Abstract
The main goal of this study is to investigate numerically the thermal performances of thermal management systems (TMSs) for an electric vehicle battery pack. Three different TMSs were considered i.e. natural convection TMS mode, passive TMS using phase change material (PCM) and hybrid liquid coolant-PCM TMS mode. A lumped thermal dynamic model is developed based on transient energy balances and then validated with measured data from the literature. An in-depth parametric study is carried out where the influence of several design and operation parameters on the TMS’s performances is presented and analyzed. When compared to passive PCM and natural convection, the results demonstrate that combining PCM with liquid cooling for battery thermal management reduces the maximum battery temperature by around 38 and 4.5℃, respectively.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The transportation industry is one of the greatest producers to greenhouse gas emissions, and as such, it will also be an important factor in determining whether or not countries are able to achieve the Net Zero objective by 2050 [1, 2]. The deployment of battery-powered electric vehicles is one of the potential zero-carbon alternatives in the transportation sector. This is due to the fact that their deployment will restrict CO2 emissions and provide flexibility to the national electrical grid of the country [3,4,5]. Because of this, it has gained widespread acceptance in the transportation industry as an environmentally friendly technology that may improve pollution levels and provide energy security, particularly when driven by renewable energy sources [6, 7].
Li-ion batteries emit three types of heat throughout the charging and discharging processes: irreversible heat, Joule heating from ohmic losses, and reaction heat from an increase or decrease in entropy [8]. Therefore, the capacity, longevity, and safety of the battery may be negatively impacted if the heat created during the operation of the battery pack is not removed effectively, resulting in an increase in the overall the system’s temperature. Maintaining a safe and reliable battery temperature while the EV is in motion is a top priority [9]. Lithium-ion batteries perform best when stored in an environment with a constant temperature of 15 to 35℃ [10]. Many different thermal management systems (BTMS) have been designed, evaluated, and experimentally investigated in order to address these issues. The division of BTMS can be done in a number of different ways, including by type (internal, exterior), operation (cooling, heating), and power use (either active or passive). In comparison to the other methods, PCM BTMS stands out as an innovative and efficient method for cooling batteries because it eliminates heat from the battery when it is in phase transition without draining the battery’s power source. This makes it a standout among battery cooling methods. PCM-based BTMS’s key advantages lie in their straightforward structure, high-temperature control capability, cheap, chemically stable property, and wide range of potential uses [11]. In the present work, the three BTM systems presented and analyzed use either natural air flow convection mode, PCM mode, or a hybrid liquid coolant-PCM mode to dissipate heat. For each BTM mode, we develop a thermal dynamic model and validate it against published experimental data. This study’s key contribution is a thorough examination of the thermal performance of the BTMS in simulated charging and discharging cycles, as well as under the real-world conditions of electric vehicles.
2 BTMS Description
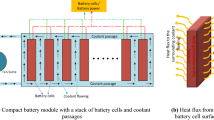
The investigated battery pack system is made up of 24 units of 21,700 Li-ion LiNiMnCoAlO2 (NMC) batteries that are connected in series (6S4P). This commercial Li-ion battery was chosen because there is a lot of interest in this format on the market right now, and because it has a lot of energy per cell, almost 50% more than the 18,650 cells. In case 1, this battery pack keeps its temperature under control by letting air flow between the battery cells. In the second scenario, PCM is added around the battery cells to control the thermal response and absorb generated heat removal from the pack throughout charge/discharge processes. In Table 1, the basic specs of the battery cell and PCM material are shown. In the third case study, a hybrid BTMS is used. Nine liquid-cooling tubes are built into the battery pack, and a PCM is also included (Fig. 1). It is interesting to note that the used PCM is a paraffine type from Rubitherm Company and a mixture of water and ethylene glycol is used as the coolant.
3 Modelling Method and Validation
Transient energy balance modeling is used to simulate the used TMS in battery pack. Because of the similarities between energy transfer and electric transfer, this thermal modeling allows use of the lumped approach. Effective heat capacity is used to model the phase transition process under the assumption that the PCM is homogeneous. Moreover, it is presumed that the liquid coolant flows in a laminar, incompressible, and unidirectional manner. The thermal energy input and output rates is given in the unsteady energy balance for each part of the battery pack. It is possible to express the total amount of heat produced by the battery pack during its charging and discharging processes as:
The internal resistance of a battery, denoted by the symbol R, varies with its temperature in the following way [12]:
The state of charge (SOC) in charging/discharging of batteries is used to figure out the entropy change \(\Delta S,\) which is given as:
It’s interesting that the charging current is negative and the current used for discharge process is positive. The following formula is used to figure out the SOC of the system:
where \(C_{{{\text{nom}}}}\) is the nominal capacity of batteries (Ah) and \({\text{SOC}}\left( {t_{0} } \right)\) is the initial state of charge of the battery pack.
As previously mentioned, the heat capacity of PCM is calculated as a function of temperature so that the phase change process can be considered. This expression of heat capacity of PCM, which has been tested in the experiments from Ref. [13] and it contains latent and sensible heat capacities:
where:
\(L_{s}\) is the latent heat and \(f\left( T \right)\) the fraction of the liquid of PCM. The latter varies between 0 and 1. Following is an expression that can be used to describe the heat capacity of PCM in terms of its liquid fraction [13]:
Both the liquid and solid states of PCM have heat capacities of C0,s and C0,l, respectively. If the temperature of PCM is smaller than the fusion point, then f(T) is a zero value. On the other hand, f(T) = 1 if Tpcm > Tm. The fraction of liquid PCM throughout the phase transition can be tracked with the help of a temperature-dependent function, as shown below [14]:
In this expression, T is the PCM temperature, and \(\gamma\) is the tilt, which is taken equal to 3.1 [14]. It’s worth mentioning that Eq. (9) also describes why the heat capacity of PCM goes up after a phase change, so Eq. (5) can be written like this:
The previous PCM heat capacity expression is integrated in the energy balance equation for PCM as the following:
where Tb is the average temperature of battery cells, Tpcm is the average PCM temperature and Tc is the coolant fluid temperature. It is important to note the for case 1 where only exterior air is used as the TMS, the term \({{\left( {T_{b} \, - \,T_{{{\text{pcm}}}} } \right)} \mathord{\left/ {\vphantom {{\left( {T_{b} \, - \,T_{{{\text{pcm}}}} } \right)} {R_{{b\, - \,{\text{pcm}}}} }}} \right. \kern-0pt} {R_{{b\, - \,{\text{pcm}}}} }}\, + \,{{\left( {T_{c} \, - \,T_{{{\text{pcm}}}} } \right)} \mathord{\left/ {\vphantom {{\left( {T_{c} \, - \,T_{{{\text{pcm}}}} } \right)} {R_{{{\text{pcm}}\, - \,c}} }}} \right. \kern-0pt} {R_{{{\text{pcm}}\, - \,c}} }}\) is equal to zero. For the second case study where both PCM and exterior air are used as TMS, only \({{\left( {T_{c} \, - \,T_{{{\text{pcm}}}} } \right)} \mathord{\left/ {\vphantom {{\left( {T_{c} \, - \,T_{{{\text{pcm}}}} } \right)} {R_{{{\text{pcm}}\, - \,c}} }}} \right. \kern-0pt} {R_{{{\text{pcm}}\, - \,c}} }}\) is equal to zero.
To predict the average battery pack temperature, the following energy balance is used:
where \(P_{{{\text{gene}}}}\) is the heat generation term given in Eq. (1). It is interesting to note that for the hybrid BTMS, the energy used by the coolant pump can be made as efficient as possible by controlling the coolant liquid flowrate. This is controlled and its value is based on the average battery pack temperature:
All the previous coupled equations were solved numerically by adopting Runge–Kutta 4th order Method and using Python Language. To prove that the proposed numerical approach for BTMSs is valid, The findings from the computations were compared with those from the simulations and experiments coming from Ref. [15]. Figure 2 shows that the proposed model can simulate the BTMSs functioning during continuous charging/discharging processes. The proposed model is able to replicate battery temperatures with an average absolute error of 0.27℃ and a maximum difference of 2℃.
Numerical results versus CFD and Experimental from Ref. [15]: Variation of average battery temperature during the charging-discharging operation
4 Results and Discussion
Figure 3 depicts how the examined electrical current varies as a function of the battery pack’s charge level during continuous charging and discharging activities. The functionality of the examined BTMSs mode is analyzed with this electric current profile. The time-dependent changes in the battery cells throughout the ongoing charging/discharging process are depicted in Fig. 4. It is demonstrated that, for all three scenarios, battery cell temperatures rapidly rise during fast-discharging periods before gradually falling off again. It is also shown that the battery pack’s temperature reaches a maximum of 68℃ under the employed conditions. To emphasize, in the latter stage (instance 1), batteries are directly in contact with the surrounding, which serves as the TMS. When battery packs reach 68℃, their storage capacity decreases dramatically over time and the risk of battery runaway increases dramatically. This issue can be avoided by maintaining the battery pack within a temperature range of roughly 20 to 30℃. Thus, a BTMS is mandatory.
The model was used to run simulations on case 2 to determine the impact of employing PCM as TMS. The results demonstrate that the maximum temperature of the battery is much lower when utilizing PCM rather than the air flow option (case1). By dissipating and storing the heat produced by the battery cells, the thermal management modes (cases 2) and (PCM-Liquid coolant) (case 3) were able to outperform the natural convection mode (case 1). At the completion of the 3-C discharging procedure, the battery reaches its maximum temperature, which in case 3 (liquid coolant-PCM mode) is around 30℃ and in case 2 (passive PCM mode) is around 34.5℃. The hybrid thermal control option improves the other two in keeping battery temperatures within the optimal range by reusing the heat already stored in the power conversion module (PCM).
5 Conclusion
In the present work, the thermal performances of three different thermal management systems (TMSs) for battery packs are presented and compared. The considered case studies are natural convection TMS mode (case 1), passive TMS using phase change material (PCM) (case 2) and hybrid liquid coolant-PCM TMS mode (case 3). A thermo-electrical dynamic model was developed and validated with existing results in the literature. Based on obtained results, it was shown that using case 3 for battery TMS is more suitable than case 2 and case 1 as it allows to recover efficiently the generated heat by the batteries and to maintain their temperatures at the suitable range. The obtained maximal battery temperature was 68, 34.5 and 30℃ for case 1, case 2 and case 3 respectively.
References
Zhang, R., Zhang, J.: Long-term pathways to deep decarbonization of the transport sector in the post-COVID world. Transp. Policy 110, 28–36 (2021). https://doi.org/10.1016/J.TRANPOL.2021.05.018
Liimatainen, H.: Decarbonizing Road Freight Transport. In: International Encyclopedia of Transportation, pp. 395–401 (2021). https://doi.org/10.1016/B978-0-08-102671-7.10277-5
Zhao, G., Wang, X., Negnevitsky, M., Zhang, H.: A review of air-cooling battery thermal management systems for electric and hybrid electric vehicles. J. Power. Sources 501, 230001 (2021). https://doi.org/10.1016/j.jpowsour.2021.230001
Lebrouhi, B.E., Khattari, Y., Lamrani, B., Maaroufi, M., Zeraouli, Y., Kousksou, T.: Key challenges for a large-scale development of battery electric vehicles: a comprehensive review. J. Energy Storage. 44, 103273 (2021). https://doi.org/10.1016/j.est.2021.103273
Yi, F., Jiaqiang, E., Zhang, B., Zuo, H., Wei, K., Chen, J.: Effects analysis on heat dissipation characteristics of lithium-ion battery thermal management system under the synergism of phase change material and liquid cooling method. Renew. Energy 181, 472–489 (2022). https://doi.org/10.1016/j.renene.2021.09.073
Boulakhbar, M., Lebrouhi, B., Kousksou, T., Smouh, S., Jamil, A., Maaroufi, M., Zazi, M.: Towards a large-scale integration of renewable energies in Morocco. J. Energy Storage 32, 101806 (2020). https://doi.org/10.1016/j.est.2020.101806
Goel, S., Sharma, R., Rathore, A.K.: A review on barrier and challenges of electric vehicle in India and vehicle to grid optimisation. Transp. Eng. 4, 100057 (2021). https://doi.org/10.1016/j.treng.2021.100057
Chen, J., Kang, S., Jiaqiang, E., Huang, Z., Wei, K., Zhang, B., Zhu, H., Deng, Y., Zhang, F., Liao, G.: Effects of different phase change material thermal management strategies on the cooling performance of the power lithium ion batteries: a review. J. Power Sources 442, 227228 (2019). https://doi.org/10.1016/j.jpowsour.2019.227228
Kim, J., Oh, J., Lee, H.: Review on battery thermal management system for electric vehicles. Appl. Therm. Eng. 149, 192–212 (2019). https://doi.org/10.1016/j.applthermaleng.2018.12.020
Tete, P.R., Gupta, M.M., Joshi, S.S.: Developments in battery thermal management systems for electric vehicles: a technical review. J. Energy Storage 35, 102255 (2021). https://doi.org/10.1016/j.est.2021.102255
Lamrani, B., Lebrouhi, B.E., Khattari, Y., Kousksou, T.: A simplified thermal model for a lithium-ion battery pack with phase change material thermal management system. J. Energy Storage 44, 103377 (2021). https://doi.org/10.1016/j.est.2021.103377
He, F., Li, X., Ma, L.: Combined experimental and numerical study of thermal management of battery module consisting of multiple Li-ion cells. Int. J. Heat Mass Transf. 72, 622–629 (2014). https://doi.org/10.1016/j.ijheatmasstransfer.2014.01.038
Medved, I., Trník, A., Vozár, L.: Modeling of heat capacity peaks and enthalpy jumps of phase-change materials used for thermal energy storage. Int. J. Heat Mass Transf. 107, 123–132 (2017). https://doi.org/10.1016/j.ijheatmasstransfer.2016.11.024
Halimov, A., Lauster, M., Müller, D.: Validation and integration of a latent heat storage model into building envelopes of a high-order building model for Modelica library AixLib. Energy Build. 202, 109336 (2019). https://doi.org/10.1016/j.enbuild.2019.109336
Kong, D., Peng, R., Ping, P., Du, J., Chen, G., Wen, J.: A novel battery thermal management system coupling with PCM and optimized controllable liquid cooling for different ambient temperatures. Energy Convers. Manag. 204, 112280 (2020). https://doi.org/10.1016/j.enconman.2019.112280
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Lamrani, B., Lebrouhi, B.E., Bekkioui, N., Ndukwu, M.C., Kousksou, T. (2024). Thermal Management of Li-Ion Battery Pack for Electrical Vehicles: A Comparative Study. In: Ali-Toudert, F., Draoui, A., Halouani, K., Hasnaoui, M., Jemni, A., Tadrist, L. (eds) Advances in Thermal Science and Energy. JITH 2022. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-43934-6_46
Download citation
DOI: https://doi.org/10.1007/978-3-031-43934-6_46
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43933-9
Online ISBN: 978-3-031-43934-6
eBook Packages: EngineeringEngineering (R0)