Abstract
Endometriosis is a complex disease by itself. When considering fertility, corrective surgeries may compromise the outcome of these women. Different protocols with varying success rates have been evaluated. The long-standing debate between the long protocols, which offers the benefits of a prolonged suppression of the pituitary, or the antagonist protocols, which are shorter and have the added safety benefit, is slowly addressing to the antagonists. The great advances observed with vitrification techniques have led to the use of newer methods of ovarian stimulation (OS) like the progesterone primed only (PPOS) protocol which offers the safety benefit while also being cheaper with better patient compliance. No protocol has been proved to be superior to the others. Early intervention with in vitro fertilization, irrespective of the protocol, can give these women early and good results.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Endometriosis is an estrogen-dependent disease of high morbidity, infertility being one of symptoms. Briefly, three different entities have been described, namely peritoneal, ovarian (endometrioma) or deep infiltrating, and these frequently coexist. Due to the lack of a reliable noninvasive method for its diagnosis, it is difficult to estimate its true prevalence. Studies report its prevalence to be about 10% in the general population and a contributing factor in causing infertility in approximately 40% of women. It is also estimated that about 50% of women with endometriosis have difficulty in getting pregnant [1].
Although a direct causal relationship with infertility cannot be made, it is shown that the fecundity rate of untreated women can go as low at 2% [2]. The impact exerted by the disease on oocyte quality/quantity and ultimately on the embryos makes this pathology a subject of constant study and interest for infertility specialists. It is assumed that this generalized disease causes damage due to the production of cytotoxic chemicals and also by disturbing the pelvic anatomy. Focal lesions like endometrioma can be more harmful due to its additional space occupying effect. It is also found that the disease itself and its surgery can damage the ovarian reserve and hence this disease is of interest [3].
Dr. Carl Wood of the Monash in vitro fertilization (IVF) team in Melbourne reported the first IVF pregnancy in 1973, although it resulted in an early miscarriage, started a new era. Medical history was made on July 25, 1978, with the birth of the world’s first “test tube baby” by performing a natural cycle IVF. Trounson et al. in 1981 introduced ovarian stimulation (OS) in IVF and this led to higher pregnancy rates [4]. These ovarian stimulations consist basically of the administration of urinary or recombinant gonadotropins, used alone or in conjunction with Letrozole or Clomifene. Premature luteinizing hormone (LH) peak is usually prevented with the use of gonadotropin-releasing hormone (GnRH) analogs (agonists and antagonists) or more recently by the use of oral progesterone [5, 6]. Thus, discussions have arisen over the years about which is the best OS protocol for these patients with endometriosis when undergoing fertility treatments. This population also frequently undergoes ovarian surgery to remove the endometriotic cysts, and, therefore, may also present impairment of the ovarian reserve [6]. Optimizing treatments and seeking the best protocols in order to obtain satisfactory amounts of oocytes and embryos of good quality is crucial to achieve reproductive success.
Preparations prior to OS have also been proposed, with the aim of obtaining a more synchronous follicular development; limit the growth of the endometriotic implants and reducing the chronic pelvic inflammatory process, which supposedly could negatively impact treatments. These have also been used post OS but prior to performing a frozen embryo transfer (FET) with the same purpose. These protocols include the use of long periods of oral contraceptives, depot GnRH agonists, and/or even intrauterine hormonal devices [7, 8].
This chapter will have a special emphasis on the peculiarities and results of using the aforementioned protocols, comparing them with each other and with patients without endometriosis.
2 Background/Impact of the Disease
Decades after the first reports on the association between endometriosis and infertility, it has yet to be fully understood. Distortion of pelvic organs with a structural and functional loss of ovarian function due to toxic metabolites has been suspected to play an important role [9].
It was previously thought that just like every other pathology, surgical removal of this disease will also lead to a decline in its side effects including infertility. It is true to some extent as in some cases precise laparoscopic excision of the endometriotic lesions while avoiding damage to the normal tissues does reduce pain and improve quality of life. This is evident as spontaneous pregnancy after such corrective surgery in cases with severe endometriosis had reached even up to 73% in young patients. But, this might not be the case for all infertile women and there are strong drawbacks of the surgery as witnessed by the declining AMH levels. For this population, waiting for a spontaneous pregnancy might not be advisable due to their advanced maternal age, or other reasons [10]. Hence, they are subjected to OS to get early and promising results with a faster and maybe even cheaper time and cost to pregnancy rates.
A meta-analysis in 2002 included 22 studies and compared results of over 2300 IVF cycle in women with endometriosis to more than 4300 controls. After adjusting for confounding factors, statistically significant reductions were found in implantation and pregnancy rates in patients with endometriosis, as well as a lower number of oocytes obtained by ovarian stimulation. Another comparison carried out within the same work, assessed the impact of disease severity on reproductive outcomes, and concluded that patients who suffer from severe/advanced forms obtain significantly lower amounts of oocytes, in addition to even lower rates of implantation and pregnancy compared to minimal stages. This study therefore demonstrated that women with endometriosis have a reduction of up to 54% in pregnancy rates when compared to patients undergoing IVF for other reasons, such as tubal factor [11].
A more recent meta-analysis studied the reproductive outcomes in these women. The authors confirmed that endometriosis is associated with a considerable decrease in the likelihood of success for these patients in their reproductive treatments. The negative influence on the number of oocytes obtained, embryos generated, on the rates of fertilization and pregnancy in this population was evident. Severe forms of the disease had a negative effect on all treatment processes, and when present in the ovary, a significantly smaller number of mature oocytes are aspirated [9].
The impact exerted by the inflammatory cytokines present there on steroidogenesis and ovarian folliculogenesis seems to be evident, corroborating with the publications that showed lower oocyte mitochondrial content, anomalous oocyte morphology and higher rates of embryo granulation/fragmentation. The concentrations of reactive oxygen and interleukin species present in the follicular fluid are also associated with a higher percentage of immature oocytes [9, 11, 12].
After all efforts, the end result which is important for the patient is the cumulative live birth rate. To address that, a recent retrospective study by Boucret et al. compared 1124 COS cycles performed in patients with and without the disease. They too confirmed that patients with endometriosis had reduced AMH and AFC values, even without undergoing surgical procedures. Due to this low reserve, these women had significantly fewer oocytes retrieved and even fewer mature oocytes (7.0 vs 9.7 and 4.8 vs 6.9, respectively). As a result of this, they had fewer embryos formed. Though the maturation rate and cleavage rates were in the same in both groups, which signifies only a quantitative loss of ovarian function, the number of embryos which could be frozen for future use were less. Due to this, the affected group had a reduced cumulative live birth rate (32.1% vs 50.7%, p = 0.001) [13].
Hence, while these women frequently undergo OS and IVF, doubt still persists about the ideal stimulation protocol as there can be an ill effect on IVF outcomes as reflected by a lower oocyte yield and quality. Several studies report that women with endometriosis have high levels of oxidative stress markers and low levels of antioxidant markers even in the follicular fluid. This indirectly is supposed to create a lower number of good quality embryos which can hamper results. This assumption is supported by the finding that oocytes from women with endometriosis have a different profile related to oxidative stress and cell growth regulation. These also show a different transcriptome behavior when compared with controls [14]. Though this is true, its clinical relevance is questioned as American Society of Reproductive Medicine (ASRM) studies have shown that the not oocyte quality but only quantity is hampered and even the aneuploidy rates are similar [10]. Hence, early intervention with OS and IVF is still thought to be the best option to achieve a pregnancy for most infertile women (Fig. 1).
3 Protocols and Results
Keeping the distinctive nature of this progressive disease in mind, fertility specialists have tried various OS protocols over the years in order to get good results.
3.1 Natural or Modified Natural Cycle IVF
IVF was originally performed in natural cycles with hCG trigger. In a Norwegian study, the authors tried this method in couples with minimal endometriosis associated infertility and compared it to patients with unexplained and tubal factor infertility. The prospectively recruited couples were given a maximum of 5 cycles with a natural IVF before proceeding to conventional IVF. In spite of having a lower pregnancy rate per initiated cycle, the pregnancy rate per embryo transfer was 23.5% in the endometriosis group and it was higher compared to the other groups [15]. The clinical relevance is questionable, but this method is a cheap and safe alternative when compared to conventional IVF.
3.2 GnRH Agonist Vs Antagonist Cycle IVF
The long protocols with GnRH agonist for OS were pioneers in contemporary reproductive medicine, being used in clinical practice even in the 1980s [16]. These treatments are based on the suppression of pituitary function by desensitizing their receptors, resulting in cycles with greater follicular synchrony, and decreased risk of premature LH rise. In contrast, the recently implemented protocols for OS with GnRh antagonists cause suspension of the pituitary function immediately after its administration, culminating in shorter treatments and with lower necessary dosages of gonadotropins. Both these regimens are routinely used in fertility practice, but studies comparing these two in a specific endometriosis population are limited.
It was thought that the long GnRH agonist protocol would be helpful in cases with endometriosis as longer suppression could decrease the local inflammatory processes and could improve oocyte quality. As opposed to this, due to the short suppression with the antagonist protocol, these benefits were lost. Pabuccu et al. conducted a prospective randomized study to elucidate the differences in these protocols in 246 patients. These women were initially divided into 3 groups: those who had mild/moderate endometriosis confirmed by laparoscopy, those who underwent cystectomies prior to OS and women with ovarian endometriomas without surgical intervention. Results showed a nonsignificant improvement with the agonist cycle and they concluded that OS with both GnRH antagonist and GnRH-a protocols may be equally effective in patients with mild-to-moderate endometriosis and endometrioma who did and did not undergo ovarian surgery [17].
Another retrospective observational study published in 2013 also compared patients with endometriosis and infertility who underwent OS with these two protocols. In total, 1180 women who were diagnosed with endometriosis surgically or by ultrasound were analyzed, and when the confounding factors were adjusted, no strategy was shown to be superior with regard to pregnancy rates [5].
When analyzing whether the severity of endometriosis could predict the results of OS in different protocols, a retrospective study published 5 years later compared the use of GnRH agonists and antagonists in 386 patients with the disease, dividing them into two groups according to the severity of disease. In patients with grades I and II endometriosis, a higher percentage of clinical pregnancies and live births (42.8% vs. 26.7%) were reported using agonists. In patients with advanced disease, the overall results were worse, but they were equivalent among the protocols. All patients included in this study were diagnosed by videolaparoscopy and did not use any hormonal preparation in the 6 months prior to stimulation, thus reducing possible confounding factors. A shorter treatment time and gonadotropin dosages were reported by the group that used antagonists to suppress premature LH peaks, reflecting greater convenience during treatment [18].
In view of the relative frequency of surgical procedures to remove ovarian endometriotic cysts, a Beijing research group tried to prove the best strategy to perform OS in 342 patients undergoing cystectomy. These women were divided into three groups: those submitted to depot GnRH agonist protocol (3.75 mg agonist in the menstrual cycle prior to stimulation), flare cycles with GnRH agonist (0.1 mg agonist since the beginning of ovarian stimulation), or classical cycles with fixed-onset GnRH antagonists. Differences were not statistically significant. The number of oocytes and embryos obtained also did not differ between the groups studied [19].
Apart from these studies comparing the two protocols, Cao et al. recently performed a meta-analysis on the effectiveness of the GnRH-a ultra-long protocol, GnRH-a long protocol, and GnRH-a short protocol in infertile women with endometriosis. As it was assumed that the longer the suppression, the better would be the results as the inflammation would be reduced. The analysis concluded that the GnRH-a ultra-long protocol can improve the clinical pregnancy rate of the patients with stages III–IV endometriosis. This conclusion was made only based on randomized controlled trials (RCTs) studies which were included in the analysis. However, subgroup analysis showed that the different down-regulation protocols provided no significant difference in improving clinical outcomes in the non-RCT studies. Hence, it is advised not to draw conclusions yet, as randomized studies would be beneficial [20].
Therefore, according to the small number of studies published with this purpose to date, it is not clear whether there is any significant difference in the results between OS cycles with protocols with GnRH agonists and antagonists in patients with endometriosis. Some studies indicate a higher amount of aspirated oocytes, implantation rates, pregnancy, and live birth with agonist protocols. However, no prospective study was able to show statistically significant differences between them, and thus both are considered equally effective in daily clinical practice. Antagonist protocols can result in lower rates of treatment dropout, given the lower amount of gonadotropins used and significantly shorter treatment duration.
The concept of freeze-all has also been challenged in women with endometriosis. It was hypothesized that the OS might generate further uterine inflammation, especially in the endometrium, and this might compromise successful embryo implantation. We have recently published a large retrospective analysis where we did not find any difference in implantation, pregnancy, and miscarriage rate whether the embryo transfer was performed in a fresh or in a subsequent frozen embryo replacement.
3.3 Progestin Primed Ovarian Stimulation (PPOS)
PPOS was initially described for fertility preservation in women with cancer; however, this protocol is not extensively studied in women with endometriosis. The rationale of using progestins was that they were equally effective in preventing the premature LH spike compared to antagonists. As this regimen could only be used in cycles where a fresh transfer was not done, these are less used for routine IVF stimulation. The advances of vitrification and equal or even superior results in FET cycles have made this option a strong candidate. This might be even more effective in women with endometriosis as a fresh transfer is less preferred due to the flare-up caused by gonadotropins.
Various progestin preparations have been tried and are found to be equally superior in stimulations. In a pioneer and recent study done by d’Argent et al., this PPOS protocol was compared to the antagonist protocol women with endometriosis. Women in the PPOS group were started on progestin desogestrel on the second day of their menstrual cycle and stimulation was started. The presence of deep versus superficial endometriosis alone, the location of endometriosis, the presence of endometrioma during the stimulation, and the size of endometriomas were not associated with the number of retrieved oocytes. The study demonstrated that there were no significant differences in the oocytes retrieved and the mature oocytes between the groups [21].
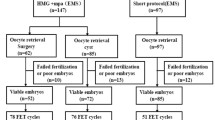
This protocol combines the benefits of antagonist protocol in terms of lower stimulation and duration, while also giving additional benefits of a lower cost and fewer injections. The drawback is that a fresh transfer, which is as such generally a less preferred option in these women with endometriosis, is not possible (Fig. 2).
4 Conclusion
Infertile women with endometriosis frequently undergo OS for IVF due to its progressive nature, with or without corrective surgery. Evidence also suggests that decline in fertility in women with endometriosis is more related to quantitative damage than qualitative. OS was initially performed with GnRH agonists but then GnRH antagonists replaced almost completely the agonists due to its shorter duration. Available studies suggest that OS using antagonist or agonist protocols yield similar results in terms of oocyte quantity and usable embryos. If an FET is planned for different reasons, PPOS appear promising and can yield similar results. Overall, available literature strongly suggests one thing – that it is early intervention with IVF for good results, irrespective of the OS protocol used.
References
Hodgson RM, Lee HL, Wang R, Mol BW, Johnson N. Interventions for endometriosis-related infertility: a systematic review and network meta-analysis. Fertil Steril. 2020;113:374–382.e2.
Practice Committee of the American Society for reproductive Medicine T. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98:591–8.
Younis JS, Shapso N, Fleming R, Ben-Shlomo I, Izhaki I. Impact of unilateral versus bilateral ovarian endometriotic cystectomy on ovarian reserve: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:375–91.
Parikh FR, Athalye AS, Naik NJ, Naik DJ, Sanap RR, Madon PF. Preimplantation genetic testing: its evolution, where are we today? J Hum Reprod Sci. 2018;11:306–14.
Rodriguez-Purata J, Coroleu B, Tur R, Carrasco B, Rodriguez I, Barri PN. Endometriosis and IVF: are agonists really better? Analysis of 1180 cycles with the propensity score matching. Gynecol Endocrinol. 2013;29:859–62.
de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–8.
Ferrero S, Gillott DJ, Remorgida V, Anserini P, Ragni N, Grudzinskas JG. GnRH analogue remarkably down-regulates inflammatory proteins in peritoneal fluid proteome of women with endometriosis. J Reprod Med. 2009;54:223–31.
Sallam HN, Garcia-Velasco JA, Dias S, Arici A, Abou-Setta AM, Jaafar SH. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev. 2006;2021:CD004635.
Horton J, Sterrenburg M, Lane S, Maheshwari A, Li TC, Cheong Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:592–632.
Garcia-Fernandez J, García-Velasco JA. Endometriosis and reproduction: what we have learned. Yale J Biol Med. 2020;93:571–7.
Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–55.
Garcia-Velasco JA, Arici A. Is the endometrium or oocyte/embryo affected in endometriosis? Hum Reprod. 1999;14:77–89.
Boucret L, Bouet P-E, Riou J, Legendre G, Delbos L, El HH, et al. Endometriosis lowers the cumulative live birth rates in IVF by decreasing the number of embryos but not their quality. J Clin Med. 2020;9:2478.
Ferrero H, Corachan A, Aguilar A, Quiñonero A, Carbajo-Garcia MC, Alama P, et al. Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum Reprod. 2019;34:1302–12.
Omland AK, Fedorcsák P, Storeng R, Dale PO, Åbyholm T, Tanbo T. Natural cycle IVF in unexplained, endometriosis-associated and tubal factor infertility. Hum Reprod. 2001;16:2587–92.
Anon. Handbook of In Vitro Fertilization. 4th ed. Boca Raton: CRC Press; 2017.
Pabuccu R, Onalan G, Kaya C. GnRH agonist and antagonist protocols for stage I-II endometriosis and endometrioma in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2007;88:832–9.
Drakopoulos P, Rosetti J, Pluchino N, Blockeel C, Santos-Ribeiro S, de Brucker M, et al. Does the type of GnRH analogue used, affect live birth rates in women with endometriosis undergoing IVF/ICSI treatment, according to the rAFS stage? Gynecol Endocrinol. 2018;34:884–9.
Zhao F, Lan Y, Chen T, Xin Z, Liang Y, Li Y, et al. Live birth rate comparison of three controlled ovarian stimulation protocols for in vitro fertilization-embryo transfer in patients with diminished ovarian reserve after endometrioma cystectomy: a retrospective study. J Ovarian Res. 2020;13:23.
Cao X, Chang HY, Xu JY, Zheng Y, Xiang YG, Xiao B, et al. The effectiveness of different down-regulating protocols on in vitro fertilization-embryo transfer in endometriosis: a meta-analysis. Reprod Biol Endocrinol. 2020;18:16.
Mathieu D’Argent E, Ferrier C, Zacharopoulou C, Ahdad-Yata N, Boudy AS, Cantalloube A, et al. Outcomes of fertility preservation in women with endometriosis: comparison of progestin-primed ovarian stimulation versus antagonist protocols. J Ovarian Res. 2020;13:18.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Banker, J., D’Allagnol, H., Garcia-Velasco, J.A. (2024). IVF Stimulation Protocols and Outcomes in Women with Endometriosis. In: Ferrero, S. (eds) Endometriosis-related Infertility. Springer, Cham. https://doi.org/10.1007/978-3-031-50662-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-50662-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50661-1
Online ISBN: 978-3-031-50662-8
eBook Packages: MedicineMedicine (R0)