Abstract
Geothermal reservoirs (GR) are studied by geochemical techniques to estimate their potential to produce energy or alternate usages (thermal bathing, heating, greenhouses). This study focuses on two close (< 15 km) low-temperature GR, located in the northern portion of the Michoacan state (Mexico), called “Ixtlán de Los Hervores (IXT)” and “Los Negritos (LN).” A sampling campaign was performed in 2018 for physicochemical parameters, major ions, trace elements, stable water isotopes, and noble gas isotopes. Water classification in both sites is chlorinated sodium–potassium waters, corresponding to those close to heat sources. The Giggenbach ternary diagram (Cl–SO4–HCO3) depicts a certain degree of maturity in geothermal waters. In contrast, the Na–K–Mg ternary diagram evaluates the chemical equilibrium and the use of geothermometers, existing partial equilibrium with the hosting rock in both sites, with temperatures at a depth of 180–220 °C and 200–210 °C for IXT and LN. The estimated mean value of the temperature in each GR corresponds to medium to low temperature, with the potential for exploitation and generation of electrical energy through binary cycle plants. Isotopic results of water samples show a clear difference from the meteoric waters and a slight deviation to the right, corresponding to water–rock interaction processes. While noble gas isotopes (He, Ne) are non-reactive isotopes at high temperatures, they are commonly used for establishing the contributions of different sources (crustal, upper mantle, atmosphere) to the measured samples. The results show little atmospheric He and Ne presence, which might indicate a limited mixing of geothermal fluids at depth with more recently infiltrated water.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
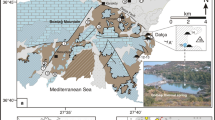
Geothermal exploitation history and its development in Mexico can be traced back to the twentieth century. In 1955, the first geothermal well was developed in Hidalgo State (Quijano-León & Gutiérrez-Negrín, 2003), and later with the development of major geothermal fields (GF) such as Cerro Prieto in Baja California (1960), Los Azufres in Michoacan (1982), and Los Humeros in Puebla (1990). Most major GR are located in the Trans-Mexicana Volcanic Belt (TMVB), such as the case of the study area (Fig. 1), located in the southeastern portion of the Lerma-Santiago basin in central Mexico. This region is characterized by various geothermal manifestations (Rosas Elguera et al., 1989), such as Ixtlán de Los Hervores (IXT) and Los Negritos (LN).
Geothermal manifestations (mud volcano, fumaroles, and thermal springs) are observed in the IXT and LN areas, reaching temperatures at a surface of 90 °C. Thermal manifestations of IXT are associated with the intersection between the Nogales and the Ixtlán-Encinal faults. At the same time, the rise of geothermal fluids in LN seems to be controlled by faults at depth (Rosas Elguera et al., 1989), covered by filled alluvial material of the quaternary age.
2 Materials and Methods
Since the objective of this work is to achieve a regional vision of the IXT and LN geothermal zones, gravimetric information from the UNAM Institute of Geophysics was used, with a Lacoste & Romberg gravimeter and calibrated at the Institute’s First Order World Gravimetric Station of Geophysics of the UNAM, where standard corrections were applied, including the topographic one, to obtain the complete Bouguer anomaly, for a density of 2.67 g/cm3.
Regarding the hydrogeochemical analysis, a sampling campaign was performed in 2018 for physicochemical parameters, major ions, stable isotopes of water (δ18O and δ2H), and noble gas isotopes (He, Ne). Seven water samples were collected in Nalgene®-type plastic bottles of 60 ml, filtered to 0.45 µm Millipore in the field, and properly preserved, stored, and transported. While for noble gas analyses, two sites in IXT and one in LN were chosen for gas sampling, collected in standard refrigeration-grade 3/8″ copper tubes, and later sealed by stainless steel clamps, following the procedure described by Pinti et al. (2017).
3 Results
3.1 Water Classification and the Use of Geothermometers
Figure 2a presents the Piper diagram with the IXT (orange diamonds) and LN (gray triangles) samples, compared to the (Molina & Banwell, 1970) (green circles).
The water classification for the samples in both sites is chlorinated sodium–potassium waters. The Giggenbach ternary diagram in Fig. 2b represents the classification of the thermal water based on the respective ions’ content (in mg/l). It indicates that samples correspond to geothermal waters with a certain degree of maturity, as they present high contents of chlorides (60–80%) and sulfates (20–40%), thus as very low bicarbonate content (0–10%). Both areas are compared with the (Molina & Banwell, 1970).
Regarding applying the Na/K-dependent formulations of Fournier, Giggenbach, and Tonani (used in cationic geothermometers) for estimating temperatures at depth, the mean values obtained for both geothermal sites were very similar, with 187, 204, and 182 °C, respectively.
3.2 Stable Isotopes of Water and Noble Gas Isotopes
Isotopic results of water samples show a clear difference from the meteoric waters shifted due to water–rock interaction processes. IXT isotopic values range from 7.64 to − 5.72 for δ18O and from 67.84 to 61.69 for δ2H. While for LN, average values are between 6.67 and 64.3 for δ18O and δ2Hn, respectively. Air-corrected helium isotopic ratios (R/Ra)c, with R = 3He/4He, were obtained and compared to other Mexican geothermal reservoirs (Fig. 3), with an average of 1.91 and 2.35 for IXT and LN, respectively, with less contribution of He and Ne from the upper mantle than from the crustal isotopes derived from fractionation.
4 Discussion
The water classification corresponds to those close to heat sources or hot springs, characteristic of waters from transition zones or local flows. Temperatures at depth obtained by cation geothermometers correspond to values of medium temperature GF, with potential for electrical generation through binary cycle plants.
The stable isotope values show a process of water–rock interaction, with enrichment in δ18O isotopes. Previous studies (Molina & Banwell, 1970) seem to indicate that over time the waters of these areas have gained lighter isotopes, going from values of δ18O from − 7.64 to − 5.72 and from − 67.84 to − 61.69 of δ2H, possibly derived from a greater mixture of shallower waters, as a product of groundwater extraction. In addition, these gas samples show little atmospheric He and Ne presence, which might indicate a limited mixing of geothermal fluids at depth with more recently infiltrated water, as shown in Pinti et al. (2017).
5 Conclusion
According to hydrological settings, both sites are separated into two different basins, with surface contributions that might explain the observed high values of specific ions (Mg, NO3, HCO3). Furthermore, as noble gas isotopes (He, Ne) are non-reactive isotopes at high temperatures, they are commonly used for establishing the contributions of different sources (crustal, upper mantle, atmosphere) to the measured geothermal samples. Finally, the presence of recent volcanism, together with the regional faulting and the movement of fluids, increases the possibility of the existence in the subsoil of geothermal reservoirs, which can be used for electricity generation.
References
Molina, T., & Banwell, J. G. (1970). Chemical studies in Mexican fields. Geothermics special issue. In UN symposium on the development and utilization of geothermal resources (Vol. 2, Part 2). Pisa.
Pinti, D. L., Castro, M. C., Lopez-Hernandez, A., Han, G., Shouakar-Stash, O., Hall, C. M., & Ramírez-Montes, M. (2017). Fluid circulation and reservoir conditions of the Los Humeros Geothermal Field (LHGF), Mexico, as revealed by a noble gas survey. Journal of Volcanology and Geothermal Research, 333, 104–115. https://doi.org/10.1016/j.jvolgeores.2017.01.015
Quijano-León J. L., & Gutiérrez-Negrín, L. (2003). 30 years of geothermal-electric generation in Mexico. Mexican Geothermal Development., Comisión Federal de Electricidad. Morelia, México.
Rosas Elguera, J., Urrutia-Fucugauchi, J., & Maciel, F. (1989). Geología del extremo oriental del graben de Chapala, breve discusión sobre su edad: Zonas geotérmicas Ixtlán de los Hervores-Los Negritos. México Geotermia, V5(1), 3–18.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Hernández Hernández, M.A., Morán, T.G. (2023). Geophysical and Hydrogeochemical Aspects of Two Low-Temperature Geothermal Reservoirs Located in Central Mexico. In: Chenchouni, H., et al. Recent Research on Hydrogeology, Geoecology and Atmospheric Sciences . MedGU 2021. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-031-43169-2_26
Download citation
DOI: https://doi.org/10.1007/978-3-031-43169-2_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43168-5
Online ISBN: 978-3-031-43169-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)