Abstract
With the increasing proportion of the elderly in the population, age-related hearing loss (ARHL) impacts the daily life of seniors and increases society's economic and medical burden. However, the change in the cochlear structures with age is still unknown. The emergency of Optical Coherence Tomography (OCT) makes it possible to observe the cochlea in vivo, which can provide high-resolution and high-imaging speed. In this article, we obtained the cochlear OCT image from mice of different ages, acquired the changes in the thickness of cochlear bone and modiolus, and analyzed the signal intensity of the round window and stria vascularis. We found that the thickness of the cochlear bone became thicker while the signal intensity became lower with the growth of age and the degeneration of hearing. These results not only prove the feasibility of OCT which can observe the structural changes of the cochlea but also indicate that age may affect the overall structure of the cochlea, especially stria vascularis, which may deepen our understanding of ARHL.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
According to the 《World Report on Hearing》published by World Health Organization, over 1.5 billion people suffer from varying degrees of hearing loss, which may reach 2.5 billion in 2050. Among this, more than 65% of adults above 60 are troubled by hearing loss [1]. Thus, age-related hearing loss (ARHL), also known as presbycusis, is caused by the accumulation of aging in the hearing system, which is affected by genetic predispositions, physiological stressors, and lifestyle behaviors. It is defined as progressive, bilateral, symmetrical sensorineural hearing loss and has become the main reason that adults older than 70 live with disability [2, 3]. Additionally, it will affect the patients’ social communication and cognitive ability and increase the social burden [4, 5]. With the development of ARHL, the hearing threshold will gradually worsen, resulting in the degeneration of the cochlea and nervous system. However, it is still challenging to observe and quantify the structural changes of the cochlea in vivo at different ages. The emergence and evolution of Optical Coherence Tomography (OCT) technology make it possible to handle this problem.
OCT based on low-coherence interference is a novel imaging technology that can obtain a noninvasive three-dimensional image with high resolution and high speed [6]. The earliest use of OCT to observe the cochlea can be traced back to 2007, Steven L. Jacques et al. obtained the image of the Organ of Corti (Ooc) at the apex of the cochlea and successfully measured the vibration of BM at 14-kHz based on time-domain OCT [7]. In 2011, John S. Oghalai et al. proved that OCT could observe the soft tissue structures and anatomical changes of the cochlea during growth and development [8]. After that, more and more researchers take advantage of OCT to observe the auditory response of the cochlea [9,10,11]. However, the structural changes of the cochlea during ARHL are still unclear. The cochlear function of mice is similar to that of human beings, and C57BL/6N mice are born with genetic ARHL. In this article, we used OCT to obtain a cochlear image at the apex of mice with different ages in vivo, calculated and qualified the thickness of cochlear bone and modiolus, analyzed the signal intensity of round window (RM) and stria vascularis, hoping to enhance our comprehension of ARHL.
2 Materials and Methods
2.1 OCT System Description
The schematic of the swept-source OCT (SS-OCT) system is shown in Fig. 1. This system uses swept laser source (MEMS-VCSEL, Thorlabs, USA) with 1300 nm center wavelength and 100 nm bandwidth. The axial and transverse resolution was 9.8 \(\mu m\) and 9.84 \(\mu m\) in air, respectively. The sweep rate of this system is 200 kHz, and the three-dimensional image of 500 \(\times \) 1000 \(\times \) 1024 pixels can be obtained in five seconds. The maximum depth and width of this system are 5 mm and 3.8 mm, respectively. The average output power of the laser is 25 mw, while the power focused on the sample is 18 mw.
2.2 Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Tianjin University, Tian Jin, China. A total of ten C57BL/6N mice were used, and all of them were male. The age of five mice was 8 weeks (young group), and the others were 8 months (old group). All of them were fed routinely in low noisy environments.
2.3 Surgery Preparation
The mice were anesthetized with urethane first and positioned on a heating pad to maintain the temperature of 37 \(^\circ{\rm C} \). . The additional overdose was supplemented every half hour. The skins and muscles that covered the bulla were removed. Part of the masseter muscle was removed to create an ideal imaging angle, while the bleeding must be controlled strictly. Then the bulla was opened carefully, resulting in the exposure of the apex of the cochlea. The whole surgery was operated under the microscope (Stemi 508, Zeiss, Germany) and followed the article written by John S. Oghalai [12]. Finally, the mice were fixed in the hold header and placed on the platform below the sweep light source. The mice were killed after all experiments.
2.4 Image Analysis
The OCT image was analyzed using ImageJ and MATLAB. No adjustments were made. We measured the thickness of the modiolus, the bone in the apex of the cochlea, the average signal intensity of the round window, and stria vascularis.
2.5 Hearing Threshold
Auditory brain response (ABR) and distortion product otoacoustic emission (DPOAE) were used to evaluate the hearing level of mice. We applied click-stimulus to obtain the threshold of ABR while using tone stimulus ranging from 10 kHz to 20 kHz to measure DPOAE. All of the stimuli generated by BioSigRZ software (Tucker-Davis Technologies, Gainesville, FL) were swept from 90 to 10 dB SPL, and stepped in 5 dB when the intensity was below 60 dB SPL.
2.6 Statistical Analysis
Statistical analysis and data plotting was performed using GraphPad Prism (version 9.5.0, GraphPad Software Inc., La Jolla, CA). All data sets were tested by Student t test, and a p < 0.05 was considered statistically significant. The results were represented in mean \(\pm \) standard error mean (SEM).
3 Results
3.1 Hearing Threshold
Compared with the young group, the ABR threshold of old mice apparently increased, as shown in Fig. 2a. Besides, the latency of ABR wave I of old mice under 60 dB SPL shown in Fig. 2b was prolonged significantly. Both threshold and latency had a statistical difference which proved the influence of age on hearing. The result of DPOAE is shown in Fig. 2c, which indicates that the threshold of DPOAE in the young group is better than in the old group under different frequencies. Furthermore, when the frequency exceeded 14 kHz, the difference between the two groups gradually became obvious with the increasing frequency. However, the threshold of DPOAE had no significant difference, which may be due to individual differences in hearing loss in older mice.
3.2 Cochlear Structure
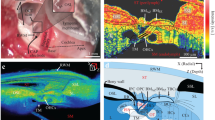
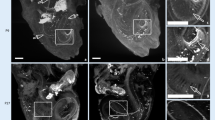
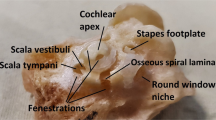
Through SS-OCT system, we acquired the cochlea image of mice. A representative result of X-Z cross-section and A-line is shown in Fig. 3. In the cross-section, the different scales of the cochlea can be identified clearly, and there are visible correlations between the cross-section and reflectivity in the A-line. The marker we made was based on the previous plastic-embedded section and OCT image [13, 14].
-
Thickness of bone
To analyze the effect of ARHL on cochlear structure, we first calculate the thickness of the cochlear bone and modiolus. The results indicated that the thickness of cochlear bone in old mice became thicker compared with young mice. The thickness is 111.58 \(\pm \) 8.45 \(\mu m\) in the young group and 97.98 \(\pm \) 6.64 \(\mu m\) in the old group, as shown in Fig. 4a. In addition, in Fig. 4b, the difference between the thickness of the modiolus was greater. The thickness of modiolus is 129.83 \(\pm \) 13.11 \(\mu m\) and 99.66 \(\pm \) 8.77 \(\mu m\), respectively. The thicker bone of the cochlea and modiolus probably demonstrated the degeneration of the cochlear structure with the growth of age and the occurrence of ARHL.
-
Reflection intensity of soft tissue in the cochlea
Then we analyze the signal intensity of soft tissue in the cochlea. As shown in Fig. 3, the round window and stria vascularis can be identified clearly. Thus, we calculated the average intensity of these two parts. The results are expressed in Fig. 5. Compared with young mice, the average intensity of round window and stria vascularis from old mice both decreased.
4 Discussion
ARHL in older people is very common, however, our understanding of it is limited. C57BL/6N mice have the characteristics of ARHL. The related characteristics gradually appear after 16 weeks of born, and with increasing age, their ABR threshold gradually increases, and wave I latency is significantly prolonged, which has been reported many times [15]. In our study, we found the degeneration of cochlear bone. This degeneration not only involves the damage of outer hair cells and auditory nerve [16], which may make an impact on the whole cochlea. Apart from the auditory system, bone mass and strength decline due to age-related changes, such as oxidative stress, may also play a crucial role in this change [17]. Besides, previous studies show that the aged animals' cells become sparse, the vascular density in stria vascularis decreases, and the permeability of the blood–the labyrinth barrier increases [18, 19]. Based on the principle of the OCT system, the signal intensity reflects tissues’ reflective properties, which depend on the composition and structure of tissues. Therefore, the reduced signal intensity can explain the sparsity of cells and blood vessels to a certain extent. Recently, many studies also have shown that oxidative stress, inflammation, and autophagy stress in the cochlea are the key mechanisms of ARHL [20, 21]. The research also stressed that the stria vascularis is an early site corresponding to the degeneration of the cochlea [22]. In vivo imaging of stria vascularis is helpful for further comprehending the change progress of stria vascularis under different ages, thereby clarifying its mechanisms. Additionally, gender also affects the degree of ARHL, such as the high-frequency threshold shift is more evident in female mice [23].
Furthermore, the observation of cochlear structure was previously based on the technology of histological sections, which requires the animals to be killed before the operation. OCT avoids image artifacts caused by complicated histological progress and overcomes dehydration-induced shrinkage [8]. Angiography based on OCT may also create new vitality for the cochlea, which can evaluate blood vessel density and blood flow velocity. Moreover, the vibrometry with OCT can measure the vibration of the OoC, which may reveal the cochlear response mechanism with ARHL.
5 Conclusion
OCT can observe the cochlea structure of mice at different ages, which can provide sufficient resolution to quantitatively measure the soft tissue in the cochlea. Based on OCT, we observed the changes in the thickness of the cochlear bone and signal intensity of the round window and stria vascularis, which reveals the influence of ARHL on the cochlea and may provide guidance for exploring its underlying mechanisms.
References
W. H. Organization (2021) World report on hearing
Bowl, M.R., Dawson, S.J.: Age-related hearing loss. Cold Spring Harb. Perspect. Med. 9(8), a033217 (2019). https://doi.org/10.1101/cshperspect.a033217
Carpena, N.T., Lee, M.Y.: Genetic hearing loss and gene therapy. Genomics Inform. 16(4) 2018
Huddle, M.G., et al.: The economic impact of adult hearing loss: a systematic review. JAMA Otolaryngol. Neck Surg. 143(10), 1040–1048 (2017). https://doi.org/10.1001/jamaoto.2017.1243
Thomson, R.S., Auduong, P., Miller, A.T., Gurgel, R.K.: Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig. Otolaryngol. 2(2), 69–79 (2017)
Drexler, W.: Optical Coherence Tomography: Technology and Applications Conference on Lasers and Electro-Optics Europe and International Quantum Electronics Conference (2013)
Chen, F., Choudhury, N., Zheng, J., Matthews, S.K., Nuttall, A.L., Jacques, S.L.: In vivo imaging and low-coherence interferometry of organ of Corti vibration. J. Biomed. Opt. 12(2), 021006 (2007). https://doi.org/10.1117/1.2717134
Gao, S.S., et al.: Quantitative imaging of cochlear soft tissues in wild-type and hearing-impaired transgenic mice by spectral domain optical coherence tomography. Opt. Express 19(16), 15415–15428 (2011). https://doi.org/10.1364/OE.19.015415
Frost, B.L., Strimbu, C.E., Olson, E.S.: Using volumetric optical coherence tomography to achieve spatially resolved organ of Corti vibration measurements. J. Acoust. Soc. Am. 151(2), 1115–1124 (2022). https://doi.org/10.1121/10.0009576
Cooper, N.P., Vavakou, A., van der Heijden, M.: Vibration hotspots reveal longitudinal funneling of sound-evoked motion in the mammalian cochlea. Nat. Commun. 9(1), 1–12 (2018)
Lee, H.Y., Raphael, P.D., Park, J., Ellerbee, A.K., Applegate, B.E., Oghalai, J.S.: Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea. Proc. Natl. Acad. Sci. 112(10), 3128–3133 (2015). https://doi.org/10.1073/pnas.1500038112
Jawadi, Z., Applegate, B.E., Oghalai, J.S.: Optical coherence tomography to measure sound-induced motions within the mouse organ of Corti in vivo. In: Auditory and Vestibular Research, pp. 449–462. Springer (2016). https://doi.org/10.1007/978-1-4939-3615-1_24
Johns, J.D., Adadey, S.M., Hoa, M.: The role of the stria vascularis in neglected otologic disease. Hear. Res. 108682 (2022).https://doi.org/10.1016/j.heares.2022.108682
Gao, S.S., et al.: Vibration of the organ of Corti within the cochlear apex in mice. J. Neurophysiol. 112(5), 1192–1204 (2014). https://doi.org/10.1152/jn.00306.2014
Cai, R., Montgomery, S.C., Graves, K.A., Caspary, D.M., Cox, B.C.: The FBN rat model of aging: investigation of ABR waveforms and ribbon synapse changes. Neurobiol. Aging 62, 53–63 (2018). https://doi.org/10.1016/j.neurobiolaging.2017.09.034
Jeng, J.Y., et al.: Age-related changes in the biophysical and morphological characteristics of mouse cochlear outer hair cells. J. Physiol. 598(18), 3891–3910 (2020)
Manolagas, S.C., Parfitt, A.M.: What old means to bone (in eng). Trends Endocrinol Metab 21(6), 369–374 (2010). https://doi.org/10.1016/j.tem.2010.01.010
Neng, L., et al.: Structural changes in thestrial blood–labyrinth barrier of aged C57BL/6 mice. Cell Tissue Res. 361(3), 685–696 (2015). https://doi.org/10.1007/s00441-015-2147-2
Gratton, M., Schmiedt, R., Schulte, B.: Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear. Res. 102(1–2), 181–190 (1996)
Kalinec, G.M., Lomberk, G., Urrutia, R.A., Kalinec, F.: Resolution of cochlear inflammation: novel target for preventing or ameliorating drug-, noise-and age-related hearing loss. Front. Cell. Neurosci. 11, 192 (2017). https://doi.org/10.3389/fncel.2017.00192
Menardo, J., et al.: Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid. Redox Signal. 16(3), 263–274 (2012). https://doi.org/10.1089/ars.2011.4037
Lang, H., et al.: The stria vascularis in mice and humans is an early site of age-related cochlear degeneration, macrophage dysfunction, and inflammation. Macrophage Dysfunct. Inflamm.
Henry, K.R.: Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hear. Res. 190(1–2), 141–148 (2004). https://doi.org/10.1016/S0378-5955(03)00401-5
Acknowledgment
This work was supported by grants from the Key Technologies Research and Development Program of China (No.2022YFF1202400) and the National Natural Science Foundation of China (No.81971698).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Zheng, S., Bai, Y., Ni, G. (2024). Effect of Age-Related Hearing Loss on Mice Cochlear Structures Based on Optical Coherence Tomography. In: Wang, G., Yao, D., Gu, Z., Peng, Y., Tong, S., Liu, C. (eds) 12th Asian-Pacific Conference on Medical and Biological Engineering. APCMBE 2023. IFMBE Proceedings, vol 103. Springer, Cham. https://doi.org/10.1007/978-3-031-51455-5_29
Download citation
DOI: https://doi.org/10.1007/978-3-031-51455-5_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-51454-8
Online ISBN: 978-3-031-51455-5
eBook Packages: EngineeringEngineering (R0)