Abstract
Results of recent magnetic resonance imaging studies suggest that meditation may be associated with region-specific structural neuroplasticity. To test the hypothesis that meditation-related brain function predicts site-specific structural changes in meditators, we conducted two meta-analyses: one of studies localizing brain activity during meditation, and a second of studies measuring differences in brain structure between meditators and non-meditators. Activation Likelihood Estimation (ALE) meta-analysis of five studies measuring brain activation during meditation revealed the greatest clusters of activity to be in the left frontal cortex and left precuneus. ALE of four studies measuring the differences in brain structure between meditators and controls revealed that meditators tended to have greater brain volume in the left inferior temporal gyrus. Thus, brain activity during meditation did not predict region-specific structural differences between meditators and non-meditators. This finding may reflect recognized limitations in neuroimaging methodology rather than the refutability of the hypothesis itself. Future efforts aimed at understanding the relationship between brain activity and structural changes in the brain should focus on improving neuroimaging experimental design and incorporating evidence from other branches of neurocognitive science. Progress in these areas promises to elucidate the connection between mind-body practices, and brain structure and function.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Studies Measuring Brain Activity
- Left Inferior Temporal Gyrus
- Activation Likelihood Estimation (ALE)
- Mind-body Practices
- Green Cluster
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Recent magnetic resonance imaging (MRI) studies demonstrate that training in some skills such as music, language, and spatial navigation is associated with increased gray matter in regions relevant to the learned task (Gaser and Schlaug 2003; Machelli et al. 2004; Maguire et al. 2000). Intensive training in juggling, for example, leads to increased gray matter in regions known to be important in perceptual-motor coordination (Draganski et al. 2004). In addition, increases in gray matter occur in less time than previously recognized. A 2007 study aimed at defining the usage dependent time scale of structural changes found that architectural alterations in the brain were detectable within a week of the intervention (May et al. 2007).
Meditation is a mind-body practice which originated in ancient religious and spiritual traditions. In Western culture, its secular clinical application falls under the rubric of complementary and alternative medicine (CAM). Meditation incorporates a diverse array of practices including various combinations of breathing, mantra recitation, focused attention, and practices related to spiritual beliefs. It has been extensively studied as a method of relaxation and has been associated with numerous physiological benefits (Benson 1984; Davidson et al. 2003; Kabat-Zinn et al. 1992, 1998). It has also been used as a method of fostering attention and emotional self-regulation (Jha et al. 2007; Ospina et al. 2007).
Functional magnetic imaging studies of meditation demonstrate that meditation is associated with brain activation in a number of cortical and sub-cortical regions including the frontal and parietal cortices, known to be important in the mental process of attention, and the insula, known to be important in emotional regulation (Lazar et al. 2000; Holzel et al. 2007). Some authors have found structural differences between the brains of meditators and non-meditator controls and speculate that differences are due to a meditation-associated training effect and structural neuroplasticity (Lazar et al. 2005; Holzel et al. 2008; Luders et al. 2009).
While common areas of activation have been identified among neuroimaging studies exploring the brain during meditation, there is some inconsistency across studies. Moreover, studies exploring differences in the brain structure of meditators versus controls fail to demonstrate consistent differences between the two groups.
Meta-analysis, specifically coordinate-based meta-analysis (CBMA), offers a method of pooling neuroimaging study results, and thus can be used to summarize and clarify these two sub-categories of MRI studies (i.e., studies of brain activation associated with meditation, and studies measuring structural differences between meditators and non-meditators). Results of such meta-analyses are amenable to comparison and would help determine if brain activity during meditation predicts differential brain structure between meditators and non-meditators. To date, no such studies have been published.
To explore the hypothesis that meditation mediates region-specific structural neuroplasticity, we reviewed the literature to identify fMRI studies measuring brain activity during meditation, and performed Activation Likelihood Estimation (ALE) meta-analysis of the pooled coordinates. Studies measuring brain structure in meditators were considered separately and coordinates were pooled in a second ALE meta-analysis (Laird et al. 2005; Turkeltaub et al. 2002). We then compared the results of these meta-analyses to see if brain activation predicted region-specific structural differences between meditators and non-meditators.
Methods
Two independent reviewers completed a multilevel systematic literature search. Using the key words “meditation AND mri”, databases (PubMed, EMBASE, Cinahl, Cochrane Database for Systematic Reviews, and PsychINFO) were searched for MRI studies of meditation published since 2000. (Because “yoga” is synonymous to “meditation” in Cinahl, the key words “yoga AND mri” were also used to search this database). Our gray literature search included searches of metaRegister of Controlled Trials, Cochrane Central Register of Controlled Trials, and Google Scholar. References of identified studies were hand searched.
For a study to be included in our review, it had to meet the following inclusion/exclusion criteria: (1) the study had to be an interventional or observational study; case reports, case series, letters, and editorials were excluded; (2) the study had to use magnetic resonance imaging to measure neural activity during meditation (state), or to measure brain structure of individuals with meditation experience versus controls (trait); (3) studies measuring differential responses to stimuli (pain, sound, etc.) during meditation versus rest, or in meditators versus non-meditators were not included in this review. In instances where studies focused primarily on stimulus–response but also included information on activation during meditation, pertinent information was extracted from the study and included in our review; (4) studies had to be published between 2000 and 2009 (2000 documents the first study of this kind); and (5) while subject description was required, there was no restriction on the study population. Disagreements between reviewers with regard to inclusion/exclusion of individual studies were resolved by consensus.
Activation Likelihood Estimation (ALE) meta-analysis was performed using GingerALE v1.1 software and the following parameters: a full-width half maximum (FWHM) of 10 mm, permutations of 5000, q-value of 0.05, and a minimum cluster size of 100 mm3 (Laird et al. 2005). Once the thresholded ALE map was generated using GingerALE v1.1, an anatomical underlay (in Talairach space) was downloaded and images were generated using Multi-Image Analysis Graphical User Interface (Mango) (Kochunov et al. 2002) (Lancaster and Martinez Accessed 24 May 2009).
Results
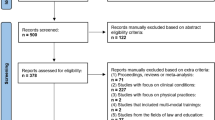
Using the inclusion criteria outlined, a total of 1,116 titles/abstracts were reviewed and 1,065 were excluded. From the remaining 51 abstracts, the full articles were reviewed in more detail and 13 articles were considered for inclusion. Four studies were excluded from meta-analysis because Montreal Neurologic Institute (MNI) coordinates were not reported. This resulted in nine studies eligible for meta-analysis, five were studies of brain activation during meditation, and four were studies comparing brain structure between meditators and non-meditators (see Fig. 1).
Systematic review of the 13 included studies, which used MRI to study meditation/meditators, revealed that such studies are generally small, ranging in size from 5 subjects to 44 subjects. Most studies included controls. However, two of the studies measuring brain activation during meditation used the non-meditative state as the control. The age of participants ranged from 22 years to 71 years (mean 34–53 years). Overall, there was a preponderance of males studied. In the 11 of 13 experiments where both sexes were represented, investigators took care to ensure that equal numbers of each sex were included in the experimental and control groups. Most participants were right handed. Handedness was matched in studies reviewed. The type of meditation practiced and the duration of practice varied between studies (2–46 years). While attention was given to meditation type and the possible impact that this might have on MRI results, from the perspective of neuroimaging, meaningful subcategories have yet to be delineated and meditation type was not considered here (i.e., all meditation types were included in meta-analysis).
In addition to the potential covariates listed in Table 1, inconsistently reported covariates include ethnicity/race, and level of education. Very few studies reported on the general health of participants and controls. Only 1 out of 13 studies included information about medication use. With regard to image acquisition and statistical analysis, the majority of studies reviewed included information about scanners and software utilized. Only studies which reported statistically significant coordinates were included in meta-analysis (see Table 1).
Brain Activation During Meditation
Two of the studies reviewed measured brain activation during meditation as compared to rest (Shimomura et al. 2008; Lazar et al. 2000); these studies revealed consistently greater activation in the frontal cortex during meditation. The parietal and cingulate cortices were also activated during meditation, but this finding was less consistent. (Lazar et al.) found additional areas of activation in the midbrain and putamen (Lazar et al. 2000).
Three studies measured the difference in brain activity during meditation between meditators and non-meditators. (Lutz et al. 2008); (Brefczynski-Lewis et al. 2007); and (Hölzel et al. 2007) found consistently greater activation during meditation in meditators versus controls in the frontal, parietal, occipital/temporal cortices and hippocampus and, less consistently, greater activation in the cingulate cortex and other subcortical regions (Lutz et al. 2008; Brefczynski-Lewis et al. 2007; Holzel et al. 2007). These authors infer that activation of specific brain areas is related to attention and emotional regulation and that the differential activation between meditators and non-meditators represents a measurable training effect.
Activation Likelihood meta-analysis of studies measuring brain activation during meditation revealed the greatest clusters of activity to be in the left superior frontal gyrus, left medial frontal gyrus, left middle frontal gyrus, and the left precuneus. Areas reported to demonstrate activation in more than one of the individual studies but which did not result in significant clusters of activity on ALE include the cingulate and insula (see Table 2, Figs. 2 and 3). (See Appendix 1 for details of extracted coordinates.)
Mango images of ALE activation clusters (Table 2): brain activation during meditation – axial view
Mango images of ALE activation clusters (Table 2): brain activation during meditation – sagittal view
Differences in Brain Structure Between Meditators and Controls
Pagnoni and Cekic (2007) found that the total gray matter volume showed a marginally significant negative correlation with age in the control group but not the meditators. Meditators showed significantly less age-related gray matter volume decline in the left putamen than controls (Pagnoni Gand Cekic 2007).
Hölzel et al. (2008) used voxel-based morphometry to measure the gray matter concentration in meditators versus non-meditators. Gray matter concentration (GMC) was found to be greater in meditators than non-meditators in the right hippocampus and the right anterior insula. Concentration in the left inferior temporal gyrus showed a trend toward significance. The GMC of the medial orbitofrontal cortex was positively correlated with hours of practice. There was a positive correlation between total hours of training in meditation and mean GMC within the left inferior temporal gyrus. This suggests a causal relationship (Holzel et al. 2008).
Vestergaard-Poulsen et al. (2009) found greater gray matter density in meditators versus controls in the medulla oblongata, left superior frontal gyrus, left inferior frontal gyrus, anterior cerebellum, and the left fusiform gyrus. Structural differences were also seen in the dorsal medulla gray matter/dorsal motor nucleus of the vagal nerve (regions of autonomic respiratory control and vagal tone). There was no correlation between structure and hours of practice (Vestergaard-Poulsen et al. 2009).
Luders et al. (2009) reported that there were significantly greater gray matter volumes in meditators versus controls in the right orbitofrontal cortex, thalamus, posterior superior parietal lobule, and inferior temporal gyrus as well as the right and left paracentral lobule. Significantly larger hippocampal volumes were also seen. Global cerebral measurements were the same for meditators and controls. Type of meditation practiced had no impact on results. Number of years practiced did not correlate with GM volume (Luders et al. 2009).
In summary, among studies measuring differences in brain structure between meditators and controls, differences are noted most frequently in the frontal and temporal cortices as well as subcortical regions including the hippocampus, thalamus, brainstem, and cerebellum.
ALE meta-analysis of studies measuring the difference between meditators and non-meditators revealed the greatest cluster to be in the left inferior temporal gyrus. The second greatest cluster was found in the brainstem (noted visually) but fell outside of the normalized brain used here. Multiple other areas of differential cluster size were found, but these were much smaller than the two largest clusters (see Figs. 4 and 5, Table 3). (See Appendix 2 for details of extracted coordinates.)
Mango images of ALE volume clusters (Table 3): differences in brain structure between meditators and controls – axial view
Mango images of ALE volume clusters (Table 3): differences in brain structure between meditators and controls – sagittal view
Discussion
Neuroplasticity refers to the ability of the nervous system to adapt to internal and external stimuli. Magnetic resonance imaging allows assessment of meditation-associated brain activity, differential brain activation in meditators versus non-meditator controls (a training effect), and subtle in vivo structural differences between meditators and controls.
In the studies of brain activation reviewed here, fMRI demonstrates that meditation is associated with activation in a variety of areas, most consistently the frontal and parietal cortices, and the hippocampus. Meta-analysis revealed the most significant clusters of brain activation during meditation to be in the left frontal and parietal cortices. This result is consistent with the individual studies. Authors of the studies reviewed here speculate that meditation is associated with a training effect and that activation in reported areas is associated with the cognitive tasks required during meditation: attention/focus, management of discursive thoughts, and emotional regulation.
Four studies (130 subjects’ total) measured structural differences in the brains of meditators and controls (Vestergaard-Poulsen et al. 2009; Holzel et al. 2008; Luders et al. 2009; Pagnoni and Cekic 2007). These studies found greater volumes of cortical, hippocampal, and subcortical gray matter in meditators as compared to controls. Specific structural differences varied between studies. Meta-analysis appeared to adequately summarize individual study findings and demonstrated the largest structural difference to be in the inferior temporal gyrus.
In several domains, intensive training has been found to increase gray matter in regions relevant to a learned task – increased gray matter in the posterior intraparietal sulcus (known to be important perceptual-motor coordination and visual attention) in jugglers, for example. In the current analysis, regional activation during meditation (most prominent in the frontal cortex) did not predict structural differences between meditators and controls (most prominent in the inferior temporal gyrus) (see Figs. 6 and 7).
There are several potential reasons for failure of meditation-associated brain activity to predict region-specific structural differences. First, the exact relationship between neural activity and the BOLD signal has yet to be elucidated. Limits in knowledge of precisely what neuronal activity (i.e., excitation or inhibition) is reflected by the hemodynamically-dependent BOLD signal limits the degree to which inferences can be drawn from its measurement. Second, as an extension of this principle, the quantity of information processing by the brain does not always correlate with metabolic activity. Task practice can result in increased performance efficiency and simultaneous decrease in brain activity as measured by fMRI. This results in confusion as to how to interpret fMRI results. For example, in the current review Brefcyznski-Lewis, et al. found that meditators with more hours of practice showed less activation than meditators with fewer hours of practice. They attribute this to a “…quieter mental state, such that tasks…become more effortless” (Brefczynski-Lewis et al. 2007). On the other hand, Short, et al. found that long-term meditators demonstrated more activity in attention-related brain structures than short-term meditators and interpreted this to reflect greater expertise in meditation (Short et al. 2007). Thus, opposite changes in brain activation have been interpreted as reflecting the same thing – greater task proficiency. Until the relationship between brain activity and cognitive processes is better defined, interpreting the cognitive processes represented by fMRI-based brain activity will remain obscure. Third, results from fMRI studies of meditation potentially reflect experimental design more than the cognitive process of meditation itself. Specifically, the switching of attention necessary with the boxcar design used in these studies could be as responsible for activity in attention-related brain structures as meditation itself. Fourth, neuroscience has yet to fully characterize neuroplasticity at the cellular level. Some brain regions and cell types may respond to training with region specific change in volume while others do not. Fifth, it is also possible that neuroplasticity associated with meditation is not related to a region-specific training effect, but rather to a generally healthier physical environment created by another meditation related factor such as down-regulation of the hypothalamic-pituitary-adrenal (HPA) axis. The findings of Pagnoni et al., that total gray matter volume showed a negative correlation with age in controls but not in mediators, supports this notion. Finally, it is important to remember that meditation is a psychological construct. It can be difficult to operationalize and exists potentially without a definable physical locus or network of loci. Whether or not such complicated thought processes can be characterized by localized brain changes has yet to be determined.
This study includes a number of limitations. First, the total number of studies analyzed here is small. Additional data from future experiments has the potential to significantly impact these results. Second, while ALE meta-analysis is appropriate for evaluation of fMRI studies measuring brain activation and MRI based structural studies separately, there is no precedence in the literature for comparing results from different study subtypes. This practice should be considered speculative at this time. Third, meta-analysis depends on the quality and reporting of constituent studies. Studies evaluated here were inconsistent in reporting potentially important covariates such as general health, and medication use.
The notion that meditation could train the brain in such positive mental attributes as attentiveness, self-regulation, and emotional descrimination and that there attributes could be instantiated structurally has profound implications that warrant further exploration. The study of meditation-associated neuroplasticity would be moved forward most expediently by performing a randomized controlled trial of participants before and after introduction of a meditation program. Recent data indicating shorter usage dependent time-scales for structural change makes such studies more feasible than previously recognized. Simultaneous collection of neurophysiological data including measures of attention and emotional regulation would help to corroborate or refute conclusions drawn from neuroimaging studies, and avoid the need (and trap) of reverse inference. While the authors of the studies reviewed here focus on the cognitive process of attention and emotional regulation during meditation, surprisingly little attention is paid to brain activity related to the hypothalamic-pituitary-adrenal (HPA) axis and relaxation during meditation. Building on the neurophysiological model of the HPA axis proposed by (Newberg et al. 2003) would help to identify relevant brain structures and allow correlation of neuroimaging and hormonal test results (Newberg and Iverson 2003). Improved modeling would also help to determine whether possible meditation-mediated neuroplasticity is related to a general improvement in health or to activation-specific stimuli.
In conclusion, training in some domains leads to measurable structural plasticity in the brain. Previous authors have suggested that meditation is a form of mental training which is associated with region specific structural changes. While it was theorized that studies measuring brain activation during meditation would predict region-specific neuroplasticity, findings here refute this hypothesis. This may have more to do with methodology than the refutability of the hypothesis itself. The notion that mental practices can enhance neuroplasticity warrants further investigation. Such exploration promises to suggest new ways of training the brain and promoting its longevity. And, it may resurrect some old methods along the way.
References
Benson, H. 1984. The relaxation response. New York: Avon.
Brefczynski-Lewis, J.A., A. Lutz, H.S. Schaefer, D.B. Levinson, and R.J. Davidson. 2007. Neural correlates of attentional expertise in long-term meditation practitioners. PNAS 104(27): 11483–11488.
Davidson, R.J., J. Kabat-Zinn, J. Schmacher, M. Rosenkranz, D. Muller, and S.F. Santorelli. 2003. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine 65: 564–570.
Draganski, B., C. Gaser, V. Busch, G. Schuierer, U. Bogdahn, and A. May. 2004. Changes in grey matter induced by training. Nature 427: 311–312.
Gaser, C., and G. Schlaug. 2003. Brain structures differ between musicians and nonmusicians. Journal of Neuroscience 23(27): 9240–9245.
Hölzel, B.K., U. Ott, H. Hempel, A. Hackl, K. Wolf, R. Stark, et al. 2007. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters 421(1): 16–21. doi:S0304-3940(07)00451-X [pii] 10.1016/j.neulet.2007.04.074 [doi].
Hölzel, B.K., U. Ott, T. Gard, H. Hempel, M. Weygandt, K. Morgen, et al. 2008. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience 3(1): 55–61.
Jha, A.P., J. Krompinger, and M.J. Baime. 2007. Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience 7(2): 109–119.
Kabat-Zinn, J., A.O. Massion, J. Kristeller, L.G. Peterson, K.E. Fletcher, L. Pbert, et al. 1992. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. The American Journal of Psychiatry 149(7): 936–943.
Kabat-Zinn, J., E. Wheeler, T. Light, A. Skillings, M.J. Scharf, T.G. Cropley, et al. 1998. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosomatic Medicine 60(5): 625–632.
Khushu, S., S.S. Kumaran, S. Telles, R.P. Tripathi, K.V. Naveen, and T.L. Matthew. 2005. Functional mapping of human brain during meditation: An fMRI study. Biomedicine Trivandrum Then Taramani 25(2): 40–47.
Kochunov, P., J.L. Lancaster, P. Thompson, A.W. Toga, P. Brewer, J. Hardies, et al. 2002. An optimized individual target brain in the Talaraich coordinate system. NeuroImage 17(2): 922–927.
Laird, A.R., M. Fox, C.J. Price, D.C. Glahn, A.M. Uecker, J.L. Lancaster, et al. 2005. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping 25: 155–164.
Lancaster, J.L., and M.J. Martinez. Multi-image analysis GUI. http://ricuthscsaedu/mango/. Accessed 24 May 2009.
Lazar, S.W., G. Bush, R.L. Gollub, G.L. Fricchione, G. Khalsa, and H. Benson. 2000. Functional brain mapping of the relaxation response and meditation. NeuroReport 11(7): 1581–1585.
Lazar, S.W., C.E. Kerr, R.H. Wasserman, J.R. Gray, D.N. Greve, M.T. Treadway, et al. 2005. Meditation experience is associated with increased cortical thickness. NeuroReport 16(17): 1893–1897. doi:00001756-200511280-00005 [pii].
Luders, E., A.W. Toga, N. Lepore, and C. Gaser. 2009. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes. NeuroImage 45(3): 672–678.
Lutz, A., J. Brefcyznyski-Lewis, T. Johnstone, and R.J. Davidson. 2008. Regulation of the neural circuitry of emotion by compassion meditation: Effects of meditative expertise. PLoS One 3(3): 1–10.
Machelli, A., J.T. Crinion, J.T. Noppeney, J. O'Doherty, J. Ashburner, R.S. Frakowiak, et al. 2004. Neurolinguistics: Structural plasticity in the bilingual brain. Nature 431(7010): 757.
Maguire, E.A., D.G. Gadian, I.S. Johnsrude, C.D. Good, J. Ashburner, R.S. Frackowiak, et al. 2000. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences 97(8): 4398–4403.
May, A., G. Hajak, S. Gansbauer, T. Steffens, B. Langguth, T. Kleinjung, et al. 2007. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cerebral Cortex 17: 205–210.
Newberg, A.B., and J. Iverson. 2003. The neural basis of the complex task of meditation:neurotransmitter and neurochemical considerations. Medical Hypotheses 61(2): 282–291.
Ospina, M.B., K. Bond, M. Karkhaneh, L. Tjosvold, B. Vandermeer, Y. Liang, et al. 2007. Meditation practices for health: State of the research. Evidence Report/Technology Assessment 155: 1–263.
Pagnoni, G., and M. Cekic, 2007. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiology Aging 28(10): 1623–1627. doi:S0197-4580(07)00243-6 [pii]10.1016/j.neurobiolaging.2007.06.008 [doi].
Ritskes, R., M. Ritskes-Hoitinga, H. Stodkilde-Jorgensen, K. Baerentsen, T. Hartman, and S. Denmark. 2003. MRI scanning during Zen meditation: The picture of enlightenment. Constructivism in the Human Sciences 8(1): 85–89.
Shimomura, T., M. Fujiki, J. Akiyoshi, T. Yoshida, M. Tabata, H. Kabasawa, et al. 2008. Functional brain mapping during recitation of Buddhist scriptures and repetition of the Namu Amida Butsu: A study in experienced Japanese monks. Turkish neurosurgery 18(2): 134–141.
Short, E.B., S. Kose, Q. Mu, J. Borckhart, A. Newberg, M.S. George, et al. 2007 Regional activation during meditation shows time and practice effects: An exploratory fMRI study. http://creativecommonsorg:1–7.
Turkeltaub, P.E., G.F. Eden, K.M. Jonesm, and T.A. Zeffirom. 2002. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage 16: 765–780.
Vestergaard-Poulsen, P., M. van Beek, J. Skewes, C.R. Bjarkam, M. Stubberup, and J. Bertelsen, et al. 2009. Long-term meditation is associated with increased gray matter density in the brain stem. NeuroReport 20(2): 170–174. doi:10.1097/WNR.0b013e328320012a [doi].
Acknowledgments
Many thanks to Angela Laird, PhD, University of Texas, San Antonio and her staff for support in the use of GingerALE and Mango; Allison Rollins, Librarian, Uniformed Services University of the Health Sciences (USUHS) for assistance in our literature search; and to Wayne Jonas, MD, and Joan Walter of The Samueli Institute, as well as Roger Gibson DVM, Tomoko Hooper, MD, Cara Olsen, PhD, and Daniel Burnett, MD, of USUHS for their support and direction in this project. This work is supported by Uniformed Services University of the Health Sciences under Contract No MDA 905-03-C-0003. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official USU position, policy or decision unless so designated by other documentation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Appendix 1 Extracted Coordinates: Brain Activation During Meditation
Author | Year | Hemisphere | Anatomic Region | x | y | z | Z max | t | p value |
|---|---|---|---|---|---|---|---|---|---|
Shimomura et al. | Left | Superior frontal gyrus | 0 | 5 | 62 | 4.71 | <0.05 | ||
Left | Superior frontal gyrus | −8 | 1 | 63 | 3.89 | <0.05 | |||
Left | Superior frontal gyrus | −4 | −8 | 67 | 3.41 | <0.05 | |||
Left | Superior frontal gyrus | −2 | 6 | 51 | 4.14 | <0.05 | |||
Left | Medial frontal gyrus | −2 | 14 | 49 | 4.56 | <0.05 | |||
Left | Medial frontal gyrus | 0 | 27 | 41 | 3.82 | <0.05 | |||
Left | Middle frontal gyrus | −44 | 14 | 42 | 5.2 | <0.05 | |||
Left | Middle frontal gyrus | −55 | 19 | 29 | 4.05 | <0.05 | |||
Left | Middle frontal gyrus | −50 | 6 | 44 | 3.49 | <0.05 | |||
Right | Supramarginal gyrus | 51 | −53 | 36 | 4.95 | <0.05 | |||
Right | Supramarginal gyrus | 61 | −43 | 33 | 4.12 | <0.05 | |||
Right | Angular gyrus | 44 | −61 | 33 | 4.14 | <0.05 | |||
Right | Medial frontal gyrus | 2 | 14 | 47 | 4.59 | <0.05 | |||
Left | Inferior frontal gyrus | −32 | 35 | 7 | 4.11 | <0.05 | |||
Left | Inferior frontal gyrus | −44 | 43 | −2 | 3.59 | <0.05 | |||
Left | Inferior frontal gyrus | −35 | 24 | 6 | 3.58 | <0.05 | |||
Left | Middle frontal gyrus | −40 | 12 | 51 | 3.82 | <0.05 | |||
Left | Middle frontal gyrus | −32 | 12 | 55 | 3.72 | <0.05 | |||
Left | Middle frontal gyrus | −28 | 5 | 55 | 3.61 | <0.05 | |||
Brefczynski-Lewis et al. | Left | Middle frontal gyrus/Inferior frontal gyrus | −49 | 29 | 19 | 3.2 | <0.005 | ||
Right | Superior frontal gyrus | 31 | 42 | 31 | 2.4 | <0.05 | |||
Left | Middle frontal gyrus/dorsal lateral prefrontal cortex | −21 | 6 | 50 | 2.5 | <0.05 | |||
Left | Rectal gyrus | −0.5 | 43 | −26 | 3.4 | <0.005 | |||
Left | Precentral, dorsal lateral prefrontal cortex | −34 | −2 | 36 | 3 | <0.01 | |||
Left | Intraparietal sulcus, superior parietal, supramarginal gyrus | −24 | −61 | 46 | 3.2 | <0.005 | |||
Right | Superior parietal | 14 | −62 | 54 | 3.8 | <0.005 | |||
Right | Cuneus | 22 | −85 | 11 | 4 | <0.005 | |||
Left | Middle temporal gyrus, inferior frontal gyrus | −38 | −7 | −26 | 5.1 | <0.005 | |||
Right | Middle temporal gyrus | 54 | −12 | −8 | 3.2 | <0.005 | |||
Fusiform | −42 | −55 | −16 | 3.5 | <0.005 | ||||
Left | Putamen | −30 | −20 | 3 | 2.8 | <0.01 | |||
Right | Lentiform, parahippocampus | 29 | −42 | 11 | 2.9 | <0.01 | |||
Cerebellum, declive, culmen | −4 | −56 | −14 | 3.3 | <0.005 | ||||
Left | Cerebellar tonsil | −22 | −39 | −40 | 3.3 | <0.005 | |||
Left | Anterior middle frontal gyrus | −26 | 43 | 7 | −3.17 | <0.01 | |||
Hölzel et al. | Left | Anterior cingulate cortex | −12 | 42 | 12 | 5.11 | 0.002 | ||
Right | Anterior cingulate cortex | 9 | 48 | 9 | 3.97 | 0.025 | |||
Left | Dorsal medial prefrontal cortex | −12 | 45 | 15 | 4.48 | 0.017 | |||
Left | Dorsal medial prefrontal cortex | 0 | 48 | 39 | 4.2 | 0.031 | |||
Right | Dorsal medial prefrontal cortex | 6 | 51 | 3 | 4.36 | 0.017 | |||
Right | Dorsal medial prefrontal cortex | 3 | 48 | 39 | 4.1 | 0.029 | |||
Left | Inferior temporal | −51 | −3 | −42 | 13.43 | 0.000 | |||
Left | Inferior orbital frontal | −45 | 36 | −21 | 8.26 | 0.000 | |||
Right | Cerebellum | 36 | −84 | −42 | 7.98 | 0.000 | |||
Right | Rectus | 15 | 21 | −21 | 7.92 | 0.000 | |||
Left | Cerebellum | −24 | −93 | −36 | 7.73 | 0.000 | |||
Left | Superior medial frontal | −9 | 63 | 6 | 7.45 | 0.000 | |||
Lazar et al. | Anterior cingulum | 6 | 33 | 0 | <0.001 | ||||
Basal ganglia(putamen) | 28 | −15 | −6 | <0.001 | |||||
Midbrain | −15 | −15 | −15 | <0.001 | |||||
Midbrain | 0 | −12 | −9 | <0.001 | |||||
Parahippocampal gyrus | −25 | −24 | −15 | <0.001 | |||||
Superior frontal gyrus | −6 | 24 | 50 | <0.001 | |||||
Middle frontal gyrus | −40 | 30 | 37 | <0.001 | |||||
Medial frontal gyrus | 12 | 48 | 9 | <0.001 | |||||
Parietal lobule | −21 | −48 | 53 | <0.001 | |||||
Superior parietal lobule | −21 | −63 | 53 | <0.001 | |||||
Superior parietal lobule | −31 | −57 | 53 | <0.001 | |||||
Superior parietal lobule | −28 | −54 | 43 | <0.001 | |||||
Superior/inferior parietal lobule | 40 | −60 | 46 | <0.001 | |||||
Inferior parietal lobule | −34 | −36 | 43 | <0.001 | |||||
Superior temporal gyrus | 59 | −60 | 28 | <0.001 | |||||
Middle temporal gyrus | 59 | −57 | 3 | <0.001 | |||||
Parahippocampal gyrus | −28 | −21 | −12 | <0.001 | |||||
Precentral gyrus | 46 | −12 | 53 | <0.001 | |||||
Postcentral gyrus | −25 | −39 | 62 | <0.001 | |||||
Paracentral lobule | −6 | −33 | 65 | <0.001 | |||||
Lutz et al. | L/R | Precuneus | −5 | −55 | 52 | 5.8 | <0.0005 | ||
Right | Supramarginal gyrus | 52 | −43 | 37 | 6.8 | <0.0005 | |||
L/R | Inferior parietal lobule | 46 | −40 | 47 | 6.1 | <0.0005 | |||
L/R | Anterior insula | 37 | 15 | 1 | 4.8 | <0.0005 | |||
L/R | Superior temporal sulcus | 54 | −38 | 14 | 5.7 | <0.0005 | |||
L/R | Superior temporal gyrus | 54 | 5 | −7 | 5.2 | <0.0005 | |||
Right | Superior parietal lobule | 34 | −60 | 46 | 6.1 | <0.0005 | |||
L/R | Posterior cingulate gyrus | 6 | −40 | 40 | 5.5 | <0.0005 | |||
Right | Middle temporal gyrus | 58 | −47 | −3 | 6.4 | <0.0005 | |||
L/R | Parahippocampus | 12 | −39 | 4 | 5.3 | <0.0005 | |||
L/R | Fusiform gyrus | 48 | −38 | −17 | 4.93 | <0.0005 | |||
L/R | Cerebellum | 16 | −48 | −12 | 6.1 | <0.0005 | |||
Right | Anterior cingulate gyrus | 5 | 24 | 37 | 4.1 | <0.005 | |||
Right | Medial frontal gyrus | 9 | 6 | 42 | 4.1 | <0.005 | |||
Right | Mid frontal gyrus | 22 | 9 | 58 | 3.4 | <0.005 | |||
L/R | Brainstem | 3 | −22 | −6 |
Appendix 2 Extracted Coordinates: Meta-Analysis of Differences in Brain Volume Between Meditators and Controls
Author | Year | Study measure | Hemisphere | Anatomic region | x | y | z | Z max | t | p value |
|---|---|---|---|---|---|---|---|---|---|---|
Luders et al. | GM volume | Right | Orbito-frontal cortex | 28 | 41 | −3 | 0.0001 | |||
Left | Inferior temporal lobe | −45 | −8 | −28 | 0.0003 | |||||
Right | Thalamus | 21 | −22 | 14 | 0.0003 | |||||
Left | Paracentral lobe | −12 | −9 | 54 | 0.0004 | |||||
Right | Paracentral lobe | 22 | −23 | 48 | 0.0005 | |||||
Hölzel et al. | GM concentration | Right | Hippocampus | 38 | −32 | −12 | 0.027 | |||
Right | Anterior insula | 36 | 12 | 6 | 0.022 | |||||
Left | Inferior temporal gyrus | −49 | −9 | −28 | 0.058 | |||||
Medial | Orbitofrontal cortex | 1 | 45 | −16 | 0.023 | |||||
Vestergaard-Poulsen et al. | GM density | Right | Medulla oblongata | 3 | −37 | −58 | 5.24 | <0.05 | ||
Left | Superior frontal gyrus | −24 | 50 | 4 | 4.29 | <0.05 | ||||
Left | Inferior frontal gyrus | −44 | 28 | 6 | 3.96 | <0.05 | ||||
Bilateral | Cerebellum | 33 | −59 | −36 | 4.14 | <0.05 | ||||
Right | Medulla Oblongata | 5 | −45 | −51 | 4.89 | <0.05 | ||||
Left | Medulla Oblongata | 5 | −46 | −51 | 5.49 | <0.05 | ||||
Left | Fusiform gyrus | −52 | −19 | −29 | 4.75 | <0.05 | ||||
Cerebellum | −29 | −58 | −37 | 4.14 | <0.05 | |||||
Pagnoni et al. | GM volume | Left | Putamen | −33 | −5 | 2 | 5.45 | 0.001 |
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Clausen, S.S., Crawford, C.C., Ives, J.A. (2014). Does Neuroimaging Provide Evidence of Meditation-Mediated Neuroplasticity?. In: Schmidt, S., Walach, H. (eds) Meditation – Neuroscientific Approaches and Philosophical Implications. Studies in Neuroscience, Consciousness and Spirituality, vol 2. Springer, Cham. https://doi.org/10.1007/978-3-319-01634-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-01634-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-01633-7
Online ISBN: 978-3-319-01634-4
eBook Packages: Behavioral ScienceBehavioral Science and Psychology (R0)