Abstract
The use of coke will be greatly reduced by much pulverized coal injection. The pulverized coal injection rate of blast furnace can be greatly increased by local oxygen-enrichment method. However, the cooling effect of room-temperature oxygen delays the devolatilization process and the entire coal combustion process will be delayed. To increase the pyrolysis rate will weaken the cooling effect of room-temperature oxygen on coal combustion. The pyrolysis rate increases and activating energy decreases with Fe2O3 addition. In this study, the effect of Fe2O3 on coal combustion in the blast furnace was simulated. The effect of Fe2O3 without oxygen enrichment on coal burnout is unobvious. For local oxygen enrichment, the coal burnout greatly increases and has a maximum increase of 22.03%.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The blast furnace is still a main ironmaking process due to its high production efficiency and energy utilization rate [1, 2]. However, high amount of coke is consumed during blast furnace production process. The price of coke continues to rise due to shortage of coking coal resources, which leads to increase of blast furnace production costs. In addition, much energy will be consumed and a large amount of pollutants will be generated in the coking process. Under the pressure of economy and environment, the use of coke must be reduced in the blast furnace production process. The consumption of coke can be effectively reduced by much coal injection. However, to achieve above goals, the most important issue is the full combustion of coal in the raceway region.
There are many factors that affect the combustion of coal, such as blast temperature, coal type, coal particle size, and oxygen concentration. Shen et al. [3, 4] investigated the effect of blast temperature on coal combustion and found that the coal burnout has an increase of about 1.5% for every increase of 100 °C. However, the blast temperature of advanced blast furnace has widely reached 1200 °C. It is difficult to achieve a higher blast temperature. Zhou et al. [5, 6] investigated the effect of coal type on coal combustion and found that high volatile coal has a higher burnout rate. But its heat value is lower, which cannot meet the heat requirements of the blast furnace. Liu et al. [7] investigated the effect of oxygen concentration in hot blast on coal combustion and found that the coal burnout only has an increase of about 1.5% for every increase of 1% oxygen content. The traditional oxygen enrichment method have cannot meet the requirement of higher coal injection rate. Zhou et al. [8] found that the oxygen concentration around coal particles can be greatly increased by the local oxygen enrichment method. However, the cooling effect of room-temperature oxygen delays the coal combustion process. The effect of oxygen enrichment will be weaker and even be invalid. Fu et al. [9] investigated the effect of different catalysts on coal pyrolysis and found that the devolatilization process is accelerated. Wu et al. [10] investigated the effect of Fe2O3 on coal pyrolysis and found that the activation energy of pyrolysis process is reduced.
In order to overcome the problems of previous research on blast furnace coal injection, in this paper, the effect of Fe2O3 on coal combustion in the blast furnace is investigated using the computational fluid dynamics (CFD) method. In other study, the activation energy of pyrolysis process is reduced by adding Fe2O3. The effect of Fe2O3 on coal combustion process is simulated. It found that the coal combustion process of traditional condition is greatly advanced, but the change of final burnout is not obvious. Furthermore, the effect of Fe2O3 on coal combustion process under local oxygen enrichment was investigated. When the addition amount of Fe2O3 is 5 and 10%, the coal burnout is greatly increased. The coal burnout has an increase of 21.59% and 22.03%, respectively. This study has important guiding significance for the development of coal injection technology in blast furnaces.
Geometric Model and Simulation Conditions
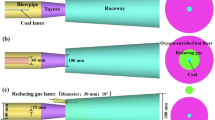
Figure 1 is the schematic diagram of geometric model. The model simulated the lance, blowpipe, tuyere, and raceway region of a blast furnace. The depth of the raceway region is 700 mm. The raceway region was simplifying designed considering flow and combustion characteristics of the coal plume. The structure of base case is used to simulate the coal combustion without oxygen enrichment, and the structure of local oxygen enrichment is used to simulate the coal combustion with oxygen enrichment. The blast temperature is 1200 °C and the oxygen content in hot blast is 21%. For local oxygen enrichment, the total oxygen concentration is 24%, and the remaining oxygen is injected into the blowpipe by the oxygen lance.
Model Description
The gas-particle flow and reaction in the drop tube furnace were calculated based on the framework of the software package ANSYS-FLUENT. In this model, the gas phase is treated with an Eulerian frame and described by the steady-state Reynolds-averaged Navier–Stokes equations closed by the k–ε turbulence model [11]. The governing equations for the gas phase includes mass, momentum, energy, gas species, turbulent kinetic energy, turbulent dissipation rate [12,13,14].
Particles of pulverized coal are treated as discrete phase, modelled using the Lagrangian method, where the trajectories of the discrete particles are determined by integrating Newton’s second law of motion. The drag force (fD) and turbulent dispersion are included. Full coupling of mass, momentum, and energy of particles with the gas phase is implemented. The change of particle temperature is governed by three physical processes: convective heat transfer, latent heat transfer associated with mass transfer, and radiative heat transfer. The governing equations for the particle phase are summarized in Table 1.
The devolatilization process releases volatiles (CαHβOγNδ) and char (C(s)). The coal devolatilization process is simulated using the single-rate devolatilization model [15].
The rate constants k are expressed in the Arrhenius format [16]. The activating energy of pyrolysis with 0%, 5%, and 10% Fe2O3 addition amount is 65.6 kJ/mol, 51.3 kJ/mol, and 40.7 kJ/mol, respectively.
For the char reactions, the heterogeneous surface reaction model is used. The following reactions are considered during the coal combustion process:
The reaction rate for char is expressed as follows [17]:
Results and Discussion
Effect of Fe2O3 Addition on Coal Combustion
Figure 2 shows the effect of Fe2O3 addition amount on final coal burnout. The final coal burnout does not gradually increase with increase of Fe2O3 addition amount. The coal burnout is 74.83% without Fe2O3 addition. When the Fe2O3 addition amount is 5%, the coal burnout is 80.91% with an increase of 6.08%. But the coal burnout of 10% Fe2O3 addition amount is 78.91% decreased by 2% than that of 5% Fe2O3 addition. The activation energy of pyrolysis process decreases with the increase of Fe2O3 addition amount. The pyrolysis rate will increase and the combustion process will be advanced. Therefore, the char will have longer distance to combust. However, the oxygen around the coal particles is limited. The coal combustion process is enhanced in the early stage and will be delayed in the late stage due to the lack of oxygen. Finally, the total coal burnout changes little.
To further understand the effect of Fe2O3 on coal combustion, the coal burnout at different positions was investigated, as shown in Fig. 3. The coal combustion process is greatly advanced with increase of Fe2O3 addition amount, and the combustion rate rapidly increases in the early stage. The coal burnout slowly increases in the early stage without Fe2O3 addition and begins to rapidly increase at the distance of 0.4 m from the coal lance tips. The coal burnout begins to rapidly increase at 0.2 m distance under 5% Fe2O3 addition amount. The distance is only 0.1 m under 10% Fe2O3 addition amount. The coal burnout still remains rapid increase at the later stage of raceway region without Fe2O3 addition. However, the combustion process slows at the later stage of raceway region with Fe2O3 addition. Finally, the gap in coal burnout gradually decreases at the end of raceway region. The reasons causing aforementioned phenomenon are as follows. The activation energy of pyrolysis process decreases with the increase of Fe2O3 addition amount, and the coal rapidly combusts in the early stage. However, the combustion process greatly weakens due to the lack of oxygen in the later stage. In general, the main reasons causing the little change of final coal burnout are the lack of oxygen.
Effect of Fe2O3 on Coal Combustion Under Local Oxygen Enrichment
The oxygen concentration around coal particles will be greatly increased by local oxygen enrichment, and the coal burnout will significantly increase. Therefore, the effect of Fe2O3 addition on coal combustion under local oxygen enrichment was investigated. The effect of Fe2O3 addition amount on coal burnout under local oxygen enrichment is shown in Fig. 4. The coal burnout significantly increases under local oxygen enrichment. The coal burnout without Fe2O3 addition under local oxygen enrichment is 91.06% with an increase of 16.23% than that of base case. The coal burnout of 5% Fe2O3 addition amount is 96.42% with an increase of 21.59% than base case. The coal burnout of 10% Fe2O3 addition amount is 96.86% and most coal particles have full combusted.
To further reveal the effect of Fe2O3 on coal combustion under local oxygen enrichment, the coal burnout at different positions is investigated, as shown in Fig. 5. The coal combustion process is delayed without Fe2O3 addition due to the cooling effect of room temperature. However, the coal burnout rapidly increases at the 0.6 m distance from the coal lance tips. This is because the room-temperature oxygen competes for heat with coal particles, and the devolatilization process is mainly affected by temperature. The char rapidly combusts at the later stage under rich oxygen concentration. The coal combustion process is not delayed with Fe2O3 addition and is greatly advanced with increase of Fe2O3 addition amount. This is because the pyrolysis rate increases with Fe2O3 addition, which makes up the cooling effect of room temperature.
Figure 6 shows the flow and combustion characteristics of coal plume. In general, the coal particles are more dispersed under local oxygen enrichment. The oxygen stream flows into the inner region of coal plume, and the coal particles in the center region are pushed toward surroundings. More coal particles will contact with oxygen, which is beneficial for coal combustion. The coal burnout without Fe2O3 addition in the early stage is lower. Much coal particles of higher burnout can be found in the early stage of the raceway region under Fe2O3 addition. Furthermore, the particles’ concentration in the center region of coal plume is higher and the burnout is lower. This is because large amount of coal particles are concentrated in the center region of coal plume, and the oxygen surrounding the particles cannot meet the combustion of whole particles. For local oxygen enrichment, the coal burnout in the center region is high, but the burnout around coal plume is lower. This is because the oxygen flows into the center region of coal plume, and the oxygen concentration greatly increases.
Conclusions
The effect of Fe2O3 on coal flow and combustion characteristics in the blast furnace was investigated using the CFD method. The main conclusions are as follows:
-
(1)
The effect of Fe2O3 on coal burnout is unobvious without oxygen enrichment, but the coal combustion process is advanced. The main reasons are that the activation energy of pyrolysis process is reduced, but the further combustion is limited by oxygen.
-
(2)
For local oxygen enrichment, the coal burnout greatly increases with Fe2O3 addition. The coal burnout is 96.86% with the addition of 10% Fe2O3, which is an increase of 22.03% than base case.
-
(3)
The coal combustion process is delayed due to the cooling effect of room-temperature oxygen. However, the cooling effect is offset by the addition of Fe2O3. This is because of the significant increase of pyrolysis rate that ensures the full utilization of oxygen.
References
Suopajarvi H, Pongracz E, Fabritius T (2014) Bioreducer use in Finnish blast furnace ironmaking—analysis of CO2 emission reduction potential and mitigation cost. Appl Energ 124:82–93
Wang H, Zhao W, Chu M, Feng C, Liu Z, Tang J (2017) Current status and development trends of innovative blast furnace ironmaking technologies aimed to environmental harmony and operation intellectualization. J Iron Steel Res Int 8(24):751–769
Shen Y, Guo B, Yu A, Zulli P (2009) Model study of the effects of coal properties and blast conditions on pulverized coal combustion. ISIJ Int 6(49):819–826
Shen Y, Maldonado D, Guo B, Yu A, Austin P, Zulli P (2009) Computational fluid dynamics study of pulverized coal combustion in blast furnace raceway. Ind Eng Chem Res 23(48):10314–10323
Zhou C, Liu G, Wang X, Qi C (2016) Co-combustion of bituminous coal and biomass fuel blends: thermochemical characterization, potential utilization and environmental advantage. Bioresour Technol 218:418–427
Tiwari H, Das A, Singh U (2018) Novel technique for assessing the burnout potential of pulverized coals/coal blends for blast furnace injection. Appl Therm Eng 130:1279–1289
Liu Y, Shen Y (2019) CFD study of charcoal combustion in a simulated ironmaking blast furnace. Fuel Process Technol 191:152–167
Zhou Z, Xue Q, Li C, Wang G, She X, Wang J (2017) Coal flow and combustion characteristics under oxygen enrichment way of oxygen-coal double lance. Appl Therm Eng 123:1096–1105
Fu Y, Guo Y, Zhang K (2016) Effect of three different catalysts (KCl, CaO, and Fe2O3) on the reactivity and mechanism of low-rank coal pyrolysis. Energ Fuels 3(30):2428–2433
Wu L, Zhou J, Yang R, Tian W, Song Y, Zhang Q et al (2021) Enhanced catalytic microwave pyrolysis of low-rank coal using Fe2O3@ bluecoke absorber prepared by a simple mechanical ball milling. J Energ Inst 95:193–205
Launder BE, Spalding DB (1972) Lectures in mathematical models of turbulence
Wu D, Zhou P, Yan H, Shi P, Zhou CQ (2019) Numerical investigation of the effects of size segregation on pulverized coal combustion in a blast furnace. Powder Technol 342:41–53
Zhou Z, Huo H, Wang G, Xue Q, She X, Wang J (2017) Effect of oxygen-coal lance configurations on coal combustion behavior. Steel Res Int 1(88):1600197
Shen Y, Shiozawa T, Austin P, Yu A (2014) Model study of the effect of bird’s nest on transport phenomena in the raceway of an ironmaking blast furnace. Miner Eng 63:91–99
Ubhayakar SK, Stickler DB, Von Rosenberg CW, Gannon RE (1977) Rapid devolatilization of pulverized coal in hot combustion gases. In: Symposium (international) on combustion. Elsevier, pp 427–436
Shen YS, Guo BY, Yu AB, Maldonado D, Austin P, Zulli P (2008) Three-dimensional modelling of coal combustion in blast furnace. ISIJ Int 6(48):777–786
Wijayanta AT, Alam MS, Nakaso K, Fukai J, Kunitomo K, Shimizu M (2014) Numerical study on pulverized biochar injection in blast furnace. ISIJ Int 7(54):1521–1529
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Zhou, Z., Wan, Z. (2024). Effect of Fe2O3 on Blast Furnace Coal Combustion Under Local Oxygen Enrichment. In: Iloeje, C., et al. Energy Technology 2024. TMS 2024. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-50244-6_20
Download citation
DOI: https://doi.org/10.1007/978-3-031-50244-6_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50243-9
Online ISBN: 978-3-031-50244-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)