Abstract
This study aimed to characterization of anthocyanins (3-monoglucosides, 3-acetylglucosides, and 3-coumaroylglucosides), pyranoanthocyanins and hydroxyphenyl-pyranoanthocyanins in Vranec wines during aging of three years. The HPLC-DAD–ESI-MSn technique was applied for identification of anthocyanins and derived stable pigments pyranoanthocyanins. All anthocyanins presented mass spectra characterized with two signals, molecular ion M+ and fragment ions [M-162]+, [M-204]+ and [M-308]+ resulting from elimination of glucose, acetylglucose and p-coumaroylglucose moieties, respectively. From the group of pyranoanthocyanins, A-type and B-type vitisins, as well as hydroxyphenyl-pyranoanthocyanins have been determined according to their molecular ions (M+) and characteristic fragments.
I. Hermosín-Gutiérrez—In memoriam.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Colour of wine is one of the most important sensorial attribute, firstly perceived by the consumers. Anthocyanins are the responsible colored compounds, synthesized in the skins of red grape berries and transformed to the wine. The main anthocyanins in Vitis vinifera L. grape varieties are delphinidin, cyanidin, petunidin, peonidin and malvidin in a form of 3-O-glucosides, 3-O-acetylglucosides, 3-O-p-coumroylglucosides [1, 2]. Among all them, derivatives of malvidin are the most abundant colored compounds in red grapes and wines.
When maceration will start, anthocyanins are the first compounds which are extracted from red grape skins, reaching maximum levels after few days of maceration (usually 3 to 4 days). Afterwards, their content is followed with decreasing during the end of fermentation, stabilization and storage of wine. It has been determined that there are many reasons for decrease of anthocyanin’s content, including their adsorption on yeast cell walls, co-precipitation with proteins and tartarates, participation in various chemical reactions in which new and stable compounds are formed. Moreover, their content decreases during filtration and finning process [3, 4]. In particular, anthocyanins participate in formation of anthocyanin-derived pigments during wine storage and ageing, and thus, contribute to a progressive change of the red–purple colour towards a more red–orange colour, especially evident during aging. In fact, anthocyanins participate in cycloaddition reaction at the O-5 and C-4 positions with fermentation metabolites and other grape and wine phenolic compounds [5,6,7], forming new stable anthocyanin-derived pigments, and named pyranoanthocyanins. This group includes vitisins, hydroxyphenyl-pyranoanthocyanins and flavanyl-pyranoanthocyanins, playing an important role in colour stabilization and sensory properties [7,8,9,10].
In the past decades, different techniques have been used for analysis of pigments and studying their structure, such as high-performance liquid chromatography (HPLC) and ultrahigh-performance liquid chromatography (UPLC) coupled to diode array detection (DAD) and/or mass spectrometry (MS) [1,2,3,4,5,6,7,8, 11], matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS) [12, 13], nuclear magnetic resonance (NMR). Recently, high performance liquid chromatography combined with low-field drift tube ion mobility time-of-flight mass spectrometry (HPLCxIMS-TOFMS) was used for characterization and fingerprinting of Macedonian red wines [14].
In this study, the focus of the work was set on the identification and quantification of individual anthocyanins and pyranoanthocyanins, the most important compounds which determine the wine colour and stability. For that purpose, wines produced from Vranec variety, the most widespread and important grape variety for Macedonia and for the Balkans, characterized with deep red colour, have been analyzed with HPLC coupled to DAD and ESI-MS (Ion Trap) in order to identify and investigate the changes of pigments during wine aging.
2 Materials and Methods
2.1 Chemicals and Reagents
Commercial standard of malvidin 3-glucoside was purchased from Phytolab (Vestenbergsgreuth, Germany). Standards of pyranoathocyanins, including vitisin A or 10-carboxy-pyranomalvidin-3-glucoside, pinotin A or 10-(4′′′-monohydroxyphenyl)-pyanomalvidin-3-glucoside and 10-(3′′′, 4′′′-dihydroxyphenyl)-pyranomalvidin-3-glucoside, have been isolated in laboratory conditions [6]. All other solvents used were of HPLC quality and purity (> 99%) and all chemicals of analytical grade (p.a). Water used for analyses was of Milli-Q quality.
2.2 Wine Samples
Red wine samples from Vranec V. vinifera L. variety (vintages: 2006, 2007 and 2008) were kindly provided by Tikveš Winery, Kavadarci. Wines from the three vintages have been produced by same winemaking protocol in triplicates. Thus, harvested grapes (maturity of 22 to 24 °Brix) were processed with electrical inox crusher/destemmer, then supplemented with SO2 (ca. 60 mg/L total concentration), and after few hours, Saccharomyces cerievisiae yeast was inoculated to start the alcoholic fermentation. Maceration time of 8–10 days at 23 ± 2 C was applied, with pumping over and delastage once to two times per day. Wines produced with the same technological treatment, from three tanks were mixed in order to obtain representative samples for analysis.
2.3 HPLC-DAD-ESI-MSn Analysis
Analyses of pigments have been performed with instrumentation supplied by Agilent: An Agilent 1100 Series system (Agilent, Germany) coupled to DAD (G1315B) and a LC/MSD Trap VL (G2445C VL) electrospray ionization mass spectrometry (ESI-MSn) system. An Agilent ChemStation (version B.01.03) software was used for data processing and Agilent LC/MS Trap software (version 5.3) was used for mass spectra processing. Before analyses, wines were diluted with 0.1 M HCl solution (1:4, V/V), filtrated (0.20 μm, polyester membrane, Chromafil PET 20/25, Macherey-Nägel, Düren, Germany) and then were injected into the HPLC system. Separation of the analytes was performed on a Zorbax Eclipse XDB-C18 column (250 × 4.6 mm; 5 μm particle size; Agilent, Germany) at 40 ℃. The mobile phase consisted of solvent A: water/acetonitrile/formic acid (87:3:10, V/V/V, solvent A) and solvent B: water/acetonitrile/formic acid (40:50:10, V/V/V), at flow rate of 0.63 mL/min. Proportions of solvent B were as follows: 0 min, 6%; 15 min, 30%; 30 min, 50%; 35 min, 60%; 38 min, 60%; 46 min, 6% [3].
Identification of pigments was performed in a positive ionization mode. Nitrogen was the drying gas (flow rate of 11 L/min), the drying temperature was set at 350 ℃, the pressure of the nebulizer was 65 psi, the capillary at 2500 V, capillary exit offset at 70 V, skimmer 1 at 20 V; skimmer 2 of 6 V and the compound stability at 100%. The mass spectra were recorded in m/z range of 50–1200. DAD chromatograms were recorded at 520 nm [3].
2.4 Statistical Analyses
XLSTAT software, version 7.5.2, Addinsoft (Paris, France) was used for calculation of means, standard deviation and relative standard deviation. Each wine was analyzed in three replicates.
3 Results and Discussion
3.1 Identification of Anthocyanins and Anthocyanin-Derivatives
Identification of individual anthocyanins and anthocyanin-derivatives in Vranec wines (vintage 2006, 2007 and 2008) was performed with HPLC–DAD–ESI-MSn technique. In total, 25 pigments were determined, including 14 anthocyanins, 5 pyranoanthocyanins and 6 hydroxyphenyl-pyranoanthocyanins. Identification of analyte peaks was performed comparing the UV/Vis spectra and the retention times of compounds for which standards were available. Moreover, obtained ESI-MS and MS/MS data were compared with those found in the relevant literature [5,6,7,8,9,10]. Table 1 contains the data for the molecular and fragment ions of the identified compounds.
Anthocyanins. In total, 14 anthocyanins were identified in analyzed Vranec wines, present in a form of 3-O-glucosides, 3-O-acetylglucosides and 3-O-p-coumaroylglucosides of delphinidin, cyanidin, petunidin, peonidin and malvidin. Identification was based on two characteristic signals, molecular ion M+ and aglycone fragments [M–162]+, [M–204]+ and [M–308]+ as a result of elimination of a glucose moiety, acetylglucoside group and p-coumaroylglucoside group, respectively [1,2,3].
Pyranoanthocyanins. Pyranoanthocyanins are formed in a reaction of cycloaddition between anthocyanins and pyruvic acid, caffeic acid, and p-coumaric acid. These compounds are called 10-carboxy-pyrano-anthocyanins or A-type vitisins [10]. In this study, three A-type vitisins have been identified in wines, as follows: vitisn A (10-carboxy-pyranomalvidin-3-glucoside), acetyl-vitisin A (10-carboxy-pyranomalvidin-3-acetylglucoside) and p-coumaroyl-vitisin A (10-carboxy-pyranomalvidin-3-p-coumaroylglucoside). These three compounds show same characteristic fragment ([M + H]+ = m/z 399) which corresponds to 10-carboxy-pyranomalvidin aglycone [3]. Thus, the mass spectrum of vitisin A contains the molecular ion M+ at m/z 561 and fragment ion at m/z 399, resulting from the loss of glucose. The mass spectra of acetylvitisin A and coumaroylvitisin A show M+ at m/z 603 and 707, respectively, and fragment ions at m/z 399, as a result of the elimination of acetyl- and p-coumaroyl groups, respectively.
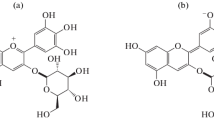
B-type vitisins are formed in a cycloaddition reaction between anthocyanins and acetaldehyde. In this study, following B-type vitisins were identified: vitisin B (pyranomalvidin-3-glucoside) with molecular signal M+ at m/z 517 and fragment ion at m/z 355 by loss of glucoside (162 Da) and acetyl-vitisin B (pyranomalvidin-3-acetylglucoside) with molecular signal M+ at m/z 559 and fragment ion at m/z 355 by loss of acetylgucoside (204 Da) [3, 15]. Fragmentation of vitisin A and vitisin B is presented in Fig. 1.
Hydroxyphenyl-Pyranoanthocyanins. Hydroxyphenyl-pyranoanthocyanins are compounds form in a reaction of caffeic acid and anthocyanins. In this study, three hydroxyphenyl-pyranoanthocyanins have been determined, observing molecular ions at m/z 625, 667 and 771 and identified as 10-(3′′′, 4′′′-dihydroxyphenyl)-pyranomalvidin-3-glucoside (known as pinotin A) [6], 10-(3′′′, 4′′′-dihydroxyphenyl)-pyranomalvidin-3-acetylglucoside and 10-(3′′′, 4′′′-dihydroxyphenyl)-pyranomalvidin-3-p-coumaroylglucoside, respectively. MS/MS fragmentation of molecular ions gave fragment ion at m/z 463 corresponding on elimination of glucose, acetylglucoside and p-coumaroylglucoside groups, respectively [15].
Moreover, compounds originated in a reaction of p-coumaric acid and anthocyanins are called hydroxyphenyl-pyranoanthocyanins. MS/MS analysis of Vranec wines showed presence of molecular ions at m/z 609, 651 and 755, which were identified as 10-(4′′′-monohydroxyphenyl) derivatives of pyranomalvidin-3-glucoside, pyranomalvidin-3-acetylglucoside and pyranomalvidin-3-p-coumaroylglucoside, respectively, all producing fragment ion at m/z 447 [15].
3.2 Influence of Aging on Pigments Content
Table 2 summarize the data for the individual anthocyanins, pyranoanthocyanins and hydroxyphenyl-pyranoanthocyanins quantified in Vranec wine. Quantitative analysis of pigments was performed on a basis of peak area calculations in the HPLC-DAD chromatograms which were recorded at 520 nm. UV/Vis chromatogram of one Vranec wine is presented in Fig. 2.
UV-Vis chromatogram of Vranec wine sample (vintage 2007) recorded at 520 nm. Abbreviations: Dp: delphinidin; Cy: cyanidin; Pt: petunidin; Pn: peonidin; Mv: malvidin; glc: 3-glucoside; acglc: 3-(6″-acetyl)-glucoside; cmglc: 3-(6″-coumaroyl)-glucoside; 10-DHP: 10-(3′′′, 4′′′-dihydroxyphenyl); 10-MHP: 10-(4′′′-monohydroxyphenyl); pymv: pyranomalvidin; vitisin A: 10-carboxy-pyrmv-3-glc; vitisin B: 10-H-pymv-3-glc; A-type vitisin: 10-carboxy-pyranoanthocyanins.
Malvidin derivatives were present in highest content in wines, as it was expected. Thus, malvidin-3-glucoside was the main anthocyanin in wines from all three years of production (47.6 to 50.2%, on a molar basis), regardless the year of production, as it is already known for most of the V. vinifera cultivars, followed by petunidin-3-glucoside (10.6 to 14%, on a molar basis), whereas the cyanidin-3-glucoside (1.03 to 2.34%, on a molar basis) was present in lowest content in all wines (Table 2). Obtained results were in accordance to previous work focused on phenolic analysis of Macedonian red wines [4].
With regards to pyranoanthocyanins, vitisin A was the dominant compound in all wines, present in a relatively high amount (39.5 to 69%), followed by acetyl-vitisin A (12.6 to 19.7%) and p-coumaroyl-vitisin A (6.67 to 28.4%). Vitisin B ranged from 6.67 to 28.4%, on a molar basis, while acetyl-vitisn B was not quantified since it was detected below the limit of detection. Concerning the group of hydroxyphenyl-pyranoanthocyanins, 10-DHP-pyranomalvidin-3-glucoside (pinotin A) and 10-MHP-pyranomalvidin-3-glucoside ranged from 25.2 to 45.3% and 38 to 54.7%, respectively (Table 2), while 10-DHP-pyranomalvidin-3-p-coumaroylglucoside was present in a very low amount (lower than the determined limit of detection).
Regarding the influence of aging, it is well known that anthocyanin content in red wines declines constantly, as a results of various mechanisms, such as adsorption on yeast cell, oxidation, degradation, adsorption and precipitation with tartarates, proteins, polysaccharides or condensed tannins, as well as their participation in stable and complex anthocyanin derived pigments. In this view, it was observed that total anthocyanins were highest in wine produced in 2008 (508 mg/L), followed by intense reduction in wines from 2007 (53.6 mg/L) and 2006 (16.1 mg/L). Similar trend was observed for vitisins and hydroxyphenyl-pyranoanthocyanins content. Highest amount was noticed in the wine sample produced in 2008 (53.1 mg/L total vitisins and 7.37 mg/L total hydroxyphenyl-pyranoanthocyanins), followed by reduction of their content in wines from 2007 and 2006 (Table 2). Generally, pyranoanthocyanins and hydroxyphenyl-pyranoanthocyanins are considered as important pigments in wine, which also decrease during aging, similarly as anthocyanins.
4 Conclusion
In this study, 25 pigments (anthocyanins and anthocyanin derivatives) have been identified and quantified in Vranec wines applying HPLC-DAD-ESI-MSn technique. Malvidin-3-glucoside and its derivatives (3-acetylglucoside and 3-p-coumaroylglucoside) were the major compounds in all analyzed wines. Vitisin A and 10-MHP-pyranomalvidin-3-glucoside were the dominant compounds in the group of derived pigments. Regarding the year of production, wine from vintage 2008 presented highest levels of all pigments analyzed. In general, anthocyanins dominated in all wines, regardless the year of production, followed by pyranoanthocyanins and hydroxyphenyl-pyranoanthocyanins.
References
Ivanova, V., et al.: Polyphenolic content of Vranec wines produced by different vinification conditions. Food Chem. 124, 316–325 (2011)
Ivanova, V., et al.: Identification of polyphenolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening. Food Res. Int. 44(9), 2851–2869 (2011)
Ivanova-Petropulos, V., et al.: Phenolic compounds and antioxidant activity of Macedonian red wines. J. Food Compos. Anal. 41, 1–14 (2015)
Ivanova-Petropulos, V., Ricci, A., Nedelkovski, D., Dimovska, V., Parpinello, G.P., Versari, A.: Targeted analysis of bioactive phenolic compounds and antioxidant activity of Macedonian red wines. Food Chem. 171, 412–420 (2015)
Marquez, A., Serratosa, M.P., Merida, J.: Pyranoanthocyanin derived pigments in wine: structure and formation during winemaking. J. Chem. 2013, 1–15 (2013). Article ID: 713028
Blanco-Vega, D., Gómez-Alonso, S., Hermosín-Gutiérrez, I.: Identification, content and distribution of anthocyanins and low molecular weight anthocyanin-derived pigments in Spanish commercial red wines. Food Chem. 158, 449–458 (2014)
He, F., et al.: Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 17(2), 1483–1519 (2012)
Bakker, J., Timberlake, C.F.: Isolation, identification and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 45, 35–43 (1997)
Schwarz, M., Hofmann, G., Winterhalter, P.: Investigations on anthocyanins in wines from Vitis vinifera cv. Pinotage: factors influencing the formation of pinotin A and its correlation with wine age. J. Agric. Food Chem. 52(3), 498–504 (2004)
Fulcrand, H., Cameira dos Santos, P.J., Sarni-Manchado, P., Cheynier, V., FavreBonvin, J.: Structure of new anthocyanin-derived wine pigments. J. Chem. Soc. Perkin Trans. 1(7), 735–739 (1996)
Wang, Z., Zhang, L., Li, Y., Liu, Q., Yuan, C.: Non-acylated and acylated anthocynins in red wines of different ages: color contribution and evaluation. J. Food Compos. Anal. 115, 104951 (2023)
Ivanova, V., et al.: Rapid MALDI-TOF-MS detection of anthocyanins in wine and grape using different matrices. Food Anal. Methods 4, 108–115 (2011). https://doi.org/10.1007/s12161-010-9143-7
Ivanova Petropulos, V., et al.: Application of a novel small-scale sample cleanup procedure prior to MALDI-TOF-MS for rapid pigment fingerprinting of red wines. Food Anal. Methods 7, 820–827 (2014). https://doi.org/10.1007/s12161-013-9687-4
Causon, T.J., Ivanova-Petropulos, V., Petrusheva, D., Bogeva, E., Hann, S.: Fingerprinting of traditionally produced red wines using liquid chromatography combined with drift tube ion mobility-mass spectrometry. Anal. Chim. Acta 1052, 179–189 (2019)
Blanco-Vega, D., López-Bellido, F.J., Alía-Robledo, J.M., Hermosín-Gutiérrez, I.: HPLC–DAD–ESI-MS/MS characterization of pyranoanthocyanins pigments formed in model wine. J. Agric. Food Chem. 59, 9523–9531 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Ivanova-Petropulos, V., Nikolić, D., Vojnoski, B., Hermosín-Gutiérrez, I. (2023). Identification of Anthocyanins and Anthocyanin-Derivatives in Vranec Wines During Aging. In: Brka, M., et al. 32nd Scientific-Expert Conference of Agriculture and Food Industry. Agriconference 2022. Lecture Notes in Bioengineering. Springer, Cham. https://doi.org/10.1007/978-3-031-47467-5_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-47467-5_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-47466-8
Online ISBN: 978-3-031-47467-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)