Abstract
Basic oxygen furnace slag (BOFS) with high content of free CaO (f-CaO) can be utilized as a kind of building materials after carbonation. However, the degree of carbonation varies greatly depending on the particle size of slag. In this study, the carbonation process of BOFS with different particle sizes was evaluated in an autoclave. The highest CO2 sequestration and f-CaO consumption reached 6.49% (64.9 g CO2/kg slag) and 99.81%, respectively, when the particle size of BOF slag was less than 61 μm. Additionally, the size of particles had a minor impact on f-CaO consumption but significantly affected CO2 sequestration. With CO2 sequestration as an indicator, the results showed that the carbonation of BOFS was accurately described by the shrinking core model and the carbonation process was proved to be controlled by internal diffusion. The effectiveness and environmental benefits of this method make it worth promoting for the carbonation and stabilization of steel slag.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Basic oxygen furnace slag (BOF slag)
- Direct carbonation
- Free CaO consumption
- CO2 sequestration
- Kinetic study
Introduction
The steel slag is a solid byproduct generated during the metallurgical industry’s steel production process, representing approximately 15–20% of global steel production [1]. In 2022, the worldwide crude steel production was 1,885 million tons, with China being the largest contributor. Each ton of steel production is associated with emissions equivalent to 1.8 tons of CO2 on average. Consequently, CO2 emissions from this industry account for about 6–7% of total global CO2 emissions [2]. Notably in China, it ranks as the third-largest emitter after power and cement industries and contributes to around 10% of domestic CO2 emissions [3].
Numerous valuable applications for steel slag has been identified, including road construction, cement manufacturing, and water engineering [4, 5]. However, the excessive presence of f-CaO in steel slag poses a challenge to its potential usage due to unfavorable expansion, thus hindering the sustainable and efficient utilization of steel slag [6]. Consequently, many studies have been conducted on reducing the f-CaO content through CO2 reaction. The sequestration of CO2 by steel slag can be classified into two main types: direct carbonation and indirect carbonation. Compared to indirect carbonation, direct carbonation is more convenient and has the potential for widespread application [7]. Direct carbonation can be achieved by the reaction of steel slag with CO2 either in the aqueous or gaseous phase, without the need for solvent extraction of active components (such as Ca2+ or Mg2+) from the steel slag. The main chemical reactions of direct carbonation of steel slag can be expressed as follows [8, 9]:
The CO2 sequestration by steel slag can be influenced by various factors, including reaction parameters and material properties. Among these properties, particle size plays a vital role in the carbonation process and is more important than the elemental composition of the initial material. Smaller particle size results in a larger surface area that benefits the extraction of calcium ions and carbonation of steelmaking slag, as it shortens the element diffusion distance from particle interior to surface [10, 11].
Baciocchi et al. [12] conducted a study on the carbonation process of stainless EAFS with varying grain sizes and observed a decrease in the carbonation reaction as the particle size increased. Renato et al. [12] conducted a study on the CO2 storage capacity of different particle sizes of steel slag under mild operating conditions. The results indicated that the finest grain sizes had maximum CO2 uptakes of 130 g CO2/kg slag, which decreased with increasing particle size due to differences in reacting species availability and specific surface area. Liu et al. [13] carried out an experiment to investigate the impact of slag particles on the carbonation process of converter steel slag. The findings suggested that changes in particle size of steel slag affected the transportation of gas and absorption of CO2 in carbonated steel slag blended mortars during curing, thereby influencing microstructure, strength, and leaching properties. Additionally, in a study conducted by Li et al. [12], they investigated the effects of particle size on the environmental, economic, and engineering performances of aqueous carbonated steel slag powders. The findings indicated that reducing the particle size of steel slag had a positive impact on CO2 sequestration (particle sizes ranging from 22.4 to 112.6 μm resulted in sequestering approximately 88.5–37.9 kg CO2 per ton of steel slag) and enhanced the leaching process for mineralization due to improved extraction of metal ions. Wu et al. [14] conducted a study on the economic and rational grading of carbonated steel slag products. They formulated different ratios of steel slag micronized powder, coarse powder, and coarse particles. The weight gain and compressive strength were then analyzed after carbonation. The findings indicated that increasing the proportion of slag coarse powder to slag coarse particle up to 15% resulted in a decrease in the rate of carbonation. Similarly, the trend observed for compressive strength was consistent with that of the carbonation weight gain rate. Based on these results, it can be concluded that the most cost-effective gradation consists of 45 wt.% fine powder, 40 wt.% coarse powder, and 15 wt.% coarse granules.
However, there have been few studies on the direct carbonation of gas–solid reactions with low L/S above 500 °C under pressurized conditions. Additionally, the high f-CaO content in BOF slag poses challenges for its direct utilization, yet there is a lack of quantitative studies investigating the consumption of f-CaO after the carbonation reaction. This study is therefore aimed to examine the direct carbonation reaction of BOF slag of different particle size. The f-CaO consumption was analyzed using ethylene glycol method, while the CO2 sequestration and reaction products were evaluated by Thermogravimetric analysis (TGA; TGA/DSC1/1600LF, Switzerland). The kinetics of f-CaO consumption and CO2 sequestration were investigated using the shrinking core model to determine kinetic equations and the rate-limiting step in the carbonation process.
Materials and Methods
Basic Oxygen Furnace Slag (BOF Slag)
The BOF slag, obtained from Xinxing Pipes Corporation (Anhui, China), underwent a drying and calcination process at 800 °C for 30 min in an oven to eliminate moisture, hydroxide, and carbonate. Subsequently, the slag was ground and divided into four types based on particle size. D1 had a particle size smaller than 61 μm, D2 ranged from 61 to 75 μm, D3 ranged from 75 to 100 μm, and D4 ranged from 100 to 300 μm.
Experimental Procedure
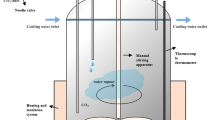
The carbonation experiment was conducted in a homemade autoclave reactor, as depicted in Fig. 1.
In each experiment, the crucible containing 25 g of slag was placed inside the reactor and heated to a temperature of 700 °C. Subsequently, CO2 was introduced into the reactor to maintain a pressure of 0.35 Mpa. Following that, water was injected into the reactor with a liquid-to-solid ratio of 0.2 mL·g-1 and the slag was stirred at a speed of 15 rpm. The entire reaction lasted for 75 min, during which six samples of slag were selected and analyzed at different time intervals.
Analysis Method
The slag samples were heated from 25 °C to 900 °C at a rate of 10 °C·min−1 under nitrogen. The mass loss between 500 °C and 900 °C was mainly due to CaCO3 decomposition. The CO2 weight (wt.%) and amount of CO2 sequestration (wt.%) were calculated using Eqs. (6) and (7):
where m105°C represents the dry weight of the slag measured at 105 °C, and △mCO2 is the CO2 weight of the slag released from CaCO3 decomposition between 500 °C and 900 °C.
The f-CaO content (wt.%) of initial and carbonated slag and the consumption of f-CaO content (%) were analyzed using a combination of ethylene glycol method [15] and TGA. Equations (8) and (9) are employed for quantifying, as follows:
where f-CaOtotal can be determined using the ethylene glycol method, △mH2O represents the loss caused by Ca(OH)2 decomposition, and MH2O is the molecular weight of H2O.
Results and Discussion
The Impact of Particle Size on CO2 Sequestration and f-CaO Consumption
The trend of f-CaO consumption and CO2 sequestration over the reaction time is illustrated in Fig. 2. It can be observed that the particle size of BOF slag had a significant impact on CO2 sequestration, with a dramatic increase as the particle size decreased. After reacting with CO2 for 75 min, D1 achieved a CO2 sequestration rate of 6.49% (64.9 g CO2/kg slag), while D4 only reached 1.45% (14.5 g CO2/kg slag) of CO2 sequestration. Conversely, the influence of particle size on f-CaO consumption was relatively weak, and the highest f-CaO consumption was 99.81%, while the lowest f-CaO consumption was 85.14%.
According to Fig. 2, initially both CO2 sequestration and f-CaO consumption increased rapidly. However, after 60 min, the value of CO2 sequestration exhibited an obvious slower rise, indicating resistance throughout the entire process. Additionally, the carbonation process of large particle size BOF slag stopped increasing in less than 60 min. Therefore, the Drozdov equation with a self-impeding coefficient was used to describe this CO2 sequestration process [16], and the equation is as follows:
where t is the reaction time, min; x is the leaching ratio, %; β is the self-impeding coefficient; and Km is the reaction ratio constant.
The results of the fitting are shown in Fig. 3 and Table 1, which clearly demonstrate a strong linear correlation (correlation coefficient > 0.999) between ln[1/(1 − x)]·t−1 and x·t−1. Additionally, it was found that both the self-impeding coefficient and reaction ratio constant increased as the particle size decreased. Notably, particles smaller than 61 μm exhibited the highest reaction ratio constant and lowest self-impeding coefficient, reaching values of 0.0022 and 0.9894, respectively.
According to previous studies [17, 18], the small particle size of BOF slag results in a larger surface area, which provides more contact areas with CO2 and facilitates the carbonation process and leads to higher CO2 sequestration and f-CaO consumption. However, compared to other alkaline constituents in BOF slag, the content of f-CaO was relatively low, approximately 5% by mass in this study. Therefore, the differences in particle size had limited influence on it.
The Kinetic Study of Carbonation Process
In this study, the carbonation kinetics of BOF slag were investigated using the shrinking core model, which has been previously validated for the carbonation process of BOF slag [19]. If the rate-limiting step is determined by the diffusion of reactants through the CaCO3 layer, then Eq. (10) can be used to express the relationship between reaction time (t, min) and CO2 sequestration (X, %). On the other hand, if it is determined by a chemical reaction between reactants, then Eq. (11) can illustrate this relationship.
where the term \({\rho }_{B}\) is the molar density of the solid, mol·cm−3. R is the radius of the slag particle, cm. De is the effective diffusivity of the gaseous reactant in the CaCO3 layer, cm2·s−1. CAg represents the concentration of CO2, mol·cm−3. K \({^{\prime\prime}}\) represents the first-order rate constant for the surface reaction, s−1.
To identify the step that limits the rate of carbonation in this study, Fig. 4 illustrates the relationships between 1–3(1−X)2/3 + 2(1−X) and t, as well as 1−(1−X)1/3 and t. The fitting results indicate that the carbonation process was governed by internal diffusion, with R2 values ranging from 0.9403 to 0.9866.
According to the assumptions of this model, the product (carbonate) layer acts as a barrier for reactant diffusion, thus becoming the rate-limiting step in carbonation process. What’s more, the kinetic equation of BOF slag of D1 was determined through a series of experiments conducted at temperatures ranging from 400 °C to 700 °C. The results are illustrated in Fig. 5a.
a Fitting results of CO2 sequestration using shrinking core model under different temperatures; b kinetics equation of carbonation process of BOF slag of D1. b shows the kinetic equations for CO2 sequestration and f-CaO consumption, which could be expressed as Eq. 13
Conclusions
The results showed that smaller particle size of BOF slag led to higher CO2 sequestration and f-CaO consumption. The highest CO2 sequestration and f-CaO consumption of 6.49% (64.9 g CO2/kg slag) and 99.81%, respectively, were achieved when the particle size was less than 61 μm. The Drozdov equation was used to describe the carbonation process, and the results showed that the self-impeding coefficient and reaction ratio constant increased with decreasing particle size. This was consistent with the experimental results. In addition, the particle size of BOF slag of less than 61 μm had the optimum self-impeding coefficient and reaction ratio constant of 0.0022 and 0.9894, respectively.
However, the impact of particle size on CO2 sequestration was more pronounced due to the lower content of f-CaO in BOF slag and f-CaO is the most reactive alkaline constituent in BOF slag and readily reacts with CO2. Therefore, there is no need to grind BOF slag into the finest powder if the purpose is only to stabilize the slag.
Additionally, the kinetics of the carbonation process could be accurately described by a shrinking core model. This model revealed that the formation of carbonate is the main reason why there was no further increase in CO2 sequestration. Furthermore, this study has proven that reactant diffusion is the rate-limiting step in the carbonation process, and a kinetic equation has been derived. As a result, this approach showed significant potential for widespread implementation as an efficient method to eliminate f-CaO, stabilize BOF slag, and capture CO2.
References
Carroll A, Przeslawski R, Radke L, Black J, Picard K, Moreau J, Haese R, Nichol S (2014) Environmental considerations for subseabed geological storage of CO2: a review. Cont Shelf Res 83:116–128
Doucet FJ (2010) Effective CO2-specific sequestration capacity of steel slags and variability in their leaching behaviour in view of industrial mineral carbonation. Miner Eng 23(3):262–269
Matsuura H, Yang X, Li G, Yuan Z, Tsukihashi F (2022) Recycling of ironmaking and steelmaking slags in Japan and China. Int J Miner Metall Mater 29(4):739–749
Amuchi M, Abtahi SM, Koosha B, Hejazi SM, Sheikhzeinoddin H (2013) Reinforcement of steel-slag asphalt concrete using polypropylene fibers. J Ind Text 44(4):526–541
Pan S-Y, Adhikari R, Chen Y-H, Li P, Chiang P-C (2016) Integrated and innovative steel slag utilization for iron reclamation, green material production and CO2 fixation via accelerated carbonation. J Clean Prod 137:617–631
Jiang Y, Ling T-C, Shi C, Pan S-Y (2018) Characteristics of steel slags and their use in cement and concrete—a review. Resour Conserv Recycl 136:187–197
Wang J, Zhong M, Wu P, Wen S, Huang L, Ning P (2021) A review of the application of steel slag in CO2 fixation. Chem Bio Eng Rev 8(3):189–199
Ko M-S, Chen Y-L, Jiang J-H (2015) Accelerated carbonation of basic oxygen furnace slag and the effects on its mechanical properties. Constr Build Mater 98:286–293
Li J, Ni W, Wang X, Zhu S, Wei X, Jiang F, Zeng H, Hitch M (2022) Mechanical activation of medium basicity steel slag under dry condition for carbonation curing. J Build Eng 50
Polettini A, Pomi R, Stramazzo A (2016) Carbon sequestration through accelerated carbonation of BOF slag: influence of particle size characteristics. Chem Eng J 298:26–35
Su T-H, Yang H-J, Shau Y-H, Takazawa E, Lee Y-C (2016) CO2 sequestration utilizing basic-oxygen furnace slag: controlling factors, reaction mechanisms and V-Cr concerns. J Environ Sci 41:99–111
Baciocchi R, Costa G, Polettini A, Pomi R (2009) Influence of particle size on the carbonation of stainless steel slag for CO2 storage. Energy Proc 1(1):4859–4866
Liu G, Schollbach K, van der Laan S, Tang P, Florea MV, Brouwers H (2020) Recycling and utilization of high volume converter steel slag into CO2 activated mortars–the role of slag particle size. Resour Conserv Recycl 160:104883
Song Q, Guo M-Z, Wang L, Ling T-C (2021) Use of steel slag as sustainable construction materials: a review of accelerated carbonation treatment. Resour Conserv Recycl 173:105740
Lee H-S, Lim H-S, Ismail MA (2017) Quantitative evaluation of free CaO in electric furnace slag using the ethylene glycol method. Constr Build Mater 131:676–681
Tong Z, Ma G, Zhou D, Yang G, Peng C (2019) The indirect mineral carbonation of electric arc furnace slag under microwave irradiation. Sci Rep 9(1):7676
Veetil SP, Hitch M (2020) Recent developments and challenges of aqueous mineral carbonation: a review. Int J Environ Sci Technol 17(10), 4359–4380
Wang Y, Liu J, Hu X, Chang J, Zhang T, Shi C (2022) Utilization of accelerated carbonation to enhance the application of steel slag: a review. J Sustain Cem-Based Mater 12(4), 471–486
Pan SY, Liu HL, Chang EE, Kim H, Chen YH, Chiang PC (2016) Multiple model approach to evaluation of accelerated carbonation for steelmaking slag in a slurry reactor. Chemosphere 154:63–71
Acknowledgements
The authors gratefully acknowledge the financial support provided by the Sichuan Science and Technology Program, project No. 2022YFQ0037, the National Natural Science Foundation of China, project No. 51904035, Fuling Science and Technology Project, project No. FLKJ,2022CBZ4061.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Wang, Z. et al. (2024). The Kinetic Study of Carbonation of BOF Slag at High Temperature: Impact of Particle Size Characteristics. In: TMS 2024 153rd Annual Meeting & Exhibition Supplemental Proceedings. TMS 2024. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-50349-8_81
Download citation
DOI: https://doi.org/10.1007/978-3-031-50349-8_81
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50348-1
Online ISBN: 978-3-031-50349-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)