Abstract
Cyanobacteria have become infamous due to their impacts on aquatic ecosystems, in particular negative consequences on ecosystem functioning and services to human populations. Observations of cyanobacterial dominance have been reported since the end of the 1800s and the global rise in industrialisation characterized by enhanced human perturbations and a warming climate. We have limited information on past cyanobacteria dynamics and geographic distribution due to the paucity of long-term monitoring data. In addition, challenges remain surrounding the detection and the identification of cyanobacterial taxa deposited in sedimentary archives.
In this chapter, we synthesized the scientific knowledge gained via studies using environmental DNA archived in lake sediments (i.e., sedimentary DNA; sedDNA; Crump 2021 ) to reconstruct the temporal changes in the ecology, habitat, and function of cyanobacteria in relation to environmental change. We begin with an overview of the general ecology and distribution of cyanobacteria, followed by a characterization of the nature of the sedDNA signal and relevant taphonomic processes, with respect to cyanobacteria. We then report the current state of knowledge of cyanobacterial past dynamics in lacustrine environments through a review of the published cyanobacteria sedDNA literature. Last, we discuss how the field of sedDNA has the potential to shed light on the timing and specific causes of environmental change in addition to guiding ecosystem restoration and management goals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sedimentary DNA
- Environmental DNA
- PCR
- High-throughput sequencing
- Metagenomics
- Eutrophication
- Climate change
- Biotic homogenization
- Cyanobacteria
- Oxyphotobacteria
- Lakes
- Paleolimnology
- Paleo-indicators
Introduction
Cyanobacteria have become infamous due to their impacts on aquatic ecosystems, in particular negative consequences on ecosystem functioning and services to human populations. Observations of cyanobacterial dominance have been reported since the end of the 1800s and the global rise in industrialisation characterized by enhanced human perturbations and a warming climate. We have limited information on past cyanobacteria dynamics and geographic distribution due to the paucity of long-term monitoring data. In addition, challenges remain surrounding the detection and the identification of cyanobacterial taxa deposited in sedimentary archives.
In this chapter, we synthesized the scientific knowledge gained via studies using environmental DNA archived in lake sediments (i.e., sedimentary DNA; sedDNA; Crump 2021) to reconstruct the temporal changes in the ecology, habitat, and function of cyanobacteria in relation to environmental change. We begin with an overview of the general ecology and distribution of cyanobacteria, followed by a characterization of the nature of the sedDNA signal and relevant taphonomic processes, with respect to cyanobacteria. We then report the current state of knowledge of cyanobacterial past dynamics in lacustrine environments through a review of the published cyanobacteria sedDNA literature. Last, we discuss how the field of sedDNA has the potential to shed light on the timing and specific causes of environmental change in addition to guiding ecosystem restoration and management goals.
Diversity and Distribution of Cyanobacteria
Cyanobacteria (a.k.a. Oxyphotobacteria) are photosynthetic bacteria (Whitton 2012). They are ancient organisms that were early colonizers of the planet and are arguably one of the most important microbial groups on Earth because of their contribution to the oxygenation of the atmosphere for the last ca. three billion years. Cyanobacteria are ubiquitous, thriving in terrestrial or aquatic (marine and freshwater) environments ranging from cold to tropical regions. They can survive under the harshest environmental conditions (e.g., geothermal springs, cold and hot deserts). The phylum Cyanobacteria exhibits a high diversity, with an estimated 6000 to 8000 species across more than 300 genera (Guiry 2012; Nabout et al. 2013; Hauer and Komárek 2022) with many species yet to be discovered, particularly in poorly studied regions (e.g., the tropics, extreme habitats). While this diversity can be seen in the range of sizes from unicellular forms below one micron to macroscopic colonies of various shapes (Bonilla and Pick 2017; Fig. 5.1), molecular analyses are leading to the discovery of more species and re-writing the phylogeny and taxonomy of phylum Cyanobacteria (Komárek 2016a, b). In addition to taxonomic diversity, cyanobacteria exhibit a range of ecological and physiological traits that enable adaptation to many environmental extremes (e.g., temperature, oxygen, nutrients, UV, salinity). Changes in the surrounding environment induce rapid physiological changes in cyanobacteria, as such they are highly adaptable and competitive.
Main morphological types of cyanobacteria. (Modified from Bonilla and Pick (2017))
Physiological and Morphological Traits of Cyanobacteria Favoring Their Dominance
Cyanobacteria have several eco-physiological traits providing a competitive advantage under diverse and changing environments. For example, in terrestrial ecosystems, tolerance to desiccation is a key trait for survival (Whitton and Potts 2014). Nitrogen control and the capacity of some species to fix nitrogen gas (N2), typically inside thick-walled specialized cells (heterocytes or heterocysts), is also an important trait in several aquatic and terrestrial habitats, providing a unique competitive advantage under low dissolved nitrogen availability (Herrero et al., 2001). In aquatic environments, key adaptations also include buoyancy regulation through gas vesicles, efficient light harvesting, tolerance to extreme solar radiation, ability to grow in warm temperatures, high affinity and high storage capacity for both phosphorus and nitrogen, akinete (resting stage) production, and toxin synthesis (Carey et al. 2012; Bonilla and Pick 2017). For example, picocyanobacteria (cells within the picoplankton size range of 0.2–2 μm) have a high nutrient uptake capacity at low nutrient concentrations, which favors their dominance both in terms of biomass and primary production in the oligotrophic ocean (Whitton and Potts 2014) as well as in oligotrophic lakes (Callieri et al. 2013). Other taxa such as the widespread Microcystis genus, which thrives in eutrophic lakes, can form large visible colonies (up to 5 mm) that contribute to dominance and bloom formation (i.e., massive accumulations of cyanobacterial cells typically observed at the water surface).
Current Trends in the Frequency and Magnitude of Cyanobacterial Blooms
Cyanobacteria can proliferate in coastal and inland waters, with accumulations characteristic of harmful algal blooms, referred to as cyanoHABs, which degrade the health of aquatic ecosystems and alter ecosystem services, raising concerns among the general public, lake managers, policymakers, and scientists. CyanoHABs can result in deoxygenation of the water column, nuisance odors and tastes, and the production of cyanotoxins, a suite of secondary metabolites potentially harmful to humans, wildlife, and domesticated animals (Chorus and Welker 2021). There are a variety of cyanotoxin types (hepatotoxins, neurotoxins, dermatoxins) with different effects (Carmichael 1994; Chorus and Batram 1999; Chorus and Welker 2021). Many species can produce multiple toxins, and the same toxin can be synthesized by many species. Overall, the factors triggering and mediating toxin synthesis in cyanobacteria, as well as the role(s) of these secondary metabolites, remain poorly understood (Chorus et al. 2021).
The incidence of cyanobacterial bloom events in lakes has risen considerably in recent decades (Ho et al. 2019). Based on sedimentary pigment analysis, the dominance of cyanobacteria relative to other phytoplankton in temperate lakes appears to have increased globally since the mid twentieth century (Taranu et al. 2015) in parallel with human population growth, urbanization and the rise in use of chemical fertilizers. The intensity and frequency of cyanoHABs is expected to continue to increase in response to rising levels of atmospheric CO2 with enhanced combined (and possible synergistic) effects of anthropogenic-induced eutrophication (Moss et al. 2011; Huisman et al. 2018; Kakouei et al. 2021). Changes in environmental conditions, such as lake trophic status, can trigger community shifts towards taxa better adapted to the new environmental conditions (Watson et al. 1997). Compositional turnover often goes unnoticed in lakes unless they are closely monitored, but they can indicate a major change in ecosystem functioning as new taxa become dominant. An increased nutrient input and/or lake water warming often leads to the seasonal dominance of large colonial and buoyant taxa, in the orders Chroococcales, Nostocales and Oscillatoriales (Bonilla and Pick 2017). These shifts in community composition can inform on the timing of past environmental changes and on whether the system has recovered after nutrient abatement or if a regime shift has occurred (Monchamp et al. 2021).
Sediment Deposition of Cyanobacteria
Lake sediments are formed by the accumulation over time of organic and inorganic matter originating from the lake itself, the catchment, and atmospheric input, creating an archive of past biota, trophic conditions, and biogeochemical processes prevailing at the time of deposition (Smol 2009). Undisturbed lacustrine (and marine) sediments therefore constitute a source of information for paleoecologists and paleoclimatologists to study past life and climatic conditions using a variety of paleolimnological tools and proxies. Living, dead or dormant cyanobacterial cells can accumulate in sediments through several processes such as aggregation on particles or concentration in fecal pellets followed by sedimentation, as well as through active migration. Some cyanobacteria are also able to grow in surficial sediments (e.g., Gaget et al. 2020), particularly in littoral areas and shallow lakes where light reaches the water-sediment interface. Most cyanobacteria have thick gelatinous cell walls making them less prone to cell degradation than other phytoplankton taxa, particularly in the case of the resting stages of Nostocales (akinetes), which have been shown to successfully germinate under laboratory conditions decades to centuries after burial in sediments (Livingstone and Jaworski 1980; Legrand et al. 2019).
Biogeochemical proxies, including lipid biomarkers and pigments (photosynthetic and UV-screening), as well as isotope composition, have been used for many decades to detect and quantify photosynthetic microbes, including cyanobacteria, in aquatic sediments (Leavitt and Hodgson 2006). More recently cyanotoxins have been quantified in sediment cores: these include the more persistent chemical species e.g., several congeners of microcystins (Zastepa et al. 2017) and the cylindrospermopsins (Waters 2016). However, such proxies offer limited information on community composition or the presence of potentially toxic taxa, because they can only provide a coarse taxonomic identification of the main phytoplankton groups. This is a challenge that sedDNA studies are currently addressing.
Taphonomic Processes: Production, Sedimentation, and Preservation of Cyanobacterial DNA in Sediments
In-lake drivers, such as grazing, parasitism, water currents and wind, can influence the rate at which phytoplankton cells are deposited in sediments. How DNA from various organisms reaches bottom sediments, is altered through diagenesis, or becomes preserved over time is still not fully understood. Investigations of the correspondence between pelagic and sediment communities provide new information that can guide the interpretation of sedDNA historical archives. For example, Nwosu et al. (2021a) compared the composition, richness, and evenness of the cyanobacterial DNA signal between pelagic water, sediment trap, and surface sediment samples, and they found no significant difference in detection of cyanobacterial taxonomic composition across sample types. This suggests minimal taxonomic bias between sediments and the water column. However, the authors reported large taxon-specific differences in relative abundances between water and sediment samples, suggesting differential deposition or preservation of the DNA signal between cyanobacterial clades in sediments. For instance, the genus Planktothrix was found in relatively low proportion compared to other cyanobacteria in both surface sediment and sediment trap samples, despite this taxon being dominant in the water column, especially near the thermocline. On the other hand, the DNA signal for colonial taxa (Aphanizomenon, Snowella) was stronger in surface sediment samples. In a comparative study of metagenomes from lake water and both contemporary and pre-industrial sediments, Garner et al. (2020) found that some dominant bacterial taxa from the pelagic zone were not detected in sediment samples, while some of the preserved microorganisms detected had never been reported from limnological surveys. Only a few such in situ studies on the efficiency of phytoplankton/microbial eukaryotes deposition and recovery in lake sediments are currently published (see Gauthier et al. 2021). Laboratory experiments suggest that, in addition to differential sedimentation behavior within the water column depending on taxa features (e.g., habitats, morphologies), there may be differences in degradation rates among major cyanobacteria taxa once settled in the sediments (Mejbel et al. 2022) as well as an impact of sediment storage on sedDNA concentration and species recovery (Brasell et al. 2022). Finally, only a few studies have investigated the congruence of the sedDNA signal between sediment cores collected at various locations in a same lake (Weisbrod et al. 2020; Thorpe et al. 2022). These works report good congruence of both cyanobacterial and other prokaryotes composition across sites.
Overall, cold and hypoxic/anoxic conditions are more favorable for DNA preservation in lake sediments (Mejbel et al. 2022 and references therein; see Chap. 2 of this volume) and thus deep cold-water lakes are generally expected to be adequate systems in which to retrieve cyanobacterial archives. However, there have been a handful of paleolimnological studies on subtropical lakes (e.g., Martínez de la Escalera et al. 2014; Huo et al. 2022a) as well as shallow temperate lakes (e.g., Picard et al. 2022a) that have successfully obtained cyanobacterial DNA records. More work that specifically aims at (1) evaluating the extent to which the limnetic biological signal reaches the sediments and (2) understanding the processes behind sinking/sedimentation/degradation rates of various planktonic forms including cyanobacteria on a range of lake types with different sediment geochemical properties remain necessary for improving ecological inferences based on sedDNA archives.
Cyanobacterial Signal in Lacustrine Sediments: Dead or Alive?
One of the challenges in the field of environmental paleomicrobiology is distinguishing between active, dormant, and dead cells in lake sediments (Capo et al. 2022). A further complication is that certain cyanobacteria taxa can overwinter in sediments and recolonize the water column the following spring under favorable environmental conditions (Brunberg and Blomqvist 2002). Nostocales can remain viable in sediments, possibly up to hundreds of years (Legrand et al. 2019) and germinate when environmental conditions improve. It is therefore difficult to disentangle the dead vs. live signal in the cyanobacterial DNA deposited to sedimentary archives, which can constitute a limitation of sedDNA-based reconstructions. Nevertheless, the recovery of cells in sediment layers, whether they are currently active or not, reflect the ecological conditions at the time of deposition and are accepted as a reasonable means of reconstructing past community composition dynamics.
Discriminating the presence of living organisms in the pool of sedDNA is not possible based on DNA analyses alone because the DNA signal can come from both active and inactive (i.e., dead) cells. Even though DNA starts degrading as soon as cell death or cell lysis occurs, it can preserve for an extended period of time in lake water and in sediments (Barnes et al. 2014; Pedersen et al. 2015). However, damage starts accumulating inside DNA molecules deposited hundreds of years ago (Dabney et al. 2013), creating a specific signature that can be authenticated bioinformatically by calculating the ratios of damaged vs. intact DNA fragments sequenced (see Chap. 3 of this volume). Finally, it has been proposed that RNA could be used to distinguish between living and dead cells because of its higher degradation rate compared to DNA. However, a recent investigation based on the recovery of ca. 200 years-old RNA molecules in lake sediments shows that cyanobacterial RNA can also be quite stable over decadal to centennial times (Pearman et al. 2022), which suggests that the potential of RNA for distinguishing between living and dead cells may extend to sediments deposited at least several hundred years ago.
SedDNA-Based Approaches: A Means of Reconstructing Past Cyanobacterial Histories
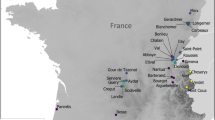
Over the last two decades, the rapid development of environmental DNA techniques and high-throughput sequencing technologies (HTS) has opened new possibilities to identify taxa within all domains of life, including cyanobacteria, in their natural habitat (Pedersen et al. 2015; Taberlet et al. 2018). Molecular genetic approaches allow for a more refined taxonomic level compared to other proxies like pigments (with some caveats), potentially at the resolution of species and subspecies/strains. Analyses of DNA preserved in aquatic sediments have expanded our knowledge of cyanobacteria temporal dynamics in various aquatic habitats around the globe. In a literature search using Web of Science (as of July 2022), and based on the list of sampling sites compiled by Von Eggers et al. (2022), we found 50 research articles exploring the long-term dynamics of cyanobacteria with sedDNA at a total of 71 lacustrine sites (Fig. 5.2). Of the 123 DNA records from aquatic sediments, 104 were from lake sediment cores (time-series), four were from lake surface sediments only, and two were obtained from sediment traps. The majority (31 out of 50) of the published cyanobacterial sedDNA papers are based on amplicon sequencing (metabarcoding; Fig. 5.3) and most studies (90%) target a region of the 16S rRNA gene to reconstruct past community diversity or quantify cyanobacterial abundance. Finally, most studies span the last ~100 to ~200 years, i.e., a period of enhanced anthropogenic impacts, but overall cyanobacterial sedDNA investigations extend from recent times (i.e., surface sediments) to 10,000 years BP (Von Eggers et al. 2022).
Global map showing the sites where cyanobacteria have been targeted using sedimentary DNA approaches in lakes (n = 71 unique sites). Sampling sites are color-coded by sample type: sediment cores (i.e., time series data) in red, surface sediments only in green, and sediment trap samples in blue. The data were compiled from the dataset published by Von Eggers et al. (2022). Theses and unpublished works were not included in this map
Barplots showing temporal changes (shown by decades) in cyanobacterial community composition based on relative abundances of molecular taxonomic units (Operational taxonomic units (OTUs) or amplicon sequence variants (ASVs)) in various lakes. Data were collected from published literature presenting metabarcoding datasets (Monchamp et al. 2018; Pilon et al. 2019; Nwosu et al. 2021; Huo et al. 2022b). Sampling sites and reference sequence databases used for taxonomic assignment are shown on the right side of the barplots (Greengenes, SILVA, or RDP Classifier)
One of the earliest studies to examine microbial sedDNA was that of Coolen and Overmann (1998) who recovered traces of DNA from purple sulfur bacteria in ~9000-year-old sediments of a Canadian meromictic lake. Subsequently, Rinta-Kanto et al. (2009) analyzed sedDNA specifically for cyanobacteria in surficial sediments collected in 2004 and 2009 in Lake Erie along with an archived surficial sediment sample (3 top centimeters collected with a Shipek grab sampler) from the 1970s. The authors successfully amplified fragments of the mcyA gene involved in the synthesis of microcystins, which were identified as produced by Microcystis spp. in both recent sediments and those retrieved in the 1970s. Savichtcheva et al. (2011) also applied quantitative PCR (qPCR) assays to study the dynamics of cyanobacterial communities and potentially toxic Planktothrix populations over a period of ca. 50 to 100 years from sediment cores collected in peri-alpine lakes. Further, Domaizon et al. (2013) analyzed ~100-years of temporal changes in Synechococcus populations in Lake Bourget (France) and reported changes in cyanobacterial composition through time as a response to climate warming and changes in lake trophic status. More studies quantifying gene copy numbers in sediments through time for either total cyanobacteria, specific taxa and/or targeted ecological functions have followed. For example, Savichtcheva et al. (2015) investigated cyanobacterial composition using qPCR over a period of ~60 years and discovered that phosphorus was the dominant driver of the relative abundance of Planktothrix rubescens, which was favored under mesotrophic conditions. The authors further reported that, despite fluctuating environmental conditions, the same Planktothrix species remained present in the lake since the 1920s. In another quantitative study, Pal et al. (2015) compared qPCR and sedimentary pigment data sets downcore in five temperate lakes located within and outside a protected area (Quebec, Canada) and found that the number of cyanobacteria 16S rRNA gene copies (in parallel with cyanobacterial pigments) had significantly increased since the 1980s across the lakes in comparison to the past ~150 years most likely in response to regional climate change and land development. More recently, a digital droplet (dd)PCR approach has been applied to quantify specific gene abundances in sediment samples as the technique has several advantages over qPCR in quantifying low DNA template (Mejbel et al. 2021). Overall, there was coherence in ddPCR and amplicon sequencing approaches, but the authors noted that research aimed at quantifying absolute abundances from complex environments are recommended using ddPCR, due to the higher tolerance of this method to PCR inhibitors when compared to HTS.
Alongside qPCR and ddPCR approaches targeting specific genes, sedDNA-based studies analyzing whole assemblages at the community level started to emerge, often combined with traditional paleo-proxies (e.g., pigments) to complement the sedDNA signal. For example, Fernandez-Carazo et al. (2013) combined denaturing gel gradient electrophoresis (DGGE) followed by cloning and sequencing, and carotenoid and chlorophyll analysis to report changes in cyanobacterial community structure in two maritime Antarctic lakes during the late Holocene. A few years later, Monchamp et al. (2016) applied for the first time sedDNA metabarcoding to reconstruct cyanobacterial communities in two peri-Alpine lakes over a period of ~60 years spanning pre- and post-eutrophication periods. The authors reported coherent compositional and diversity (richness) patterns from cyanobacterial communities sampled in sedDNA archives and in water samples (i.e., microscopy counts of cyanobacterial cells) collected as part of a long-term monitoring program.
Based on sedDNA community composition analyses of lakes published to date, community structure has shifted over the past ~50–100 years either towards dominance of Nostocales or towards greater dominance of Chroococcales (at the expense of Synechococcales, Fig. 5.3). Both these orders contain bloom-forming and toxigenic taxa. Direct comparisons of suborder changes are more difficult, given the use of different bioinformatics tools and reference sequence databases. Suborder and species level changes are also likely more context dependent on specific limnological characteristics of lakes.
Overall, paleolimnological multi-proxy studies published to date analyzing pigments, biomarkers, and hard subfossils data in addition to cyanobacteria sedDNA have collectively helped: (1) evaluate the congruence between sedDNA datasets and other proxies and (2) evaluate the past impact of environmental change on cyanobacteria (e.g., Pal et al. 2015; Tse et al. 2018; Pilon et al. 2019; Monchamp et al. 2021; Nwosu et al. 2021b; Erratt et al. 2022; Picard et al. 2022b; Nwosu et al. 2023). Such research continues to be relevant as the field of sedDNA is in its infancy and there is a need to evaluate methods and tools across a range of lake types (e.g., from shallow to deep, from acidic to alkaline) and different climatic conditions to accurately interpret sedDNA results as a proxy of past environmental and biotic changes.
An Expanded Representation of Phylum Cyanobacteria: Contribution of sedDNA Analyses
The application of HTS technologies to sequence the genomes of uncultured bacteria collected in natural environments has also led to the discovery of a previously unknown group of ancestral cyanobacteria (Di Rienzi et al. 2013; Soo et al. 2014; Soo et al. 2015). These clades are currently comprised of three described classes (determined on the basis of 16S rRNA gene analyses): Oxyphotobacteria (typically called Cyanobacteria), Melainabacteria, and the basal clade ML635J-21 for which the name Sericytochromatia has been proposed (Soo et al. 2017).
Taxa from the Melainabacteria and Sericytochromatia clades have been identified in a wide variety of anoxic and oxic environments, including subsurface groundwater (Di Rienzi et al. 2013), algal biofilms (Soo et al. 2017), animal and human intestinal tracts, and feces/animal guts (Ley et al. 2005; Di Rienzi et al. 2013; Soo et al. 2014). Several recent observations of their presence in freshwater lakes based on sedDNA and water eDNA analyses have been reported (Salmaso et al. 2018; Monchamp et al. 2019; Ibrahim et al. 2021; Nwosu et al. 2021b). Members of these clades have a range of metabolic pathways different from the Oxyphotobacteria (e.g., anoxic respiration, fermentation). However, the factors controlling their physiology, ecology, and distribution in lakes and other natural settings have yet to be explored.
The Use of sedDNA to Track Anthropogenic-Induced Change: Impact of climate and Pollution on Cyanobacteria Abundance, Community Structure and Taxonomic Composition
Growing evidence over the last few decades is confirming the impacts of climate warming and pollution by eutrophication on lake biota (Barouillet et al. 2023). As such, determining cyanobacteria dominance in freshwaters in relation to human activities is a topic that has gained a lot of interest. DNA reconstructions from lacustrine sediments have provided new insights on the timing and specific causes of environmental change, with great potential to help set restoration and management goals. From investigations of individual lakes and lake districts, climate warming, in addition to eutrophication and hydrological modifications, has likely increased the overall abundance of cyanobacteria and of specific taxa based on quantitative sedDNA analyses over the past several hundred years (Savichtcheva et al. 2011, 2015; Pal et al. 2015; Pilon et al. 2019; Yan et al. 2019; Erratt et al. 2022). Importantly, deconvoluting the effect of climate change and eutrophication is often challenging since the two drivers coincide temporally in many regions that have experienced population and land use changes (De Senerpont Domis et al. 2013; Rigosi et al. 2015). In some cases the absence of significant change in independent proxies of nutrient enrichment can point to the importance of climate change alone (Pilon et al. 2019).
One trend that has been observed worldwide is the regional homogenisation of biological communities (e.g., Rahel 2002; Dornelas et al. 2014; Magurran et al. 2015), that is the increased taxonomic similarity between communities in a given region. In freshwaters, this phenomenon is most commonly caused by alterations to the watershed as well as changes in climate and lake productivity (Petsch 2016). Studies based on sedDNA have pointed to the role of climate warming and anthropogenically-induced eutrophication as drivers of biotic homogenisation in various biological groups in freshwaters, including cyanobacteria and other photosynthetic phytoplankton (Monchamp et al. 2018; Cao et al. 2020; Zhang et al. 2021; Huo et al. 2022b), as well as heterotrophic protists (Keck et al. 2020) and ciliates (Barouillet et al. 2022). Monchamp et al. (2018) first reported cyanobacterial homogenization in 10 peri-Alpine lakes since the ~1980s due to the influence of climate warming (affecting the strength and duration of water column stratification) combined with nitrogen and phosphorus enrichment that allow cyanobacterial growth. Huo et al. (2022b) has also reported the recent homogenisation of cyanobacterial communities in north temperate lakes and reservoirs attributed mainly to enhanced nutrient pollution and warming. These findings are important because biotic homogenisation of cyanobacteria tends to be associated with the dominance of colonial and bloom-forming taxa which also include toxigenic taxa. Both observational (Salmaso et al. 2015) and sedDNA datasets (Monchamp et al. 2018) suggest the recent colonization of several large temperate lakes in northern Italy by the potentially toxic Dolichospermum lemmermannii, contributing to biotic homogenisation.
The Use of sedDNA in Evaluating the Temporal Dynamics of Potentially Toxic Cyanobacteria
Cyanotoxins are synthesized via the activation of a cluster of genes encoding the non-ribosomal peptide and polyketide synthetases (Kultschar et al. 2018). This suite of genes can be detected in sedDNA archives to inform on the temporal dynamics of potentially toxic cyanobacterial taxa in the past (Henao et al. 2019). However, detecting their presence is not sufficient to confirm the active synthesis of cyanotoxins, but rather informs on the potential toxicity of cyanobacterial species. The ability to detect and quantify these functional genes has high relevance for research aimed at identifying the timing and causal factors behind toxic cyanobacteria blooms and for the purposes of lake management (Pilon et al. 2019). Rinta-Kanto et al. (2009) developed mcyD primers allowing for quantification of microcystin synthetase gene via qPCR and applied these to surficial sediment in Lake Erie. Additional primers for microcystins and other cyanotoxins (saxitoxins, anatoxins) have since been developed and used to detect and quantify toxin genes in lake sediments. For example, Martínez de la Escalera et al. (2014) reported a compositional shift in cyanobacterial communities in a freshwater lagune (Laguna Blanca, Uruguay) between the nineteenth and the twenty-first centuries. The appearance of potentially toxic cyanobacteria was attributed to changes in trophic status. Quantitative PCR of the sxtU gene (saxitoxin synthesis) further revealed the presence of saxitoxin-producing Raphidiopsis (previously Cylindrospermopsis) raciborskii (Wołoszyńska) in recent sediment samples (e.g., from 2000).
Legrand et al. (2017) investigated the distribution of Dolichospermum macrosporum and Dolichospermum flos-aquae in sediment cores of the French Lake Aydat over a period of ~220 years by microscopy counts of akinetes PCR detection of 16S rRNA gene and both anatoxin-a (anaC) and microcystin (mcyA) biosynthetic genes. While mcyA was detected throughout the core, anaC was only detected in the most recent sediments (since the 1980s). Based on these observations and the distribution of akinetes of the two Dolichospermum species downcore, the authors concluded that the presence of anaC was most likely linked to the dominance of D. macrosporum in Lake Aydat since the mid-1980s related to anthropogenic activities.
Quantitative PCR applied to sedDNA has shown contrasting histories of toxic cyanobacteria in two temperate lakes of central Canada (Pilon et al. 2019). In Lake of the Woods (straddling the USA and Canada), Microcystis (genus specific 16S rRNA) and microcystin genes (mycD) were both detected in the samples dated to the 1980s, with climate change rather than eutrophication likely responsible for the recent rise in copy numbers of both genes. In contrast, in naturally eutrophic Lake Baptiste (Alberta, Canada) both these gene markers were found throughout the entire core and were present well before European settlement. In both lakes, the trends in sedDNA gene copies and microcystin concentrations measured in sediments were correlated, although in Lake of the Woods the detection of toxic genes preceded the detection of microcystins by ~20 years, because of the greater sensitivity of DNA techniques compared to the analytical chemical methods for microcystins. In another quantitative study tracing back both photosynthetic pigments and anatoxin-a genes in sedimentary archives, Hobbs et al. (2021) found that a change in nutrient cycling due to farming history and possibly fish stocking in Anderson Lake (Washington State, USA) caused a shift in the bacterial community and led to the dominance of anatoxin-a producing Dolichospermum sp.
Some species, such as the tropical and potentially toxic filamentous Raphidiopsis raciborskii (Wołoszyńska), are thought to have spread to several regions including northern latitudes through the 1980’s and 1990’s (Neilan et al. 2003; Hamilton et al. 2005). Dolichospermum lemmermannii expanding to the Italian peri-Alpine lakes is another example (Salmaso et al. 2015). The invasion histories of toxigenic cyanobacterial taxa remain generally unknown, but paleolimnological work based on sedDNA analyses could help elucidate the timing of invasion and the phylogeography of cyanobacteria of interest both from an ecological and a lake management perspective.
Methodological Considerations
DNA-based paleo-reconstructions remain limited by a number of methodological considerations (Capo et al. 2021). These include the low amount of starting DNA material that can be retrieved from ancient sediments, the limited knowledge of deposition and taphonomic processes, and the bioinformatics treatment of sedDNA sequence data (e.g., raw sequence preprocessing, contaminant removal, taxonomic assignment). Therefore, adhering to strict ancient DNA laboratory protocols to reduce contamination and the use of blanks (negative controls) at every step of the workflow are especially important in order to ascertain the quality of sedDNA datasets (Chap. 3 of this volume). Further, the choice of analytical methods and pipelines have an incidence on the type of ecological inferences that can be made based on these data. For example, PCR-based approaches (including amplicon sequencing and quantitative PCR methods) efficiently enhance the signal of the target organisms, but they introduce abundance biases due to the exponential nature of the PCR amplification. Furthermore, amplicon sequencing does not inform as to the patterns of DNA damage (i.e., size fractionation of DNA molecules and deamination patterns), which can be used to authenticate ancient DNA molecules (Dabney et al. 2013). Copy number variation within genes used to analyzed cyanobacterial community composition is another source of error as it can skew the abundance estimates in favor of taxa that have higher numbers of copies of these genes: presently the number of gene copies has not been determined for all taxa and a major effort of sequencing cyanobacteria whole genomes is required here. Finally, a limitation that remains is related to primer design. Indeed, designing truly cyanobacteria specific (or genus-specific) 16S primers is difficult, and consequently a high number of metabarcoding amplicons belong to other (non-target) bacterial taxa, which translates to a skewed representation of cyanobacteria in sequencing assays.
Despite some limitations, sedDNA metabarcoding has proven to be a valuable approach for reconstructing the taxonomic diversity of lake cyanobacteria and relating changes in community composition and relative abundance with anthropogenic stressors in the watershed of lakes (e.g., water-level fluctuations due to damming, nutrient pollution, climate warming) (Fig. 5.3). However, due to the lack of standardized protocols, databases, and the different molecular units used (e.g., operational taxonomic units; OTUs vs. amplicon sequence variants; ASVs), metabarcoding datasets generated by different research groups can be difficult to compare in a meaningful way.
The current paucity of reference DNA sequence databases (for cyanobacteria as well as for most biological groups, especially microbes) is another major limitation of cyanobacterial DNA studies, both in modern and paleo-environments. In a recent gap-analysis study, it was shown that approximately 50% of the cyanobacterial species identified by light microscopy were not represented in the reference databases (Salmaso et al. 2022). Finally, inconsistencies and mis-identification of cyanobacterial taxa in existing 16S rRNA reference databases have been identified which have led to the development of a curated database for cyanobacteria (Ramos et al. 2017). Cyanobacteria taxonomy is under revision with new molecular data changing nomenclature and phylogenetic assignments.
Future Directions
Similar to the study of genes responsible for the synthesis of cyanotoxins, other functional genes could be examined to monitor cyanobacteria and their specific functioning. One example is pertinent to the proliferation of benthic cyanobacteria, which are problematic because they produce taste and odor compounds as well as toxins. Gaget et al. (2020) developed qPCR assays to monitor taste and odor genes (geoA, MIB synthetase genes) in benthic biofilms. Paleo-reconstructions aimed at monitoring changes in the abundance of functional genes regulating secondary metabolites in cyanobacteria (such as toxins, taste and odor compounds) have important implications for lake and reservoir management. Other targets could also be relevant to explore, such as functional genes associated with gas vesicle formation, akinete formation, nitrogen fixation and nutrient storage unique to cyanobacteria.
The field of paleogenomics has begun exploring the possibility of recovering the RNA signal in sedimentary archives to study the taxonomic composition and function of microorganisms. The analysis of RNA can also enable the separation and comparison of total vs. “active” bacteria (Pawlowski et al. 2022), even in ancient sediments (Pearman et al. 2022). Although it is assumed that RNA degrades more quickly in the environment compared to DNA, RNA molecules can apparently preserve over long time periods in aquatic sedimentary archives and can be used to study function in past biological communities through genetic and metagenomic analyses (Lindqvist and Rajora 2019). Even more care is required in the analysis of RNA in sediments compared to sedDNA to minimize signal contamination and degradation.
A developing trend in paleo-molecular studies is to use shotgun sequencing (metagenomics) which allows retrieval of the DNA signal of all organisms in a sample in proportion to their original presence. This approach allows for the reconstruction of the entire pool of lake biota and thus to potentially explore relationships between different organisms across trophic levels (see Chap. 12 of this volume). Paleogenomics coupled with DNA enrichment or hybridization capture techniques have also proven efficient to recover planktonic algae and to track ancient DNA damage. Recently, hybridization capture was used on sediment seafloor to target both phytoplankton (including cyanobacteria) and zooplankton, demonstrating the potential of this approach to reconstruct the composition and relative abundance of various planktonic forms (Armbrecht et al. 2021). Recent work has also highlighted the potential of paleogenomic for reconstructing whole microbial time-series diversity from lake sediments metagenomes (Garner et al. 2020) and the diversity of an Ice Age algal population based on mitochondrial genomes reconstructed from lake sediments (Lammers et al. 2021). Collectively, these approaches demonstrate the power of sedDNA for paleogenomics once the potential of sediment nucleic acid analyses is fully unlocked.
Summary
The number of molecular genetic explorations of cyanobacterial past trajectories in lacustrine sediments continues to increase. The main applications of cyanobacterial sedDNA studies so far include evaluating the impact of human activities and eutrophication on community composition and dominance, determining the presence and abundance of toxin biosynthesis genes over time, and evaluating sedimentation and taphonomy processes to better interpret the cyanobacterial DNA signal in lake sedimentary archives. This new knowledge complements other findings from classical paleo-indicators, while also contributing to improving our understanding of the causes of environmental and ecological change in lakes. Additional studies comparing sedDNA-based proxies with other proxies (fossil organisms, biogeochemical markers) or modern surveys are still much needed to validate sedDNA findings in a variety of lacustrine settings and in response to environmental stressors (e.g., eutrophication, climate change, invasive species, changes in hydrology (water residence time or water levels), salinization, acidification, overfishing). In addition to the question of biotic homogenization of communities and environmental selection, sedDNA approaches can address questions of evolutionary change as a result of human activities, as well as historical biogeography distribution.
References
Armbrecht L, Hallegraeff G, Bolch CJS, Woodward C, Cooper A (2021) Hybridisation capture allows DNA damage analysis of ancient marine eukaryotes. Sci Rep 11:3220. https://doi.org/10.1038/s41598-021-82578-6
Barnes MA, Turner CR, Jerde CL, Renshaw MA, Chadderton WL, Lodge DM (2014) Environmental conditions influence eDNA persistence in aquatic systems. Environ Sci Technol 48(3):1819–1827. https://doi.org/10.1021/es404734p
Barouillet C, Monchamp M-E, Bertilsson S, Brasell K, Domaizon I, Epp LS, Ibrahim A, Mejbel H, Nwosu EC, Pearman JK, Picard M, Thomson-Laing G, Tsugeki N, Von Eggers J, Gregory-Eaves I, Pick FR, Wood SA, Capo E (2023) Investigating the effects of anthropogenic stressors on lake biota using sedimentary DNA. Freshwater biology n/a(n/a). https://doi.org/10.1111/fwb.14027
Barouillet C, Vasselon V, Keck F, Millet L, Etienne D, Galop D, Rius D, Domaizon I (2022) Paleoreconstructions of ciliate communities reveal long-term ecological changes in temperate lakes. Sci Rep 12(1):7899. https://doi.org/10.1038/s41598-022-12041-7
Bonilla S, Pick FR (2017) Freshwater bloom-forming cyanobacteria and anthropogenic change. Limnology and Oceanography e-Lectures 7(2):1–62. https://doi.org/10.1002/loe2.10006
Brasell KA, Pochon X, Howarth J, Pearman JK, Zaiko A, Thompson L, Vandergoes MJ, Simon KS, Wood SA (2022) Shifts in DNA yield and biological community composition in stored sediment: implications for paleogenomic studies. Metabarcoding and Metagenomics 6:e78128. https://doi.org/10.3897/mbmg.6.78128
Brunberg A-K, Blomqvist P (2002) Benthic overwintering of Microcystis colonies under different environmental conditions. J Plankton Res 24(11):1247–1252. https://doi.org/10.1093/plankt/24.11.1247
Callieri C, Coci M, Corno G, Macek M, Modenutti B, Balseiro E, Bertoni R (2013) Phylogenetic diversity of nonmarine picocyanobacteria. FEMS Microbiol Ecol 85(2):293–301. https://doi.org/10.1111/1574-6941.12118
Cao X, Xu X, Bian R, Wang Y, Yu H, Xu Y, Duan G, Bi L, Chen P, Gao S, Wang J, Peng J, Qu J (2020) Sedimentary ancient DNA metabarcoding delineates the contrastingly temporal change of lake cyanobacterial communities. Water Res 183:116077. https://doi.org/10.1016/j.watres.2020.116077
Capo E, Giguet-Covex C, Rouillard A, Nota K, Heintzman PD, Vuillemin A, Ariztegui D, Arnaud F, Belle S, Bertilsson S, Bigler C, Bindler R, Brown AG, Clarke CL, Crump SE, Debroas D, Englund G, Ficetola GF, Garner RE, Gauthier J, Gregory-Eaves I, Heinecke L, Herzschuh U, Ibrahim A, Kisand V, Kjær KH, Lammers Y, Littlefair J, Messager E, Monchamp M-E, Olajos F, Orsi W, Pedersen MW, Rijal DP, Rydberg J, Spanbauer T, Stoof-Leichsenring KR, Taberlet P, Talas L, Thomas C, Walsh DA, Wang Y, Willerslev E, van Woerkom A, Zimmermann HH, Coolen MJL, Epp LS, Domaizon I, G. Alsos I, Parducci L (2021) Lake sedimentary DNA research on past terrestrial and aquatic biodiversity: overview and recommendations. Quaternary 4(1):6. https://doi.org/10.3390/quat4010006
Capo E, Monchamp M-E, Coolen MJL, Domaizon I, Armbrecht L, Bertilsson S (2022) Environmental paleomicrobiology: using DNA preserved in aquatic sediments to its full potential. Environ Microbiol 24(5):2201–2209. https://doi.org/10.1111/1462-2920.15913
Carey CC, Ibelings BW, Hoffmann EP, Hamilton DP, Brookes JD (2012) Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res 46(5):1394–1407. https://doi.org/10.1016/j.watres.2011.12.016
Carmichael WW (1994) The toxins of cyanobacteria. Sci Am 270(1):78–86. https://doi.org/10.1038/scientificamerican0194-78
Chorus I, Batram J (1999) Toxic cyanobacteria in water - a guide to their public health consequences, monitoring and management
Chorus I, Fastner J, Welker M (2021) Cyanobacteria and cyanotoxins in a changing environment: concepts, controversies. Challenges Water 13(18):2463. https://doi.org/10.3390/w13182463
Chorus I, Welker M (eds) (2021) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management, 2nd edn. CRC Press, London
Coolen MJL, Overmann J (1998) Analysis of subfossil molecular remains of purple Sulfur bacteria in a Lake sediment. Appl Environ Microbiol 64(11):4513–4521. https://doi.org/10.1128/AEM.64.11.4513-4521.1998
Crump SE (2021) Sedimentary ancient DNA as a tool in paleoecology. Nat Rev Earth Environ 2(4):229–229. https://doi.org/10.1038/s43017-021-00158-8
Dabney J, Meyer M, Pääbo S (2013) Ancient DNA damage. Cold Spring Harb Perspect Biol 5(7):a012567. https://doi.org/10.1101/cshperspect.a012567
De Senerpont Domis LN, Elser JJ, Gsell AS, Huszar VLM, Ibelings BW, Jeppesen E, Kosten S, Mooij WM, Roland F, Sommer U, Van Donk E, Winder M, Lürling M (2013) Plankton dynamics under different climatic conditions in space and time. Freshw Biol 58(3):463–482. https://doi.org/10.1111/fwb.12053
Di Rienzi SC, Sharon I, Wrighton KC, Koren O, Hug LA, Thomas BC, Goodrich JK, Bell JT, Spector TD, Banfield JF, Ley RE (2013) The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to cyanobacteria. eLife 2:e01102. https://doi.org/10.7554/eLife.01102
Domaizon I, Savichtcheva O, Debroas D, Arnaud F, Villar C, Pignol C, Alric B, Perga ME (2013) DNA from lake sediments reveals the long-term dynamics and diversity of Synechococcus assemblages. Biogeosciences 10(6):3817–3838. https://doi.org/10.5194/bg-10-3817-2013
Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE (2014) Assemblage time series reveal biodiversity change but not systematic loss. Science 344(6181):296–299. https://doi.org/10.1126/science.1248484
Erratt K, Creed IF, Favot EJ, Todoran I, Tai V, Smol JP, Trick CG (2022) Paleolimnological evidence reveals climate-related preeminence of cyanobacteria in a temperate meromictic lake. https://doi.org/10.1139/cjfas-2021-0095
Fernandez-Carazo R, Verleyen E, Hodgson DA, Roberts SJ, Waleron K, Vyverman W, Wilmotte A (2013) Late Holocene changes in cyanobacterial community structure in maritime Antarctic lakes. J Paleolimnol 50(1):15–31. https://doi.org/10.1007/s10933-013-9700-3
Gaget V, Hobson P, Keulen A, Newton K, Monis P, Humpage AR, Weyrich LS, Brookes JD (2020) Toolbox for the sampling and monitoring of benthic cyanobacteria. Water Res 169:115222. https://doi.org/10.1016/j.watres.2019.115222
Garner RE, Gregory-Eaves I, Walsh DA (2020) Sediment metagenomes as time capsules of Lake microbiomes. mSphere 5(6):e00512–e00520. https://doi.org/10.1128/mSphere.00512-20
Gauthier J, Walsh D, Selbie DT, Bourgeois A, Griffiths K, Domaizon I, Gregory-Eaves I (2021) Evaluating the congruence between DNA-based and morphological taxonomic approaches in water and sediment trap samples: analyses of a 36-month time series from a temperate monomictic lake. Limnol Oceanogr 66(8):3020–3039. https://doi.org/10.1002/lno.11856
Guiry MD (2012) How many species of algae are there? J Phycol 48(5):1057–1063. https://doi.org/10.1111/j.1529-8817.2012.01222.x
Hamilton PB, Ley LM, Dean S, Pick FR (2005) The occurrence of the cyanobacterium Cylindrospermopsis raciborskii in Constance Lake: an exotic cyanoprokaryote new to Canada. Phycologia 44(1):17–25. https://doi.org/10.2216/0031-8884(2005)44[17:TOOTCC]2.0.CO;2
Hauer T, Komárek J (2022) CyanoDB 2.0 - on-line database of cyanobacterial genera. World-Wide Electron Publ
Henao E, Rzymski P, Waters MN (2019) A review on the study of cyanotoxins in Paleolimnological research: current knowledge and future needs. Toxins (Basel) 12(1):6. https://doi.org/10.3390/toxins12010006
Ho JC, Michalak AM, Pahlevan N (2019) Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 574(7780):667–670. https://doi.org/10.1038/s41586-019-1648-7
Hobbs WO, Dreher TW, Davis EW, Vinebrooke RD, Wong S, Weissman T, Dawson M (2021) Using a lake sediment record to infer the long-term history of cyanobacteria and the recent rise of an anatoxin producing Dolichospermum sp. Harmful Algae 101:101971. https://doi.org/10.1016/j.hal.2020.101971
Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JMH, Visser PM (2018) Cyanobacterial blooms. Nat Rev Microbiol 16(8):471–483. https://doi.org/10.1038/s41579-018-0040-1
Huo S, Zhang H, Wang J, Chen J, Wu F (2022a) Temperature and precipitation dominates millennium changes of eukaryotic algal communities in Lake Yamzhog Yumco, southern Tibetan plateau. Sci Total Environ 829:154636. https://doi.org/10.1016/j.scitotenv.2022.154636
Huo S, Zhang H, Monchamp M-E, Wang R, Weng N, Zhang J, Zhang H, Wu F (2022b) Century-long homogenization of algal communities is accelerated by nutrient enrichment and climate warming in lakes and reservoirs of the north temperate zone. Environ Sci Technol 56(6):3780–3790. https://doi.org/10.1021/acs.est.1c06958
Ibrahim A, Capo E, Wessels M, Martin I, Meyer A, Schleheck D, Epp LS (2021) Anthropogenic impact on the historical phytoplankton community of Lake Constance reconstructed by multimarker analysis of sediment-core environmental DNA. Mol Ecol 30(13):3040–3056. https://doi.org/10.1111/mec.15696
Kakouei K, Kraemer BM, Anneville O, Carvalho L, Feuchtmayr H, Graham JL, Higgins S, Pomati F, Rudstam LG, Stockwell JD, Thackeray SJ, Vanni MJ, Adrian R (2021) Phytoplankton and cyanobacteria abundances in mid-21st century lakes depend strongly on future land use and climate projections. Glob Chang Biol 27(24):6409–6422. https://doi.org/10.1111/gcb.15866
Keck F, Millet L, Debroas D, Etienne D, Galop D, Rius D, Domaizon I (2020) Assessing the response of micro-eukaryotic diversity to the great acceleration using lake sedimentary DNA. Nat Commun 11(1):3831. https://doi.org/10.1038/s41467-020-17682-8
Komárek J (2016a) A polyphasic approach for the taxonomy of cyanobacteria: principles and applications. Eur J Phycol 51(3):346–353. https://doi.org/10.1080/09670262.2016.1163738
Komárek J (2016b) Review of the cyanobacterial genera implying planktic species after recent taxonomic revisions according to polyphasic methods: state as of 2014. Hydrobiologia 764(1):259–270. https://doi.org/10.1007/s10750-015-2242-0
Kultschar B, Llewellyn C, Kultschar B, Llewellyn C (2018) Secondary metabolites in cyanobacteria. Secondary Metabolites - Sources and Applications. IntechOpen
Lammers Y, Heintzman PD, Alsos IG (2021) Environmental palaeogenomic reconstruction of an ice age algal population. Commun Biol 4(1):1–11. https://doi.org/10.1038/s42003-021-01710-4
Leavitt P, Hodgson D (2006) Sedimentary pigments. Dev. Paleoenviron. Res, In, pp 295–325
Legrand B, Lamarque A, Sabart M, Latour D (2017) Benthic archives reveal recurrence and dominance of toxigenic cyanobacteria in a eutrophic Lake over the last 220 years. Toxins (Basel) 9(9):271. https://doi.org/10.3390/toxins9090271
Legrand B, Miras Y, Beauger A, Dussauze M, Latour D (2019) Akinetes and ancient DNA reveal toxic cyanobacterial recurrences and their potential for resurrection in a 6700-year-old core from a eutrophic lake. Sci Total Environ 687:1369–1380. https://doi.org/10.1016/j.scitotenv.2019.07.100
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci 102(31):11070–11075. https://doi.org/10.1073/pnas.0504978102
Lindqvist C, Rajora OP (2019) Paleogenomics. Springer, Cham, Switzerland
Livingstone D, Jaworski GHM (1980) The viability of akinetes of blue-green algae recovered from the sediments of Rostherne mere. Br Phycol J 15(4):357–364. https://doi.org/10.1080/00071618000650361
Magurran AE, Dornelas M, Moyes F, Gotelli NJ, McGill B (2015) Rapid biotic homogenization of marine fish assemblages. Nat Commun 6(1):8405. https://doi.org/10.1038/ncomms9405
Martínez de la Escalera G, Antoniades D, Bonilla S, Piccini C (2014) Application of ancient DNA to the reconstruction of past microbial assemblages and for the detection of toxic cyanobacteria in subtropical freshwater ecosystems. Mol Ecol 23(23):5791–5802. https://doi.org/10.1111/mec.12979
Mejbel HS, Dodsworth W, Baud A, Gregory-Eaves I, Pick FR (2021) Comparing quantitative methods for Analyzing sediment DNA Records of Cyanobacteria in experimental and Reference Lakes. Front Microbiol 12
Mejbel HS, Dodsworth W, Pick FR (2022) Effects of temperature and oxygen on cyanobacterial DNA preservation in sediments: a comparison study of major taxa. Environ DNA 4(4):717–731. https://doi.org/10.1002/edn3.289
Monchamp M-È, Bruel R, Frossard V, McGowan S, Lavrieux M, Muschick M, Perga M-É, Dubois N (2021) Paleoecological evidence for a multi-trophic regime shift in a perialpine Lake (lake Joux, Switzerland). Anthropocene 35(100301). https://doi.org/10.1016/j.ancene.2021.100301
Monchamp M-E, Spaak P, Domaizon I, Dubois N, Bouffard D, Pomati F (2018) Homogenization of lake cyanobacterial communities over a century of climate change and eutrophication. Nat Ecol Evol 2(2):317–324. https://doi.org/10.1038/s41559-017-0407-0
Monchamp M-E, Spaak P, Pomati F (2019) Long term diversity and distribution of non-photosynthetic cyanobacteria in Peri-Alpine Lakes. Front Microbiol 9
Monchamp M-E, Walser J-C, Pomati F, Spaak P (2016) Sedimentary DNA reveals cyanobacterial community diversity over 200 years in two Perialpine Lakes. Appl Environ Microbiol 82(21):6472–6482. https://doi.org/10.1128/AEM.02174-16
Moss B, Kosten S, Meerhoff M, Battarbee RW, Jeppesen E, Mazzeo N, Havens K, Lacerot G, Liu Z, De Meester L, Paerl H, Scheffer M (2011) Allied attack: climate change and eutrophication. Inland Waters 1(2):101–105. https://doi.org/10.5268/IW-1.2.359
Nabout JC, da Silva Rocha B, Carneiro FM, Sant’Anna CL (2013) How many species of cyanobacteria are there? Using a discovery curve to predict the species number. Biodivers Conserv 22(12):2907–2918. https://doi.org/10.1007/s10531-013-0561-x
Neilan BA, Saker ML, Fastner J, Törökné A, Burns BP (2003) Phylogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Mol Ecol 12(1):133–140. https://doi.org/10.1046/j.1365-294x.2003.01709.x
Nwosu EC, Brauer A, Monchamp M-E, Pinkerneil S, Bartholomäus A, Theuerkauf M, Schmidt J-P, Stoof-Leichsenring KR, Wietelmann T, Kaiser J, Wagner D, Liebner S (2023) Early human impact on lake cyanobacteria revealed by a Holocene record of sedimentary ancient DNA. Commun Biol 6(1):1–12. https://doi.org/10.1038/s42003-023-04430-z
Nwosu EC, Roeser P, Yang S, Ganzert L, Dellwig O, Pinkerneil S, Brauer A, Dittmann E, Wagner D, Liebner S (2021a) From water into sediment—tracing freshwater cyanobacteria via DNA analyses. Microorganisms 9(8):1778. https://doi.org/10.3390/microorganisms9081778
Nwosu EC, Brauer A, Kaiser J, Horn F, Wagner D, Liebner S (2021b) Evaluating sedimentary DNA for tracing changes in cyanobacteria dynamics from sediments spanning the last 350 years of Lake Tiefer see. NE Germany J Paleolimnol 66(3):279–296. https://doi.org/10.1007/s10933-021-00206-9
Pal S, Gregory-Eaves I, Pick FR (2015) Temporal trends in cyanobacteria revealed through DNA and pigment analyses of temperate lake sediment cores. J Paleolimnol 54(1):87–101. https://doi.org/10.1007/s10933-015-9839-1
Pawlowski J, Bruce K, Panksep K, Aguirre FI, Amalfitano S, Apothéloz-Perret-Gentil L, Baussant T, Bouchez A, Carugati L, Cermakova K, Cordier T, Corinaldesi C, Costa FO, Danovaro R, Dell’Anno A, Duarte S, Eisendle U, Ferrari BJD, Frontalini F, Frühe L, Haegerbaeumer A, Kisand V, Krolicka A, Lanzén A, Leese F, Lejzerowicz F, Lyautey E, Maček I, Sagova-Marečková M, Pearman JK, Pochon X, Stoeck T, Vivien R, Weigand A, Fazi S (2022) Environmental DNA metabarcoding for benthic monitoring: a review of sediment sampling and DNA extraction methods. Sci Total Environ 818:151783. https://doi.org/10.1016/j.scitotenv.2021.151783
Pearman JK, Biessy L, Howarth JD, Vandergoes MJ, Rees A, Wood SA (2022) Deciphering the molecular signal from past and alive bacterial communities in aquatic sedimentary archives. Mol Ecol Resour 22(3):877–890. https://doi.org/10.1111/1755-0998.13515
Pedersen MW, Overballe-Petersen S, Ermini L, Sarkissian CD, Haile J, Hellstrom M, Spens J, Thomsen PF, Bohmann K, Cappellini E, Schnell IB, Wales NA, Carøe C, Campos PF, Schmidt AMZ, Gilbert MTP, Hansen AJ, Orlando L, Willerslev E (2015) Ancient and modern environmental DNA. Philosop Transact Royal Society B: Biolog Sci 370(1660):20130383. https://doi.org/10.1098/rstb.2013.0383
Petsch DK (2016) Causes and consequences of biotic homogenization in freshwater ecosystems. Int Rev Hydrobiol 101(3–4):113–122. https://doi.org/10.1002/iroh.201601850
Picard M, Pochon X, Atalah J, Pearman JK, Rees A, Howarth JD, Moy CM, Vandergoes MJ, Hawes I, Khan S, Wood SA (2022a) Using metabarcoding and droplet digital PCR to investigate drivers of historical shifts in cyanobacteria from six contrasting lakes. Sci Rep 12(1):12810. https://doi.org/10.1038/s41598-022-14216-8
Picard M, Wood SA, Pochon X, Vandergoes MJ, Reyes L, Howarth JD, Hawes I, Puddick J (2022b) Molecular and pigment analyses provide comparative results when reconstructing historic cyanobacterial abundances from Lake sediment cores. Microorganisms 10(2):279. https://doi.org/10.3390/microorganisms10020279
Pilon S, Zastepa A, Taranu ZE, Gregory-Eaves I, Racine M, Blais JM, Poulain AJ, Pick FR (2019) Contrasting histories of microcystin-producing cyanobacteria in two temperate lakes as inferred from quantitative sediment DNA analyses. Lake Reservoir Manag 35(1):102–117. https://doi.org/10.1080/10402381.2018.1549625
Rahel FJ (2002) Homogenization of freshwater faunas. Annu Rev Ecol Syst 33(1):291–315. https://doi.org/10.1146/annurev.ecolsys.33.010802.150429
Ramos V, Morais J, Vasconcelos VM (2017) A curated database of cyanobacterial strains relevant for modern taxonomy and phylogenetic studies. Sci Data 4(1):170054. https://doi.org/10.1038/sdata.2017.54
Rigosi A, Hanson P, Hamilton DP, Hipsey M, Rusak JA, Bois J, Sparber K, Chorus I, Watkinson AJ, Qin B, Kim B, Brookes JD (2015) Determining the probability of cyanobacterial blooms: the application of Bayesian networks in multiple lake systems. Ecol Appl 25(1):186–199
Rinta-Kanto JM, Saxton MA, DeBruyn JM, Smith JL, Marvin CH, Krieger KA, Sayler GS, Boyer GL, Wilhelm SW (2009) The diversity and distribution of toxigenic Microcystis spp. in present day and archived pelagic and sediment samples from Lake Erie. Harmful Algae 8(3):385–394. https://doi.org/10.1016/j.hal.2008.08.026
Salmaso N, Albanese D, Capelli C, Boscaini A, Pindo M, Donati C (2018) Diversity and cyclical seasonal transitions in the bacterial Community in a Large and Deep Perialpine Lake. Microb Ecol 76(1):125–143. https://doi.org/10.1007/s00248-017-1120-x
Salmaso N, Capelli C, Shams S, Cerasino L (2015) Expansion of bloom-forming Dolichospermum lemmermannii (Nostocales, cyanobacteria) to the deep lakes south of the Alps: colonization patterns, driving forces and implications for water use. Harmful Algae 50:76–87. https://doi.org/10.1016/j.hal.2015.09.008
Salmaso N, Vasselon V, Rimet F, Vautier M, Elersek T, Boscaini A, Donati C, Moretto M, Pindo M, Riccioni G, Stefani E, Capelli C, Lepori F, Kurmayer R, Mischke U, Klemenčič AK, Novak K, Greco C, Franzini G, Fusato G, Giacomazzi F, Lea A, Menegon S, Zampieri C, Macor A, Virgilio D, Zanut E, Zorza R, Buzzi F, Domaizon I (2022) DNA sequence and taxonomic gap analyses to quantify the coverage of aquatic cyanobacteria and eukaryotic microalgae in reference databases: results of a survey in the alpine region. Sci Total Environ 834:155175. https://doi.org/10.1016/j.scitotenv.2022.155175
Savichtcheva O, Debroas D, Kurmayer R, Villar C, Jenny JP, Arnaud F, Perga ME, Domaizon I (2011) Quantitative PCR enumeration of Total/toxic Planktothrix rubescens and Total cyanobacteria in preserved DNA isolated from Lake sediments. Appl Environ Microbiol 77(24):8744–8753. https://doi.org/10.1128/AEM.06106-11
Savichtcheva O, Debroas D, Perga ME, Arnaud F, Villar C, Lyautey E, Kirkham A, Chardon C, Alric B, Domaizon I (2015) Effects of nutrients and warming on Planktothrix dynamics and diversity: a palaeolimnological view based on sedimentary DNA and RNA. Freshw Biol 60(1):31–49. https://doi.org/10.1111/fwb.12465
Smol JP (2009) Pollution of lakes and Rivers: a paleoenvironmental perspective, 2nd edn. Wiley-Blackwell, Malden, MA
Soo RM, Hemp J, Parks DH, Fischer WW, Hugenholtz P (2017) On the origins of oxygenic photosynthesis and aerobic respiration in cyanobacteria. Science 355(6332):1436–1440. https://doi.org/10.1126/science.aal3794
Soo RM, Skennerton CT, Sekiguchi Y, Imelfort M, Paech SJ, Dennis PG, Steen JA, Parks DH, Tyson GW, Hugenholtz P (2014) An expanded genomic representation of the phylum cyanobacteria. Genome Biol Evol 6(5):1031–1045. https://doi.org/10.1093/gbe/evu073
Soo RM, Woodcroft BJ, Parks DH, Tyson GW, Hugenholtz P (2015) Back from the dead; the curious tale of the predatory cyanobacterium Vampirovibrio chlorellavorus. Peer J 3:e968. https://doi.org/10.7717/peerj.968
Taberlet P, Bonin A, Zinger L, Coissac E, Taberlet P, Bonin A, Zinger L, Coissac E (2018) Environmental DNA: for biodiversity research and monitoring. Oxford University Press, Oxford, New York
Taranu ZE, Gregory-Eaves I, Leavitt PR, Bunting L, Buchaca T, Catalan J, Domaizon I, Guilizzoni P, Lami A, McGowan S, Moorhouse H, Morabito G, Pick FR, Stevenson MA, Thompson PL, Vinebrooke RD (2015) Acceleration of cyanobacterial dominance in north temperate-subarctic lakes during the Anthropocene. Ecol Lett 18(4):375–384. https://doi.org/10.1111/ele.12420
Thorpe AC, Anderson A, Goodall T, Thackeray SJ, Maberly SC, Bendle JA, Gweon HS, Read DS (2022) Sedimentary DNA records long-term changes in a lake bacterial community in response to varying nutrient availability. Environmental DNA 4(6):1340–1355. https://doi.org/10.1002/edn3.344
Tse TJ, Doig LE, Tang S, Zhang X, Sun W, Wiseman SB, Feng CX, Liu H, Giesy JP, Hecker M, Jones PD (2018) Combining high-throughput sequencing of sedaDNA and traditional Paleolimnological techniques to infer historical trends in cyanobacterial communities. Environ Sci Technol 52(12):6842–6853. https://doi.org/10.1021/acs.est.7b06386
Von Eggers J, Monchamp M-E, Capo E, Giguet-Covex C, Spanbauer T, Heintzman PD (2022) Inventory of ancient environmental DNA from sedimentary archives: locations, methods, and target taxa. Zenodo. https://doi.org/10.5281/ZENODO.6847522
Waters MN (2016) A 4700-year history of cyanobacteria toxin production in a shallow subtropical Lake. Ecosystems 19(3):426–436. https://doi.org/10.1007/s10021-015-9943-0
Watson SB, McCauley E, Downing JA (1997) Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol Oceanogr 42(3):487–495. https://doi.org/10.4319/lo.1997.42.3.0487
Weisbrod B, Wood SA, Steiner K, Whyte-Wilding R, Puddick J, Laroche O, Dietrich DR (2020) Is a central sediment sample sufficient? Exploring spatial and temporal microbial diversity in a small Lake. Toxins 12(9):580. https://doi.org/10.3390/toxins12090580
Whitton BA (ed) (2012) Ecology of cyanobacteria II. Springer, Netherlands, Dordrecht
Whitton BA, Potts M (2014) Summary for policymakers. In: Whitton BA, Potts M (eds) Climate change 2013 – the physical science basis. Cambridge University Press, New York, NY, USA, pp 1–30
Yan D, Xu H, Yang M, Lan J, Hou W, Wang F, Zhang J, Zhou K, An Z, Goldsmith Y (2019) Responses of cyanobacteria to climate and human activities at Lake Chenghai over the past 100 years. Ecol Indic 104:755–763. https://doi.org/10.1016/j.ecolind.2019.03.019
Zastepa A, Taranu ZE, Kimpe LE, Blais JM, Gregory-Eaves I, Zurawell RW, Pick FR (2017) Reconstructing a long-term record of microcystins from the analysis of lake sediments. Sci Total Environ 579:893–901. https://doi.org/10.1016/j.scitotenv.2016.10.211
Zhang H, Huo S, Yeager KM, Wu F (2021) Sedimentary DNA record of eukaryotic algal and cyanobacterial communities in a shallow Lake driven by human activities and climate change. Sci Total Environ 753:141985. https://doi.org/10.1016/j.scitotenv.2020.141985
Acknowledgements
We thank E. Nwosu for providing the cyanobacterial OTU table for Lake Tiefer See. We also wish to thank members of the SedaDNA Scientific Society who contributed ancient DNA data from lake sampling sites to the database. We thank Paul MacKeigan for feedback on a previous version of the manuscript. Finally, we extend a special thanks to Drs. Susie Wood, Nico Salmaso, Eric Capo, John Smol and Cécilia Barouillet for their constructive reviews of this chapter. MEM was supported by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC) and FRP through an NSERC Discovery Grant.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Monchamp, ME., Pick, F.R. (2023). Cyanobacterial DNA from Lake Sediments. In: Capo, E., Barouillet, C., Smol, J.P. (eds) Tracking Environmental Change Using Lake Sediments. Developments in Paleoenvironmental Research, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-031-43799-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-43799-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43798-4
Online ISBN: 978-3-031-43799-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)