. However, the attachment of an alkyl chain such as hexyl at the C3 position allows the polymer to become soluble in common organic solvents such as chloroform or dichlorobenzene. Hydrocarbon side chains with different lengths or containing polar groups have also been exploited.
3.2.2.2 Energy Level Engineering
Tuning the energy gaps for semiconducting polymers have probably been one of the most significant factors that have propelled polymer solar cells to efficiencies that are promising for commercialization [27].
The efficiency of a solar cell is defined by (12.1)
where J sc, V oc, FF, and P in are the short circuit current density, open circuit voltage, fill factor, and power of the incident light (AM 1.5G 1000 W/m2), respectively. Since J sc is heavily influenced by the amount of light absorption, it is also important to tune the bandgap of the materials in order to achieve a broader solar spectrum absorption range, which can enhance the photocurrent. A powerful method for achieving such a goal is to include alternating electron donor/acceptor (also known as electron push/pull) units in the polymer backbone [28]. Such architecture increases the effective resonance length of the π electrons, facilitating π electron delocalization, and hence resulting in a polymer with extended absorption beyond the visible spectrum. For example, one of the most successful push/pull polymers is PBDTTT, where a strong pull unit, thieno[3,4-b]thiophene (TT) is copolymerized with a push unit, benzo[1,2-b:4,5-b′]dithiophene (BDT). Its chemical structure along with some of the other efficient molecular engineered polymers are illustrated in Fig. 12.12. Many of these polymers also provide further enhancement to the J sc by offering a more crystalline microstructure due to the planar units in the polymer backbone, which leads to higher mobility and more efficient charge transport.
Improving the V oc is also important for optimizing the solar cell efficiency. V oc is largely determined by the energy level difference between the HOMO level of the donor and the LUMO of the acceptor; therefore, one way to increase the solar cell efficiency is to design a donor polymer with a lower HOMO level. However, improvement of the J sc by narrowing the band gap often leads to a rise in the HOMO level as well, which deteriorates the V oc. Recently, elegant molecular engineering has paved the way for lowering the HOMO level while maintaining the low bandgap. For example, for PBDTTT, a strongly electron withdrawing atom such as fluorine can be attached to the electron pull TT unit, leading to lowering of the HOMO and LUMO levels simultaneously, while maintaining the bandgap [29].
Although such sophisticatedly engineered molecules have led to great success in organic solar cells, they are yet to be tested for hybrid solar cells. Since the charge transferring and separation mechanism at the organic/inorganic interfaces are still debatable for hybrid solar cells, coupled with the ad hoc synthesis and the high cost of these new polymers, benchmark materials such as P3HT or PEDOT are typically used, with P3HT for p–n junction hybrid photovoltaic devices, and PEDOT for their Schottky counterparts.
3.2.3 Synthesis of Benchmark Polymers
Since P3HT and PEDOT have both been well developed, most hybrid solar cell studies involving these polymers have utilized their commercially available forms, which can be purchased from Sigma-Aldrich® or Clevios™, among many other specialized chemical companies. The polymer typically is marketed in the forms of a solid, dispersion, or solution, which can be spin-coated onto the inorganic layer or other planar substrates. However, for studies with more controlled processes or more complex structures, it is necessary to generate the polymers in situ. A few general synthetic approaches are discusses below.
3.2.3.1 Chemical Synthesis
Conducting or semiconducting polymers can be synthesized via chemical or electrochemical routes. P3HT can be made in the regiorandom or regioregular form (Fig. 12.13). Regioregular P3HT is desired and typically used for solar cell purposes because the regularity of the hexyl attachment position not only leads to less torsion angles in the polymer backbone, providing a longer conjugated pathway for π electrons to travel, but also allows for more ordered intermolecular packing that increases the mobility of the material. Regioregular P3HT can be synthesized via a Grignard metathesis reaction, or by Reike zinc reactions [30, 31].
PEDOT can be produced chemically in organic solvents using an oxidant such as FeCl3 or Fe(OTS)3, where OTs stands for tosylate. The resulting product, however, is a black, insoluble, and infusible solid that is difficult to characterize and process. A more practical approach towards high-quality PEDOT is to polymerize the monomer in an aqueous polyelectrolyte, often poly(styrenesulfonate) (PSS) with Na2S2O8 as the oxidizing agent. The resulting PEDOT is chemically doped with PSS, which also induces dispersibility of PEDOT in water. The aqueous dispersion is dark blue in color and can be readily processed into uniform thin films. The commercially available PEDOT:PSS dispersions are produced largely based on this synthesis [25].
PEDOT can also be polymerized in the solid state [32]. When 2,5-dibromo-3, 4-ethylenedioxythiphene (DBEDOT), a white, crystalline solid, is stored at room temperature over a prolonged period (i.e. 2 years), the crystals turn dark blue/black in color, while maintaining the morphology of the starting monomer. Characterization indicates that the dark product is PEDOT doped with bromine, and possesses a rather high conductivity (80 S cm−1). Such solid-state polymerization can be greatly accelerated and completed in 24 h by heating to 60 °C. The conductivity of the resulting PEDOT, however, drops to 33 S cm−1, since a prolonged process at lower temperature typically leads to more ordered intermolecular packing.
3.2.3.2 Electrochemical Synthesis
Another powerful approach towards the synthesis of conducting polymers is electropolymerization. Using a setup similar to that for electrochemical doping discussed earlier, the monomer is oxidized on the anode, typically a metal or other conductive surface. Polymers can be formed under potentiodynamic (by scanning cyclic voltammetry passing its oxidation potential), potentiostatic (constant voltage), or galvanostatic (constant current) approaches. For example, EDOT can be readily polymerized into a sky-blue, conductive PEDOT film on the working electrode, in either organic or aqueous electrolyte solutions [25]. Electropolymerization only requires a small amount of monomer, and can yield films with controlled thicknesses. However, the polymer can only be generated on a conductive substrate, which limits the application range of polymers produced via this method.
4 Morphology
Hybrid organic–inorganic solar cells constitute a viable route to convert solar power into electricity at low manufacturing costs. The hybrid composite resulting from combining organic conjugated polymers with inorganic semiconductor nanostructures is highly dependent on the morphology of the individual constituents. One-dimensional structures can provide directional carrier transport, but are often difficult to achieve for hybrid composites. Optimum layer thickness is also important due to the short exciton diffusion length of organic materials (5–20 nm). However, the organic films optimized for exciton diffusion and charge separation are often way too thin for abundant light absorption, which requires a ~100–200 nm layer. Furthermore, it is desirable for both constituents to be single crystalline for efficient charge transport, which is readily achievable for inorganic materials, but extremely challenging for their organic counterparts. Inducing a certain level of crystallinity, however, is possible for polymers, and therefore also needs to be taken into consideration. The overall dimensionality of the device is important as well. For instance, with the same junction interfacial area, a solar cell with a 3-D structure has the dual function of light trapping, hence increasing the efficiency. From the hybrid solar cell literature, the most intensively studied devices in general can be categorized based on their morphologies/device architectures, as discussed below.
4.1 Types of Hybrid Solar Cells
Several types of hybrid solar cells have been developed based on the different methods for combining the two materials. One approach is to incorporate a photoactive semiconductor working electrode and counter electrode, both immersed in an electrolyte containing suitable redox couples, known as a dye-sensitized solar cell (DSSC). A second method is by depositing a layer of polymer film on top of an inorganic film or wafer resulting in a bilayer structure. A third approach is to form a bulk heterojunction that can be fabricated from blends of an electron-donor polymer and electron-acceptor nanocrystals, with the advantage of being solution processable and possessing an efficient, continuous charge transport pathway. The fourth method is to constitute an interpenetrating network by infiltrating or coating an organic material on an ordered inorganic semiconductor nanostructure array, where the order and the small feature size can dramatically enhance the exciton separation and transport to the respective electrodes.
4.1.1 Dye Sensitized Solar Cell (DSSC)
Dye-sensitized solar cells are attractive since the constituents are low-cost materials that can be printed in large volumes. The uniqueness of these devices relies on the fact that light absorption and electron and hole transport are each handled by separate materials [33]. Typically, the dye is attached to a wide-bandgap semiconductor (e.g. ZnO or TiO2) and when it absorbs photons, photoexcited electrons travel to the conduction band of the semiconductor, carrying the electron to one of the electrodes. A redox couple reduces the oxidized dye back to neutral and hauls the positive charge to the counter-electrode. In such a way, electric current can flow under light to an external load (Fig. 12.14).
4.1.2 Bilayer Structure
Bilayer structure is the most primitive solar cell structure. The active layer material, whether for p–n or Schottky junction, are two thin films sandwiched between the anode and cathode electrodes [34]. A typical structure is shown in Fig. 12.15, as a polymer layer is deposited by spin-coating on top of an inorganic film or wafer. Such structure is not practical for hybrid solar cells as the interfacial area is very small—only the lateral dimension of the device, and hence yields very low short-circuit currents. Furthermore, due to the delicate balance between needing a thin organic film in order to match its low exciton diffusion length and the requirement of a thick film so that an adequate amount of light can be absorbed, the thickness of the organic layer is difficult to optimize and hence, poses as another factor leading to low efficiency. However, the simplicity of the bilayer structure allows for fundamental studies of figures of merit for solar cells at the interface of the two materials, which can serve as control experiments or tools for trouble shooting more complicated structures (e.g. bulk heterojunctions or interdigitated architectures) so that the exact factors leading to higher efficiencies can be deciphered.
4.1.3 Bulk Heterojunction Solar Cells
Bulk heterojunctions (BHJs) have been adopted in polymer and colloidal nanoparticle solar cells to improve energy conversion efficiency [35]. Generally, nanowires or nanocrystals are mixed with conjugated polymers or fullerenes in the form of a solution-based blend, then spin-coated onto a substrate. A typical device structure is illustrated in Fig. 12.16. Most common blends use n-type inorganic nanostructures with p-type polymers. One of the earliest hybrid solar cells is based on such an active layer morphology, with CdSe nanorods as the acceptor blended into the P3HT donor solution, leading to a 1.7 % efficiency. Many follow up studies have been performed including GaAs/P3HT BHJ devices [36].
Despite the success of such structures in all organic solar cells, they are not as desirable for hybrid solar cells partly because of the high loading necessary for inorganic materials, especially 0-D structures, to create a continuous pathway for charge transport and collection. Also, unlike all organic blends, the phase segregation for organic/inorganic blends is difficult to control, which is crucial for this type of solar cell. Furthermore, the morphological stability of the blend film needs to remain intact over the device lifetime, which could be problematic for hybrid blends due to nanowire/particle migration over time as a result of the huge density difference between the organic and inorganic components.
4.1.4 Interdigitated/Ordered Structures
The adoption of ordered, interdigitated heterojunctions as illustrated in Fig. 12.17 has been explored as a means to improve the charge extraction before undesired recombination processes become predominant. Recent advancements in nanofabrication made sub-100 nm patterning routinely available. Vertical inorganic nanostructures (e.g. nanopillars or nanorods) that are perpendicular with respect to the substrates are advantageous because (1) the density and point-to-point distance between nanostructures can be tuned in an ordered fashion without incurring concentration gradients or inhomogeneity once reduced to a practical device, (2) the uni-directionality of all the 1-D inorganic nanostructures, which are also typically single crystalline, provides directional and rapid carrier transport for the solar cells, and (3) it unfolds an enormous opportunity for creating ordered, high interfacial area structures with organic materials, as it is difficult to create interdigitated or core/shell structures out of polymers alone due to their molecular flexibility. These architectures are advantageous compared to their planar counterparts because the active junction area is greatly enhanced, which also increases the probability of photogenerated carriers to be extracted before recombination can occur. This aspect relaxes the requirement of having ultra-high purity materials necessary for planar solar cells. Therefore, we direct our focus in the next section to these type of structures because of their vast potential for achieving high efficiencies and other unique properties.
4.2 Efficient Hybrid Photovoltaics Based on Ordered Structures
Due to the significant advantages of having an ordered structure as discussed in previous sections, photovoltaic devices based on patterned 1-D nanostructure arrays will be the focus of this section.
One significant benefit of having a 1-D nanostructure array is that the amount of active absorber can be reduced without compromising the overall optical absorption due to their light trapping effects. Nanoscale texturing stemming from ensembles of nanowires, nanodomes, or nanocones considerably improves the light absorption over a broad range of wavelengths. In standard planar solar cells, the p–n junction is formed by dopant diffusion into the crystal. This ion implantation process requires extremely high temperatures (~1,000 °C). Significant efforts have been reported to form a photoactive heterojunction at lower temperatures. Recently, a hybrid radial-junction design that combines silicon microwires with amorphous silicon shells achieved efficiencies of up to 12.2 % [37]. A nanowire-based hybrid structure exploits the enhancement in light absorption from the sub-wavelength texturing, while the conductive polymer represents the other side of the junction. In a radial geometry, the constituting material for the shell is not required to be of high-grade quality since the carriers will be collected through the thin shell along the radial direction.
A multitude of methods can be used to achieve such attractive architectures for hybrid solar cells, including infiltration of a polymer into the inorganic nanowire arrays, controlled spin-coating of a polymer solution to form a coating on nanowires, self-assembly of end-functionalized polymers onto the inorganic constituent surface, and direct electropolymerization with in situ materials engineering to create a tunable core/shell hybrid structure.
4.2.1 Infiltration
Infiltration/percolation of conjugated polymers into nanoporous materials or a nanorod array (e.g TiO2) have been exploited for many of the earlier studies aiming to achieve ordered/semi-ordered active layer structures. The main hurdle in this concept using nanoporous materials is the difficulty in filling the pores efficiently. Infiltration effectiveness can be improved by using a proper surface modifier. Filling ordered, controlled morphologies of n-type inorganic semiconductor with p-type organic polymers can be easier compared to a randomly porous structure and be more effective in charge transport.
Another efficient device is achieved with crystalline perovskites [38], which combines a transparent p-type hole conductor (e.g. spiro-OMeTAD) and mesoporous alumina (Al2O3), delivering up to 10.9 % power conversion efficiency. This class of solar cells shows open-circuit photovoltages in excess of 1.1 V, despite the narrow bandgap of 1.55 eV. The engineered structure takes advantage of mesoporous alumina that forces the electrons to be transported through the perovskite. The strength of hybrid photovoltaics resides especially in the fact that the fabrication is solution-processable, allowing reel-to-reel, flexible manufacturing integrations.
Vertically grown nanorods short in length are also appealing inorganic candidates [39]. A polymer solution can be spin-coated or drop-cast onto the nanorod array so that the polymer fills all the gaps in-between the rods, forming an interdigitated structure reminiscent of that shown in Fig. 12.17. The height of the nanorods, thus the final active layer thickness, should be kept at around 200 nm—the optimal thickness for organic solar cells absorption. However, due to the approximately equal amount of organic and inorganic materials used in this structure and the significantly higher mobility of the inorganic material, there is likely to be a build up of charges for carriers that the polymer transfers, thus leading to low efficiency. Therefore, a core/shell-like architecture is desirable for realizing efficient hybrid solar cells.
4.2.2 Controlled Spin-Coating
Perhaps the most widespread method adopted to form organic-inorganic heterojunctions is controlled spin-coating. This process involves depositing a small quantity of conjugated polymer onto the center of the sample and then spinning it at high speed. The centripetal torque will force the polymer to spread out to the edges of the sample. The resulting thickness will depend on the properties of the polymer solution (e.g. viscosity and surface tension) and the parameters of the spin process (e.g. ramp, speed, and time). One limitation of this method is that it works well for planar and low aspect-ratio surfaces (e.g. cones), but not the more demanding high aspect ratio nanowires. This is due to the fact that the centripetal force while spinning will restrict the polymer to predominantly flow horizontally, limiting the chance of pore infilling or coverage of high-aspect ratio nanostructures (e.g. nanowires). For instance, hybrid solar cells can be created by coating poly(3-hexylthiophene) (P3HT) on GaAs nanowires by carefully tuning the spin-coating conditions [40]. A standard fabrication process is presented in Fig. 12.18. The n-type GaAs nanopillars are grown in the form of ordered arrays and low-cost spin-coating is adopted to realize the p-type coating of P3HT. To complete the device, indium tin oxide is sputtered as a transparent top contact. However, the conversion efficiency greatly depends on the treatment that the GaAs surface undergoes prior to the polymer deposition.
Modifying the inorganic 1-D morphologies presents another route for achieving well controlled core/shell structures. For instance, a vertically asymmetrical architecture of the inorganic constituent, such as nanocones or nanodomes, can facilitate uniform coating via spin-coating. Since the polymer solution tends to settle near the bottom with such processing method, the significantly larger bottom of the nanocones allow for the spreading and thus a more uniform coating of the polymer (Fig. 12.19). Schottky hybrid cells comprising conformal coatings of PEDOT on periodic n-Si nanocones recently exhibited efficiencies of up to 9.6 % [41].
4.2.3 Self-assembly
Polymeric semiconductors can be self-assembled onto the surface of inorganic nanowires. A P3HT/ZnO hybrid nanowire photovoltaic device is used as an example here [42].
Very little interactions exist between the donor P3HT and the acceptor ZnO, meaning that either the ZnO surface or the P3HT needs to be functionalized in order for self-assembly to occur. Bromine-terminated regioregular P3HT was reacted with butyllithium then diethylchlorophosphate to yield a phosphonic ester-end group for the P3HT molecules (Fig. 12.20a). The phosphonic ester group has high affinity for ZnO, thus leading the P3HT chains to form ordered lamellae on the ZnO nanowire surfaces (Fig. 12.20b–d). The thickness of the polymer coating is about 7–20 nm, which is close to the exciton diffusion length in organic materials. A single nanowire solar cell was fabricated with Al in contact with the P3HT as the anode, and Au as the cathode for the ZnO end. A low device efficiency of 0.036 % was obtained. However, it is mostly due to the fact that little light can be absorbed by a single nanowire, leading to a J sc of only ~0.32 mA cm−2. A large area array containing thousands of such hybrid nanowires can lead to a much higher short circuit current density and thus improved conversion efficiency.
A single nanowire P3HT/ZnO hybrid solar cell achieved by self-assembling the end-functionalized polymer onto the nanowire surface: (a) Synthetic scheme of the phosphonic acid-terminated P3HT and its assembly onto ZnO nanowires. (b–d) TEM analysis showing the core/shell structure and the lamellar packing of the P3HT chains on the ZnO surface. A proposed packing model is shown in (e)
4.2.4 Direct Electrochemical Polymerization
All the methods discussed above share the common ground that they are based on combining the synthesized organic and inorganic components via different approaches. However, the organic conductor/semiconductor can also be directly polymerized onto ordered inorganic nanowire arrays by direct electrochemical polymerization. A one-compartment three-electrode setup similar to that for electrochemical doping discussed in Sect. 3.2.1.2 is also suitable for this purpose. A Schottky junction GaAs/PEDOT core/shell nanowire solar cell serves as an example here [43]. A highly doped n-GaAs wafer with vertically oriented nanowire arrays was used as the working electrode, while a Ag/AgCl reference electrode and a Pt sheet counter electrode complete the setup. Anodic polymerization under potentiodynamic conditions achieved by scanning cyclic voltammetry (CV) passing the oxidation potential of the EDOT monomer dissolved in an electrolyte solution led to a uniform PEDOT film on the GaAs nanowire surfaces (Fig. 12.21b, c). The film thickness was found to have a direct relationship with the number of CV cycles, offering a simple method for controlling the polymer thickness. Furthermore, by taking advantage of the in situ electrochemical doping of the PEDOT with the counterions from the electrolyte solution, the properties of the PEDOT shell can be readily tuned simply by incorporating different electrolytes. Five counteranions that can dope PEDOT were tested: poly(styrenesulfonate) (PSS−), dodecyl sulfate (DS−), perchlorate (ClO4 −), tetrafluoroborate (BF4 −), and hexafluorophosphate (PF6 −). Each dopant led to a different conductivity value for the final PEDOT film, with PEDOT/ClO4, PEDOT/BF4, and PEDOT/PF6 possessing the highest three conductivities of 68, 90, and 116 S cm−1, respectively. Slightly different HOMO energy levels results from these three films as well. The higher conductivities and the deeper HOMO level for PEDOT lead to direct improvement in all figures of merit for the solar cells, resulting in PCEs of 1.07, 2.71, and 4.11 % for n-GaAs nanowires with PEDOT/ClO4, PEDOT/BF4, and PEDOT/PF6 shells, respectively (Fig. 12.21f). This method opens up new opportunities to (1) achieve conformal coating of organic material on ordered inorganic nanowire arrays, which preserves the 3-D morphology of the final cell, (2) in situ material engineering of the organic component in terms of feature size, energy level, and electrical properties, and (3) the combination of all these factors that provides us the ability to rationally design and tune the performance of a hybrid solar cell.
Three-dimensional core/shell hybrid solar cells and device performance tuning achieved by in situ materials engineering via direct electropolymerization of an organic material onto an inorganic nanopillar arrays. (a–c) Illustrate the polymer shell thickness control using different numbers of scan cycles. (d and e) Shows the schematic of the solar cell and the SEM of the actual completed device with ITO top electrodes, respectively. The J–V characteristics of the solar cell with PEDOT doped with different dopants are presented in (f)
5 Interface Manipulation
Functionalization and surface treatment of nanostructures is an area of crucial importance in the nanotechnology field. Controlling the surface properties and tuning its interactions at the nanometer scale represent critical issue for high-performance devices. Engineering the interactions between such diverse organic and inorganic classes of materials represents an essential step towards understanding the corresponding interface properties upon the solar cell behavior.
5.1 Surface Functionalization
The study presented in Sect. 4.2.3 provides an excellent example for illustrating the importance of surface functionalization. Inorganic surfaces often do not provide a high enough affinity for the desired polymers to bond. Therefore, either the surface of the inorganic material needs to be modified, or the polymer has to be functionalized, either at the end of the chain or on the backbone. For example, in order to form a P3HT/CdSe quantum dot hybrid, the surface of the CdSe was capped with (4-bromophenyl)methyl dioctylphosphine oxide (DOPO-Br), so that the vinyl-terminated P3HT can be readily grafted onto the quantum dot [44]. Alternatively, for the study presented in Sect. 4.2.3, no surface modification was performed for ZnO, but the P3HT chain terminal needs to be converted to a phosphonic ester in order for bonding to occur. Its oxygenated groups interact with the oxygen from the ZnO surface, thus leading to grafting and self-assembly.
5.2 Surface Passivation
At the nanometer scale, surface properties become more relevant since the surface-to-volume ratio increases. The surfaces of nanostructures can be affected by electronically active surface states due to unterminated atomic chains or dangling bonds. Surface passivation aims to rebond these dangling bonds with certain passivating agents while maintaining charge neutrality after the passivation [45]. For instance, one recent study of hybrid solar cells based on poly(3-hexylthiophene) and GaAs nanopillars [40] shows that the surface passivation is crucial to alleviate the surface states on the nanopillar facets, leading to a much enhanced efficiency. Nonetheless, only simple inorganic molecules were adopted (e.g. (NH4)2S). Organo-thiol-based molecules can form self-assembled monolayers on semiconductor surfaces, which not only passivate, but also offer well-defined and tunable properties. Organic surface modifiers are currently being investigated since they promote infiltration of polymers along high-aspect ratio configurations. Surface passivation can be extended also to fully inorganic solar cells where nanostructures are exploited [46].
6 Future Directions in Hybrid Photovoltaics
Current state-of-the-art hybrid solar cells based on nanowires and pyramids are able to achieve up to 10 % power conversion efficiencies [47]. On the other side, recent advances in nanowire-based InP photovoltaics demonstrated efficiencies up to 13.8 % [48]. These numbers raise the bar for nanostructured solar cells and reduce the skepticism gap with respect to mature, mass-produced planar solar cells. Another important aspect for nanotechnology applied to photovoltaics is the reuse of substrates [49]. Considering that the active area is represented by the sole nanostructures (not the substrate as in conventional solar cells), the primary function of the mother substrate is to support a lattice-matched growth. In fact, subsequent to growth, the nanowires can be detached from their platform enabling the fabrication of vertically aligned electronic devices with metal electrodes on arbitrary supporting substrates (e.g. Kapton film, aluminum foils, and flexible plastic) [50]. Such a process eliminates any manufacturing cost related to the substrate, which can be reused consecutively. Thin-film photovoltaics are often included into flexible designs. However, a highly efficient nanowire solar cell necessitates only ~12 % of an active semiconductor material, further reducing material-associated expenses. For the organic component, aside from the benchmark materials such as P3HT and PEDOT, very few newer and molecularly engineered polymers, such as those outlined in Fig. 12.12 that have led to highly efficient organic solar cells, have been tested for hybrid photovoltaics. As the synthetic approaches become more mature and with extensive interdisciplinary collaborations, these sophisticated polymers are likely to help enhance the hybrid solar cell efficiencies as well. In fact, a new class of polymers specifically designed and produced to align with the energy levels and physical properties of the inorganic components could emerge and lead to a new altitude for photovoltaic research. Furthermore, the bendability of the 3-D structures arising from the inorganic nanowire arrays coupled with the flexibility of the organic coatings could potentially lead to low cost, flexible, possibly wearable photovoltaics. The colloidal dispersibility or solution solubility of the organic materials also allows for ink-jet printing or spray coating of polymers onto the inorganic nanowire arrays, providing golden opportunities for roll-to-roll manufacturing of hybrid solar cells.
References
Green, M.A., Emery, K., Hishikawa, Y., Warta, W., Dunlop, E.D.: Solar cell efficiency table (version 41). Prog. Photovolt. Res. 21, 1–11 (2013)
Li, G., Zhu, R., Yang, Y.: Polymer solar cells. Nat. Photon. 6, 153–161 (2012)
Jorgesen, M., Norrman, K., Gevorgyan, S.A., Tromholt, T., Andreasen, B., Krebs, F.C.: Stability of polymer solar cells. Adv. Mater. 24, 580–612 (2012)
Arici, E., Sariciftci, N., Serdar, N., Dieter, M.: Encyclopedia of Nanoscience and Nanotechnology, vol. 3, pp. 99–944. (2004)
Gunes, S., Sariciftci, N.S.: Hybrid solar cells. Inorg. Chim. Acta 361, 581–588 (2008)
Whitesides, G.M., Kriebel, J.K., Mayers, B.T.: Nanostructure Science and Technology. Springer Publisher (2005)
Murray, C.B., Sun, S., Gaschler, W., Doyle, H., Betley, T.A., Kagan, C.R.: IBM J. Res. Dev. 45, 47–56 (2001)
Allen, P.M.: Semiconductor nanocrystals: synthesis, mechanism of formation, and applications in biology. PhD Dissertation, MIT (2010)
Sargent, E.H.: Colloidal quantum dot solar cells. Nat. Photon. 6, 133–135 (2012)
Wang, X., Koleilat, G.I., Tang, J., Liu, H., Kramer, I.J., Debnath, R., Brzozowski, L., Barkhouse, D.A.R., Levina, L., Hoogland, S., Sargent, E.H.: Tandem colloidal quantum dot solar cells employing a graded recombination layer. Nat. Photon. 5, 480–484 (2011)
Wagner, R.S., Ellis, W.C.: Vapor-liquid solid mechanism of single crystal growth. Appl. Phys. Lett. 4, 89 (1964)
Kim, D.-H., Lu, N., Ghaffari, R., Rogers, J.A.: Inorganic semiconductor nanomaterials for flexible and stretchable bio-integrated electronics. NPG Asia Mater. 4, 1–9 (2012)
Ouyang, G., Yang, G., Sun, C., Zhu, W.: Small 4, 1359–1362 (2008)
Lee, J., Ju, H., Yi, Y., Lee, J., Uhm, S., Lee, J.K., Lee, H.J.: J. Phys. Chem. C 4, 2915–2918 (2012)
Gebeyehu, D., Brabec, C.J., Sariciftci, N.S., Vangeneugden, D., Kiebooms, R., Vanderzande, D., Kienderger, F., Schindler, H.: Hybrid solar cells based on dye-sensitized nanoporous TiO2 electrodes and conjugated polymers as hole transport materials. Synth. Met. 125, 279–287 (2002)
Johansson, E.M.J., Pradhan, S., Wang, E., Unger, E.L., Hagfeldt, A., Andersson, M.R.: Efficient infiltration of low molecular weight polymer in nanoporous TiO2. Chem. Phys. Lett. 502, 225–230 (2011)
MacDiarmid, A.G.: “Synthetic metals”: a novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Ed. 40(14), 2581–2590 (2001)
Zhou, Y., Geng, J., Li, G., Zhou, E., Chen, L., Zhang, W.: J. Polym. Sci. B Polym. Phys. 44, 764–769 (2006)
Bredas, J.L., Street, G.B.: Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 18(10), 309–315 (1985)
McGehee, M.D., Miller, E.K., Moses, D., Heeger, A.J.: Advances in Synthetic Metals, 1st ed. Chap. 2, pp. 98–205. In: Bernier, P., Lefrant, S., Bidan, G. (eds.) Elsevier, Switzerland (1999)
Tran, H.D., Li, D., Kaner, R.B.: One-dimensional conducting polymer nanostructures: bulk synthesis and applications. Adv. Mater. 21(14–15), 1487–1499 (2009)
Mayer, A.C., Scully, S.R., Hardin, B.E., Rowell, M.W., McGehee, M.D.: Polymer-based solar cells. Mater. Today 10(11), 28–33 (2007)
Reese, C., Bao, Z.: Organic single-crystal field-effect transistors. Mater. Today 10(3), 20–27 (2007)
Reghu, M., Cao, Y., Moses, D., Heeger, A.J.: Counterion-induced processibility of polyaniline—transport at the metal-insulator boundary. Phys. Rev. B 47(4), 1758–1764 (1993)
Groenendaal, B.L., Jonas, F., Freitag, D., Pielartzik, H., Reynolds, J.R.: Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Adv. Mater. 12(7), 481–494 (2000)
Li, D., Kaner, R.B.: How nucleation affects the aggregation of nanoparticles. J. Mater. Chem. 17(22), 2279–2282 (2007)
Liang, Y.Y., Yu, L.P.: A new class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance. Acc. Chem. Res. 43(9), 1227–1236 (2010)
Su, Y.-W., Lan, S.-C., Wei, K.-H.: Organic photovoltaics. Mater. Today 15(12), 554–562 (2012)
Chen, H.Y., Hou, J., Zhang, S., Liang, Y., Yang, G., Yang, Y., Yu, L., Wu, Y., Li, G.: Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photon. 3(11), 649–653 (2009)
Chen, T.-A., Rieke, R.D.: The first regioregular head-to-tail poly(3-hexylthiophene-2,5-diyl) and a regiorandom isopolymer: Ni vs Pd catalysis of 2(5)-bromo-5(2)-(bromozincio)-3-hexylthiophene polymerization. J. Am. Chem. Soc. 114, 10087–10088 (1992)
Mao, H., Xu, B., Holdcroft, S.: Synthesis and structure-property relationships of regioirregular poly(3-hexylthiophenes). Macromolecules 26, 1163–1169 (1993)
Meng, H., Perepichka, D.F., Wudl, F.: Facile solid-state synthesis of highly conducting poly(ethylenedioxythiophene). Angew. Chem. Int. Ed. 115(6), 682–685 (2003)
Hardin, B.E., Snaith, H.J., McGehee, M.D.: The renaissance of dye-sensitized solar cells. Nat. Photon. 6, 162–169 (2012)
Ayllon, J.A., Lira-Cantu, M.: Application of MEH-PPV/SnO2 bilayer as hybrid solar cell. Appl. Phys. A Mater. Sci. Proc. 95(1), 249–255 (2009)
Huynh, W.U., Dittmer, J.J., Alivisatos, A.P.: Hybrid nanorod-polymer solar cells. Science 295(5564), 2425–2427 (2002)
Ren, S., Zhao, N., Crawford, S.C., Tambe, M., Bulovic, V., Gradecak, S.: Heterojunction photovoltaics using GaAs nanowires and conjugated polymers. Nano Lett. 11(2), 408–413 (2011)
Gharghi, M., Fathi, E., Kante, B., Sivoththaman, S., Zhang, X.: Heterojunction silicon microwire solar cells. Nano Lett. 12, 6278–6282 (2012)
Lee, M.M., Teuscher, J., Miyasaka, T., Murakami, T.N., Snaith, H.J.: Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338, 643–647 (2012)
Kuo, C.Y., Tang, W.C., Gau, C., Guo, T.F., Jeng, D.Z.: Ordered bulk heterojunction solar cells with vertically aligned TiO(2) nanorods embedded in a conjugated polymer. Appl. Phys. Lett. 93(3), 033307 (2008)
Mariani, G., Laghumavarapu, R.B., TremoletdeVillers, B., Shapiro, J., Senanayake, P., Lin, A., Schwartz, B.J., Huffaker, D.L.: Hybrid conjugated polymer solar cells using patterned GaAs nanopillars Appl. Phys. Lett. 97, 013107 (2010)
Jeong, S., Garnett, E.C., Wang, S., Yu, S., Fan, S., Brongersma, M.L., McGehee, M.D., Cui, Y.: Hybrid silicon nanocone-polymer solar cells. Nano Lett. 12, 2971–2976 (2012)
Briseno, A.L., Holcombe, T.W., Boukai, A.I., Garnett, E.C., Shelton, S.W., Frechet, J.J., Yang, P.: Oligo- and polythiophene/ZnO hybrid nanowire solar cells. Nano Lett. 10(1), 334–340 (2010)
Mariani, G., Wang, Y., Wong, P.S., Lech, A., Hung, C.H., Shapiro, J., Prikhodko, S., El-Kady, M., Kaner, R.B., Hufraker, D.L.: Three-dimensional core-shell hybrid solar cells via controlled in situ materials engineering. Nano Lett. 12(7), 3581–3586 (2012)
Xu, J., Wang, J., Mitchell, M., Mukherjee, P., Jeffries-EL, M., Petrich, J.W., Lin, Z.Q.: Organic-inorganic nanocomposites via directly grafting conjugated polymers onto quantum dots. J. Am. Chem. Soc. 129(42), 12828–12833 (2007)
Huang, X., Lindgren, E., Chelikowsky, J.R.: Surface passivation method for semiconductor nanostructures. Phys. Rev. B 71, 165328 (2005)
Mariani, G., Wong, P.-S., Katzenmeyer, A.M., Leonard, F., Shapiro, J., Huffaker, D.L.: Patterned radial GaAs nanopillar solar cells. Nano Lett. 11, 2490–2494 (2011)
He, L., Lai, D., Wang, H., Jiang, C., Rusli: High-efficiency Si/polymer hybrid solar cells based on synergistic surface texturing of Si nanowires on pyramids. Small 8, 1664–1668 (2012)
Wallentin, J., Anttu, N., Asoli, N., Huffman, M., Aberg, I., Magnusson, M.H., Siefer, G., Fuss-Kailuweit, P., Dimroth, F., Witzigmann, B., Xu, H.Q., Samuelson, L., Deppert, K., Borgstrom, M.T.: InP nanowire array solar cells achieving 13.8% efficiency by exceeding the ray optics limit. Science (2013). doi: 10.1126/science.1230969
Spurgeon, J.M., Plass, K.E., Kayes, B.M., Brunschwig, B.S., Atwater, H.A., Lewis, N.S.: Repeated epitaxial growth and transfer of arrays of patterned, vertically aligned, crystalline Si wires from a single Si(111) substrate. Appl. Phys. Lett. 93, 032112 (2008)
Weisse, J.M., Lee, C.H., Kim, D.R., Zheng, X.: Fabrication of flexible and vertical silicon nanowire electronics. Nano Lett. 12, 3339–3343 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mariani, G., Wang, Y., Kaner, R.B., Huffaker, D.L. (2014). Hybrid Solar Cells: Materials, Interfaces, and Devices. In: Wang, X., Wang, Z. (eds) High-Efficiency Solar Cells. Springer Series in Materials Science, vol 190. Springer, Cham. https://doi.org/10.1007/978-3-319-01988-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-01988-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-01987-1
Online ISBN: 978-3-319-01988-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)