Abstract
Plastic products are essential parts of modern life as they are used in household items, packaging, electronics, building, automotive and many more. Amongst about 350 Mt of waste plastics produced worldwide, only about 20% are recycled and 80% are landfilled. The landfilled waste plastic has a serious negative impact on the environment which causes land diversity, havoc in marine, etc. Pyrolysis of waste plastics into usable energy products such as syngas, char and liquid oil can alleviate the burden of plastic waste management. While pyrolyzed liquid oil can be converted to plastic diesel through distillation and hydrotreatment processes, and char can be used for agricultural purposes, the pyrolysis syngas can be processed further through different reforming processes to produce H2. H2 is expected to dominate in fuel sector by substituting fossil fuels. In this paper, the experimental findings of waste plastics pyrolysis into oil, char and syngas are reported which shows that waste plastic can be converted to liquid oil by about 80%, remaining are approximately 10% char and 10% syngas. The possibility of H2 production from pyrolysis syngas through different reforming processes such as steam, partial oxidation, autothermal, plasma, aqueous phase, etc. are reviewed and critically analysed in this paper. The literature indicated that 530 Mt of H2 is needed to achieve net zero by 2050 worldwide. It is envisaged that converting all waste plastic into H2 will meet the demand of H2 and support net zero goal by 2050. Amongst different reforming processes, steam reforming is better than others to produce H2 from syngas. Therefore, the waste plastics can be a significant potential source of H2 and will benefit the society and the environment from negative impact and support achieving net zero by 2050.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Plastic is used in every part of daily life which includes food packaging, electrical devices, household utensils and many more because of their favorable properties. The production of plastic items is increasing day by day and the safe disposal of waste plastic has become an urgent need to all countries in the world. Global plastic production exceeds 350 million tons in 2018 (Kusenberg et al. 2022). The main types of waste plastic that are found in the municipal waste are high-density polythene (HDPE), polyvinyl chloride (PVC), low-density polythene (LDPE), polythene terephthalate (PET), polypropylene (PP), and polystyrene (PS) (Kusenberg et al. 2022). Most of the plastics are non-biodegradable and take longer time to degrade which are causing many problems in land and seas. Incineration of plastic produces noxious and toxic fumes which poses health hazard. Therefore, incineration is not a good solution for disposing the plastics. Pyrolysis can an alternative and comparatively safe process of utilising waste plastic. Pyrolysis is a process of decomposing solid wastes into oil, char, and syngas in the absence of oxygen. The oil can be used as a fuel for internal combustion engine after refinement to plastic diesel. The char can be used for soil abatement and other purposes. The Syngas are mostly neglected and released into the atmosphere.
Combustion of hydrocarbons produces CO2 and other harmful GHG gases which has detrimental effects on the environment. Researchers are searching for environmentally friendly alternative fuel, for example H2 as a clean energy which can be a suitable substitute for fossil fuel (Hazrat et al. 2022; Sarker et al. 2023). H2 has many favorable properties such as the highest calorific value and does not produce any harmful gases during the combustion. The demand of H2 in 2020 was around 88 Mt and expected to increase to 530 Mt in 2050 to achieve net zero goal (IEA 2019; PwC 2017).
The syngas, normally discarded into the atmosphere, can be converted into H2 using different reforming processes. In this paper, the pyrolysis of waste plastic is discussed first, then different types of reforming processes are briefly described to produce H2.
2 Pyrolysis of Waste Plastics
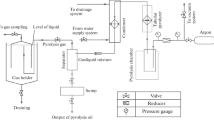
As introduced earlier, pyrolysis is a thermochemical process of decomposing the waste plastic into oil, biochar, and syngas in the absence of oxygen. There are different types of pyrolysis reactors used for pyrolysis as shown in Fig. 2.1 (Lewandowski et al. 2019; Papari and Hawboldt 2015). Authors used 20L vertical fixed bed reactor for their own research, the pictorial view of which is shown in Fig. 2.2.
In this reactor, three electrical heaters are used to heat the reactor and k-type thermocouples are used to measure the pyrolysis operational temperature. Before any experiment, nitrogen gas is purged through the system to make it inert (free of oxygen). A PID controller is used to operate the reactor at atmospheric pressure. A chiller unit is used to condense the pyrolysis vapors after decomposition of feed materials into crude oil. Polyethylene glycol solution circulated chiller unit temperature can be set − 5 to 20 °C. The mixed waste plastic was fed into the reactor through a feeding hopper and kept sealed after nitrogen was purged. Then, the data logging system and electrical heater were turned on to heat the reactor to the desired temperature and maintained at the desired time. During this period, the feedstock was converted into vapor and char. The vapor passed through a water-cooled condenser to produce crude oil which was collected from the oil collection tank. The system was turned off until the reactor temperature reduced to room temperature, then char was taken out from the bottom opening of the reactor. The crude oil can be refined through distillation process to produce oil equivalent to standard diesel to use in diesel engine. The ultimate and proximate analysis of different types of waste plastics can be found in Zhou et al. (2014). The yield from the pyrolysis of waste plastic is presented in Table 2.1. The results show that the oil yield can be found ≥ 80% including findings of the authors, except yield of PET, PE, and few mixtures (Rasul et al. 2022).
3 Production from Syngas
Syngas produced from pyrolysis of waste plastic is a mixture of H2, CO and small amount of CO2 and methane. The general equation for syngas production from pyrolysis can be expressed by:
Hydrogen can be separated from the syngas. The CO can be transformed into CO2 by producing H2 using different reforming process as shown in Fig. 2.3 (Rasul et al. 2022). Reforming is the process of converting low quality hydrocarbon to high quality hydrocarbon which has been used in industry for quite long. Reforming process can be used to produce H2 by cracking the natural gas, methane, gaseous hydrocarbon, liquid hydrocarbon, ethanol, methanol, coal, and naphtha. Brief description of different types of reforming processes are given below.
3.1 Catalytic Steam Reforming
In this process, steam at high temperatures and pressures is used to crack the hydrocarbon to produce CO and H2 rich syngas. Steam reforming process can be carried out with and without catalyst. After steam reforming process, water–gas-shift (WGS) process is carried out to convert CO into CO2 and dissociate H2 from water. This combined process is used to produce H2 from the hydrocarbon. The highest efficiency of SR can reach up to 80–90%. The reforming reactions (2.2) to (2.4) are given below:
Oxygenated hydrocarbon:
Non-oxygenated hydrocarbon:
WGS process.
Catalyst is used to enhance the efficiency of the H2 production. Nahar et al. (2015) found 94% yield efficiency at 650 °C in the presence of 10 wt.% Ni/Ce-Zr catalyst. Typical operating conditions for cracking natural gas is 3–25 bar and 700–1000 °C in the presence of a catalyst. Steam reform process requires high temperature if catalyst is not used for example CH4 cracks into various radicals (e.g., C2H4, C2H2, and C) at 1000 °C, and at over 1500 °C to produce H2 gas (Rostrup-Nielsen et al. 2011). The water–gas shift process is performed after the steam reforming process to decompose water with CO to increase H2 production. Haber–Bosch process is being used for quite long to produce H2. The combination of SR and WGS process has drawn the attention to produce H2 in recent time. Globally about 48 and 30% of the total H2 is produced using the SR and WGS methods (Ugurlu and Oztuna 2020).
3.2 Autothermal Reforming
Autothermal steam reforming (ATR) is a combination of SR and partial oxidation (POX) where fuel, steam, and water input into the reactor to produce H2. The heat is produced through POX therefore no additional heat is required. This process requires oxygen, so oxygen separation plant is needed to supply and carry out this process. WGS process is carried out to enhance the H2 concentration in the mixture. ATR process can produce H2 at a cost of $1.69–$2.55 per kg and adding the carbon capture, utilization, and storage (CCUS) techniques can make it green process (Ahmed and Krumpelt 2001). The CO2 emissions from this process is less than other processes. Catalysts can enhance the production of H2 as for example the addition of Pd-Zn/γ-Al2O3 at 400 °C can produce about 45% (v/v) of H2 efficiently (Oni et al. 2022). The typical reaction process of the ATR or OSR process is shown in Eq. 2.5.
Cortazar et al. (2022) reported the production of H2 from several waste plastics (HDPE, PP, PS, PET), mixed plastics, biomass, and HDPE and found that the highest H2 production was from PP (64.1%), and HDPE (64%).
3.3 Partial Oxidation Reforming (POX)
POX process generates heat during the reaction. This process does not require external heat like SR process. POX process produces less H2 than that of SR process. POX reforming process is carried out at sub-stoichiometric quantity of oxygen as can be seen in Eqs. 2.6 and 2.7 (Rasul et al. 2022). POX is exothermic whereas SR is endothermic process.
Hydrogen is separated using the pressure swing absorption process. Three-fourth of the total global H2 is produced usually using this technology. The generic POX reaction of hydrocarbon fuels is presented in Eq. 2.8.
The process can be carried out without catalyst therefore there is no drawback of degradation of catalyst effectiveness. The main challenge of POX is the requirement of high temperature and lower H2/CO ratio (Rasul et al. 2022). WGS is conducted after POX to convert CO to CO2. POX reaction for methanol and ethanol can be expresses as in the Eqs. 2.9 and 2.10, respectively (Rasul et al. 2022).
Catalyst can lower operating temperature. Agrell et al. (2001) used Cu (%40) Zn (%60) to conduct POX reaction of methanol at 185–215 °C and found that the H2 production increases.
3.4 Dry Reforming (DR)
In drying reforming process, CO2 and CH4 react to produce CO and H2 at 700–900 °C. The main challenge of this process is the deactivation of the catalyst and low H2/CO ratio (Uddin et al. 1997). In the dry reforming process CO2 is used to crack the hydrocarbon to produce the syngas. The dry reforming process can be expressed in Eq. 2.11 below.
The Boudouard and reverse water–gas shift (RWGS) reaction can be written as:
The efficiency of this process depends on the performance of the catalyst therefore a highly active catalyst is desired. Ru, Pt and Pd show higher catalytic activity for DR process (Medeiros et al. 2022). Ballarini et al. (2019) reported that K-L Zeolite, K-Al2O3, K-Mg/Al oxides, and MgO along with Pt-based catalyst has high stability to produce H2. The maximum yield was obtained when MgO/Pt was used. Xie et al. (2018) found that PtCo/CeO2 has high stability and effectiveness to produce H2. Hajizadeh et al. (2022) found that 48.07 kg/h biogas produces 8.11 kgH2/h in presence of Co-Ni-Al2O3 catalyst.
3.5 Aqueous Phase Reforming
In the aqueous phase reforming (APR), oxygenated/non-oxygenated hydrocarbon is cracked in aqueous solution at lower temperature to produce H2. This process operates at 200–250 °C and at 60 bar in the presence of catalyst. Platinum (Pt), tin (Sn), cobalt (Co) or nickel (Ni)-based metallic, and alumina can be used as a catalyst support (Shabaker and Dumesic 2004). The reaction can be given by Eq. 2.14.
This process consumes less energy than other processes and is termed as greener and therefore this process is an economical process to produce H2 from organic compounds. There is complexity of producing H2 directly from the biomass which can be overcome by converting the biomass into liquid as an intermediate material and then producing H2 through reforming. The stoichiometric APR reaction of sugar–alcohol sorbitol (C6O6H14) in the presence of Pt catalyst can be shown as in Eq. 2.15 (Shabaker and Dumesic 2004):
H2 and CO2 react in the presence of catalyst therefore it is essential to capture CO2 otherwise the overall efficiency of the process will decrease. In the APR process, bimetallic catalysts like PtNi, PdFe, PtFe show better performance than that of monometallic catalysts.
3.6 Plasma Reforming
Electron at high temperature in the plasma supports the decomposition of organic material. At high temperature, waste plastic and other hydrocarbon decomposes into CO, H2, and other hydrocarbons. There are different types of plasma reactors exists, such as dielectric barrier discharge (DBD) reactor, pulse plasma reactor, gliding arc plasma reactor, and microwave plasma reactor etc. (Budhraja et al. 2023). Comparative analysis of different types of plasma reactors can be found in Budhraja et al. (2023). Song et al. (2019) investigated the conversion of ammonia into methane and H2 using DBD plasma reactor and found that catalyst enhances the conversion of methane to H2. Morgan and ElSabbagh (2017) used a pulse plasma reactor to convert methane into H2 and found that 92% of methane converted into H2. Wang et al. (2019) used gliding arc reactor to convert n-heptane and found 50.1% H2. Wang et al. (2021) used MW plasma reactor and found 94% methane conversion with 74% H2 yield. The comparison of different reforming processes is given in Table 2.2.
4 Challenges and Conclusions
Waste plastic is posing a global threat for environment as only 20% of the total used plastic is disposed safely and the rest is thrown to landfill which takes more than hundred years to decompose. Incineration and other technique are not sustainable and environmentally friendly because it produces toxic gases. Literature review suggests that pyrolysis of waste plastic can produce more than 80% liquid oil which can be refined to diesel fuel for engine. The syngas produced pyrolysis of waste plastic can be used as feedstock to produce H2 through reforming processes. It is found from the literature that steam reforming process is better than other reforming processes to produce H2 which has efficiency of more than 90%. The conversion of waste plastic into H2 through pyrolysis and reforming process can solve the issue of safe disposal of waste plastics. The production of H2 can also help meet the demand of H2 and achieve the net zero by 2050. The main challenges of producing hydrogen from waste plastic using reforming process are:
-
Cost of producing hydrogen from waste plastic.
-
Scale up the production process for mass production.
-
Deactivation and reduction of catalyst effectiveness.
References
Abbas-Abadi MS, Haghighi MN, Yeganeh H, McDonald AG (2014) Evaluation of pyrolysis process parameters on polypropylene degradation products. J Anal Appl Pyrol 109:272–277
Agrell J, Hasselbo K, Jansson K, Järås SG, Boutonnet M (2001) Production of H2 by partial oxidation of methanol over Cu/ZnO catalysts prepared by microemulsion technique. Appl Catal A 211:239–250
Ahmed S, Krumpelt M (2001) H2 from hydrocarbon fuels for fuel cells. Int J H2 Energy 26:291–301
Ballarini AD, Virgens CF, Rangel MC, Miguel SRD, Grau JM (2019) Characterization and behaviour of Pt catalysts supported on basic materials in dry reforming of methane. Braz J Chem Eng 36:275–284
Budhraja N, Pal A, Mishra RS (2023) Plasma reforming for H2 production: pathways, reactors and storage. Int J H2 Energy 48(7):2467–2482
Cortazar M, Gao N, Quan C, Suarez MA, Lopez G, Orozco S et al (2022) Analysis of H2 production potential from waste plastics by pyrolysis and in line oxidative steam reforming. Fuel Process Technol 225:107044
de Medeiros FGM, Lopes FWB, Rego de Vasconcelos B (2022) Prospects and technical challenges in H2 production through dry reforming of methane. Catalysts 12:363
Fakhr Hoseini SM, Dastanian M (2013) Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J Chem 2013:1–5
Hajizadeh A, Mohamadi-Baghmolaei M, Cata Saady NM, Zendehboudi S (2022) H2 production from biomass through integration of anaerobic digestion and biogas dry reforming. Appl Energy 309:118442
Hazrat MA, Rasul MG, Jahirul MI, Chowdhury AA, Hassan NMS (2022) Techno-economic analysis of recently improved hydrogen production pathway and infrastructure. Energy Rep 8:836-844
IEA (2019) The future of H2. International Energy Agency (IEA), Paris
Kunwar B, Cheng HN, Chandrashekaran SR, Sharma BK (2016) Plastics to fuel: a review. Renew Sustain Energy Rev 54:421–428
Kusenberg M, Roosen M, Zayoud A, Djokic MR, Thi HD, De Meester S, Ragaert K, Kresovic U, Van Geem KM (2022) Assessing the feasibility of chemical recycling via steam cracking of untreated plastic waste pyrolysis oils: feedstock impurities, product yields and coke formation. Waste Manag 141:104–114
Lewandowski WM, Januszewicz K, Kosakowski W (2019) Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—a review. J Anal Appl Pyrol 140:25–53
Martínez JD, Puy N, Murillo R, García T, Navarro MV, Mastral AM (2013) Waste tyre pyrolysis—a review. Renew Sustain Energy Rev 23:179-213
Miskolczi N, Bartha L, Deák G, Jóver B (2004) Thermal degradation of municipal plastic waste for production of fuel-like hydrocarbons. Polym Degrad Stab 86(2):357–366
Morgan NN, ElSabbagh M (2017) H2 production from methane through pulsed DC plasma. Plasma Chem Plasma Process 37:1375–1392
Nahar G, Dupont V, Twigg MV, Dvininov E (2015) Feasibility of H2 production from steam reforming of biodiesel (FAME) feedstock on Ni-supported catalysts. Appl Catal B Environ 168–169:228–242
Onwudili JA, Insura N, Williams PT (2009) Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: effects of temperature and residence time. J Anal Appl Pyrol 86(2):293–303
Oni AO, Anaya K, Giwa T, Di Lullo G, Kumar A (2022) Comparative assessment of blue H2 from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions. Energy Conv Manage 254:115245
Papari S, Hawboldt K (2015) A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew Sustain Energy Rev 52:1580–1595
PwC (2017) The world in 2050: the long view—how will the global economic order change by 2050? The PwC network
Rasul MG, Sattar MA, Jahirul MI (2022) Experimental and numerical investigation of mixed waste plastics pyrolysis. ICEER 2022:12–16
Rasul MG, Hazrat MA, Sattar MA, Jahirul MI, Shearer MJ (2022) The future of H2: challenges on production, storage and applications. Energy Convers Manage 272:116326
Rehan M, Nizami AS, Shahzad K, Ouda OKM, Ismail IMI, Almeelbi T, Iqbal T, Demirbas A (2016) Pyrolytic liquid fuel: a source of renewable electricity generation in Makkah. Energy Sour Part A Recov Utiliz Environ Effects 38(17):2598–2603
Rostrup-Nielsen JR, Hansen JB (2011) Chapter 4—Steam reforming for fuel cells. In: Shekhawat D, Spivey JJ, Berry DA (eds) Fuel cells: technologies for fuel processing. Elsevier, Amsterdam, pp 49–71
Sarker AK, Azad AK, Rasul MG, Doppalapudi AT (2023) Prospect of green hydrogen generation from hybrid renewable energy sources: a review. Energies 16:1556 (Q2, MDPI)
Seo Y-H, Lee K-H, Shin D-H (2003) Investigation of catalytic degradation of high-density polyethylene by hydrocarbon group type analysis. J Anal Appl Pyrol 70(2):383–398
Shabaker JW, Dumesic JA (2004) Kinetics of aqueous-phase reforming of oxygenated hydrocarbons: Pt/Al2O3 and Sn-modified Ni catalysts. Ind Eng Chem Res 43:3105–3112
Song L, Liang T, Liu C, Li X (2019) Experimental investigation of H2 production by CH4–CO2 reforming using rotating gliding arc discharge plasma. Int J H2 Energy 44(56):29450–29459
Supriyanto PY, Richards T (2021) Gaseous products from primary reactions of fast plastic pyrolysis. J Anal Appl Pyrol 158:105248
Uddin MA, Koizumi K, Murata K, Sakata Y (1997) Thermal and catalytic degradation of structurally different types of polyethylene into fuel oil. Polym Degrad Stab 56(1):37–44
Ugurlu A, Oztuna S (2020) How liquid H2 production methods affect emissions in liquid H2 powered vehicles? Int J H2 Energy. Int J Hydrogen Energy 45(60). https://doi.org/10.1016/j.ijhydene.2020.01.250
Wang B, Peng Y, Yao S (2019) Oxidative reforming of n-heptane in gliding arc plasma reformer for H2 production. Int J H2 Energy 44(41):22831–22840
Wang Q, Wang J, Zhu T, Zhu X, Sun B (2021) Characteristics of methane wet reforming driven by microwave plasma in liquid phase for H2 production. Int J H2 Energy 46(69):3
Williams PT (2006) Yield and composition of gases and oils/waxes from the feedstock recycling of waste plastic. In: Scheirs J, Kaminsky W (eds) Feeds tock recycling and pyrolys is of waste plastics: converting waste plastics into diesel and other fuels. Wiley, West Sussex, pp 285–309
Williams PT, Slaney E (2007) Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour Conserv Recycl 51(4):754–769
Xie Z, Yan B, Kattel S, Lee JH, Yao S, Wu Q et al (2018) Dry reforming of methane over CeO2-supported Pt-Co catalysts with enhanced activity. Appl Catal B 236:280–293
Zhou H et al (2014) Classification and comparison of municipal solid waste based on thermochemical characteristics. J Air Waste Manag Assoc 64(5):597–616
Acknowledgements
The authors would like to express their gratitude to Fuel and Energy Research Group of CQUniversity, Australia for providing technical support to conduct this research. The work was supported by CRC-P8 project on “Australian Standard Diesel from Mixed Waste Plastics Waste: Maximizing Recovery from Waste”, Project No: CRCPEIGHT000194.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Rasul, M.G., Sattar, M.A., Jahirul, M.I., Hasan, M.M. (2023). Waste Plastics to Hydrogen (H2) Through Thermochemical Conversion Processes. In: Caetano, N.S., Felgueiras, M.C. (eds) The 9th International Conference on Energy and Environment Research. ICEER 2022. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-43559-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-43559-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43558-4
Online ISBN: 978-3-031-43559-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)