Abstract
The aim of this chapter is to describe the involvement of the autonomic nervous system (ANS) in the determinism of cerebrovascular pathologies both as a modulating factor of cerebral autoregulation and as a concausal risk element for the pathology. In particular, the mechanisms through which ANS dysfunction can cause the onset of the pathological manifestations of stroke and chronic vascular suffering of the central nervous system (CNS), as well as the sequelae of the acute event and the accompanying manifestations of the chronic phase of the disease, are indicated. In particular, the main changes induced by acute stroke on autonomic functions (especially cardiopressor functions), their prognostic significance, and their functional assessment by means of the most commonly used laboratory tests are described. Possible modulation and control measures on autonomic alterations before and after the acute vascular event are finally reported.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sympathetic
- Parasympathetic
- Cerebral autoregulation
- Cerebrovascular risk factors
- Stroke
- Cerebral small vessel disease
- Autonomic testing
5.1 Introduction
Autonomic nervous system and cerebrovascular pathology are closely interconnected topics, and from different perspectives: from anatomic and physiological bases to diagnostic and management implications, to therapeutic potential in pathological conditions. Autonomic functions underlie a coordinated but complex organization that is often difficult to study, especially in the presence of concomitant acute or chronic ‘disturbing’ factors that can often occusr in patients with cerebrovascular diseases [1,2,3,4,5,6].
5.2 Cerebral Autoregulation
When examining the relationship between autonomic nervous system and cerebrovascular pathology, it is essential to take into account the physiological peculiarities of the cerebral circulation, which in particular is characterized by the phenomenon of cerebral autoregulation (AR), i.e., the mechanism by which the cerebral blood flow (CBF) is kept constant when systemic arterial blood pressure remains in the range between 50 and 150 mmHg [7], and also for values above or below this range in some populations (e.g. patients with chronic hypo- or hypertension) [7,8,9,10,11]. CBF depends on arterial blood pressure (ABP), cerebral venous pressure (tends to coincide with intracranial pressure (ICP)) and cerebrovascular resistance (CVR), according to the formula CBF = (ABP − ICP) / CVR [7, 11, 12].
These criteria, however, essentially define a “static” model of autoregulation as described by Lassen [7], that subsequently has been furtherly characterized and now is known to be a more “dynamic” mechanism, potentially modified by several factors influencing the CBF during rapid changes in BP (seconds or minutes) [13] (Table 5.1).
In fact, a dynamic model of AR has the capacity to buffer changes in CBF, but it is strongly dependent on the speed of BP changes [14].
The slower the change in BP, the smaller the impact on CBF, up to a point after which CBF becomes almost unaffected. However, for more rapid changes in BP, the buffering capacity is progressively reduced, and changes in CBF become larger, up to a point after which changes in CBF become as large as the change in BP, so that CBF “passively” follows BP.
To skip a more detailed description of cerebral AR, that is not the principle aim of this text, let’s just state that the control that AR plays on CBF values to ensure an adequate functioning of brain structures is evident, together with the regulation of blood pressure at the microvascular level and the protection of the blood-brain barrier (BBB), targeted to protecting, by adequate self-regulation, both cognitive and noncognitive functions.
On the contrary, an alteration of AR (acute or chronic) can be observed in different pathological conditions, such as hypoperfusion or stroke, syncope, hyperperfusion syndromes, pressure alteration at the microvascular level, loss of BBB integrity with the appearance of edema, hemorrhage and/or micro bleedings.
The cerebrovascular system is richly innervated by adrenergic and cholinergic fibers, with a complex organization that can be simplified by subdividing the extrinsic innervation of the extraparenchymal arteries (from the cervical, otic, sphenopalatine, and trigeminal ganglia) from the intrinsic innervation of the intraparenchymal arterioles (from the truncus encephalic nuclei: locus coeruleus, nucleus of the fastigial, nucleus of the dorsal raphe).
The main sympathetic neurotransmitter is norepinephrine (NA): in the cerebral circulation, α1-adrenergic receptors are the most represented at the postsynaptic level, while α2-adrenoceptors are mainly located at the presynaptic terminal. NA release causes vasoconstriction through vascular smooth muscle contraction mediated by α1-adrenergic receptors, whereas activation of α2-adrenoceptors inhibits further NA release from the presynaptic neuron [17], creating local negative feedback that is useful in regulating NA release. The reactivity of α-adrenergic receptors proportionally decreases with the reduction in caliber of cerebral afferent vessels, being greatest at the level of large-caliber extracranial arteries, progressively decreasing through intracranial arteries, then lowest for small pial arterioles. The β-adrenergic receptors are also located post (β-1) and presynaptically (β-2), and are mainly involved in vasodilatory mechanisms (Table 5.2) [18,19,20,21,22].
A contemporary model of autoregulation, derived from the intra-patient reanalysis of 41 studies, shows essentially a reduction of the plateau and a substantial increase in the passive pressure-CBF ratio, i.e. a more effective buffering property against pressure increases or decreases [23].
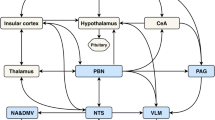
Beyond acting on the control of vascular caliber, cerebral blood flow, and cerebrovascular resistance, autonomic fibers determine changes in arterial and venous pressure parameters, thus acting also indirectly on CBF regulation. Although the contribution of the sympathetic nervous system to the resting state is estimated to be modest, its role during rapid and/or acute increases in arterial BP is very important. Several studies seem to confirm that it contributes to CBF autoregulation [24], and during acute hypertension, its vasoconstrictor effect buffers surges in microvascular pressure, thus contributing to CBF, and ideally BBB, preservation of CBF [25, 26]. Other studies suggest that intracerebral vascular response induced by cold pressor test (i.e., a marked pressure reduction obtained by pretreatment with clonidine, that is a central alpha2-agonist) may rely on a central noradrenergic mechanism, which is possibly modulated at the locus coeruleus level [27] (Fig. 5.1). The generalized sympathetic discharge determines increases in ABP and can prevent concomitant increases in CBF by acting on both small and large vessels’ resistance and compliance [28].
Effect of cold pressor test on blood flow velocity in Middle Cerebral Artery (MCA): time course and power spectra [G Micieli, 1994, personal communication]. In the figure, Middle Cerebral Artery (MCA) blood flow velocity, registered by means of Transcranial Doppler ultrasounds, shows a marked reduction during (prolonged: 5 min) cold pressor test, whereas the frequent oscillations in flow velocity can be attributable to rapid change induced in AR during the exercise
Transient systemic hypotension is accompanied in experimental models by a sympathetic-mediated response leading to vasoconstriction of cerebral afferent arteries [29, 30]. Of note, AR appears to be better adapted to transient increases in BP rather than reduction, a phenomenon also known as hysteresis [31].
Despite the anatomical presence of cholinergic nerve terminals throughout intracranial vessels, located proximal to Virchow-Robin spaces, the exact role of parasympathetic nervous system in the regulation of CBF in not clear [32, 33]. It seems to influence parenchymal neurons and the vasculature by releasing their neurotransmitters, whereas cholinergic projections induce the local release of NO [34] and the intrinsic system can be influenced by cholinergic or anticholinergic drugs.
In addition, the trigeminal sensory system represents an emerging subject in CBF autoregulation, mainly through the release of the potent Calcitonin gene-related neuropeptide (CGRP), which is currently thought to mediate most of the effects of the trigeminal nerve on vascular tone. However, despite the emerging role of trigeminal sensory nerves in acute conditions like severe hypertension, the influence of this neural system on resting CBF is estimated to be minimal [35].
In addition to neurogenic control, several other factors may affect AR and, thus, CBF: myogenic vasomotor response, arterial partial pressures of oxygen and carbon dioxide, and cerebral metabolism [23]. More specifically, partial pressures of gasses determine changes mainly in the arterial vessels with larger caliber, while the pial and cortical vessels appear to be more sensitive to variations in systemic pressure parameters and local neural stimuli.
Several pathological conditions are associated with CBF alterations because of various dysregulation phenomena, such as cerebral ischemia, both acute and “chronic” (due to atherosclerosis with hemodynamic repercussions, or small vessel disease) [9, 36,37,38], and late ischemic damage after subarachnoid hemorrhage [39, 40]. While the myogenic tone is the predominant component of AR under physiological conditions, it may be overridden by other factors promoting increased vascular tone under pathological conditions, such as cerebral vasospasm [1, 40, 41]. In these specific contexts, therapeutic intervention could aim to impede sympathetic tone.
The study of AR in response to vasoactive stimuli and, in particular, clinical contexts beyond the traditional neurosonological methods (Transcranial Doppler) could also take advantage of high-resolution magnetic resonance imaging techniques dedicated to the study of tissue perfusion parameters [1, 39].
5.3 Autonomic Nervous System and Cerebrovascular Diseases: Anatomical Peculiarities
Often, the interconnection between autonomic dysfunction and cerebrovascular pathology is also a direct consequence of close anatomic-functional contiguity [41]. In fact, in addition to the centers located within the neuraxis [41], several autonomic structures are characteristically located close to the course of the cerebral afferent vessels, thus being potentially susceptible to damage in the event of pathological phenomena affecting the latter, giving rise to some characteristic clinical syndromes whose early diagnostic recognition is crucial to set up their proper management [1,2,3,4,5,6, 42].
Indeed, in some cases, signs of autonomic dysfunction may be the only clinical expression of potentially dangerous cerebrovascular pathologies, such as aneurysms and arterial dissections [43,44,45].
Oculo-sympathetic fibers run along the walls of the internal carotid artery, forming the internal pericarotic plexus, and then distributing to the deep structures of the eye (superior tarsal muscle and pupil dilator muscle), interconnecting with the trigeminal ganglion, the abducens nerve, the sphenopalatine ganglion, and the tympanic branch of the glossopharyngeal nerve. The vaso- and sudomotor fibers separate at the carotid bifurcation. The sympathetic fibers directed to innervate the ipsilateral arterial vessels and the sweat glands of the medial portion of the forehead and nose run along the internal carotid artery, while the fibers directed to the remaining facial areas run along the external carotid artery.
Horner’s syndrome includes a combination of dysautonomic signs due to damage to the ipsilateral oculosympathetic trunk. In its full expression, it is characterized by the presence of miosis (due to inactivation of the pupil dilator muscle), eyelid ptosis with enophthalmos (due to inactivation of the superior tarsal muscle), and anhidrosis (due to inactivation of the sudomotor fibers). Sometimes, ipsilateral flushing is present, due to vasodilation of the skin vessels by interruption of the vasomotor sympathetic fibers [44]. Pathologies such as cavernous sinus thrombosis and carotid dissection may produce disruption of the peri-carotid sympathetic pathway, leading to the appearance of incomplete Horner’s syndrome without anhidrosis. In addition to neurological examination findings, anamnestic data and accompanying symptoms (mode of onset, associated symptoms such as headache, neck pain and cranial nerve palsy) may help guide the diagnostic pathway.
The harlequin sign is characterized by flushing with unilateral facial hyperhidrosis. It may be a consequence of both pre- and postganglionic damage to the vaso- and sudomotor fibers, resulting in pallor and anhydrous hemiface, and has been reported for example in association with carotid dissections, although less frequently than Horner’s syndrome [45].
On the parasympathetic side, of cerebrovascular interest is the case of the third cranial or oculomotor nerve, whose Edinger-Westphal nucleus provides parasympathetic fibers directed to the eye to control the pupil sphincter muscle (which controls pupillary constriction) and the ciliary muscle (which controls accommodation). After emergence from the brainstem, the oculomotor passes between the superior cerebellar artery and the posterior cerebral artery, near the posterior communicating artery; it then crosses the cavernous sinus, receives sympathetic fibers from the peri-carotid plexus and a communicating branch of the ophthalmic branch of the trigeminal nerve. Since the parasympathetic fibers run in the outer portion of the nerve, any extrinsic compressive element would first damage the parasympathetic component of the motor fibers. The proximity of the nerve to certain vessels of the Willis circle may therefore explain the finding of pupillary abnormalities in the case of aneurysmal dilatations (aneurysm of the apex of the internal carotid artery, aneurysm of the posterior communicating artery) [43].
5.4 Autonomic Nervous System and Cerebrovascular Diseases: Management and Therapeutic Perspectives
The profound interconnection between the autonomic nervous system and cerebrovascular pathology, particularly if acute, has been effectively described with the expression “striking reciprocity” [42, 46]. In fact, if on the one hand the cerebral damage following the cerebrovascular event can determine secondary dysautonomia with important repercussions on the prognosis, during the hyperacute phase, on the other hand the dysfunction of the ANS can play a decisive role in the development of pathological conditions that determine the occurrence of the event itself (Fig. 5.2).
Chronic risk factors, which act in the long term, and acute risk factors (triggers), which rapidly increase the risk of an ischemic event and often determine the timing of its presentation, are involved in the etiopathogenesis of stroke. Both chronic risk factors and triggers are susceptible to modulation by the ANS. Different autonomic responses are associated with different etiopathogenetic mechanisms of cerebrovascular pathology: atherosclerotic (large or small vessel occlusion, athero-arterial embolization), cardioembolism and vasospasm. A careful assessment of the activity of the SNA, although complex, could allow the identify the effects of chronic stress/risk factors and acute triggers in the different subtypes of ischemic stroke, contributing to outline a personalized risk profile useful for modulating the necessary management and pharmacological interventions.
As is well known, the NES and its various components have the task of maintaining homeostasis, which can be defined as the state of physiological equilibrium, since it is susceptible to various perturbing elements, both intrinsic and extrinsic. The term ‘stress’ was coined to describe a state, whether transient or chronic, of altered homeostasis induced by various factors (stressors), listed in Table 5.3, which in turn may be acute, episodic, or chronic.
The role of the ANS is to respond to acute stressful events by implementing countermeasures to maintain homeostasis through the activity of both its sympathetic and parasympathetic branches, which in turn act on the target organs. Although this process is physiological and aimed at maintaining the proper functioning of the organism, under conditions of chronic/acute recurrent stress its response may become maladaptive. In chronic stress, in fact, the ability of the parasympathetic nervous system to keep the stress response under control is particularly impaired, with possible anticipatory or dysregulated activation phenomena. Adaptation to stress can lead to stress-related disorders. The maladaptive phenomena of the ANS lead to secondary effects on the target organs with the consequent development of dysfunctional/dysmetabolic conditions such as hyperglycemia, dyslipidemia, and arterial hypertension, which in turn cause further damage to the ANS. The resulting vicious cycle may culminate in the occurrence of an acute cerebrovascular event [47,48,49].
As patients grow older, comorbidity in the form of multiple concurrent chronic conditions is the norm rather than the exception. One attempt to provide an answer to this phenomenon has been the introduction of the concept of allostatic load as a measure of the cumulative physiological load imposed on the body through attempts to adapt to the demands of life. Allostasis is in fact understood as the ability to adapt to challenges [50]. The concept of allostasis emphasizes the physiological imperative that, to survive, “an organism must vary the parameters of its internal environment and adapt appropriately to environmental demands”. When adaptive responses to challenge are chronically outside normal operating ranges, wear, and tear on regular systems, primarily the SNA, occurs, and allostasis accumulates, leading to progressive wear and tear on physiological systems [51] (Fig. 5.3).
5.4.1 Chronic Risk Factors
Chronic stress has been proposed as a factor favoring the occurrence of a metabolic syndrome. The metabolic syndrome, an important risk factor for ischemic stroke, cardiovascular diseases, and diabetes, is made up of a cluster of disorders (Table 5.4) which recognize a dysfunction of the sympathetic nervous system as the primum movens of cardiovascular and metabolic changes.
On the other hand, severe SNA dysregulation can lead to insulin resistance, altered lipid metabolism, and increased blood pressure. An increase in HR and a reduction in respiratory sinus arrhythmia, indicators of reduced parasympathetic activity, and a reduction in the cardiac preejection period, indicative of elevated sympathetic activity, were correlated with elevated blood pressure, hypertriglyceridemia, hyperglycemia, and increased abdominal circumference. In patients with metabolic syndrome, sympathetic overactivity and reduced parasympathetic activity have been documented. Moreover, sympathetic hyperactivity seems to be predictive, in a 2-year follow-up of an increase in the number of metabolic alterations, suggesting its role as a predictor for the development of cardiovascular disease and diabetes through dysregulation of lipid metabolism and blood pressure over time [53].
The sympathetic nervous system (SNS) plays a role in the genesis or progression of the metabolic syndrome as it can reduce insulin sensitivity by both hemodynamic mechanisms and direct cellular effects (arteriolar vasoconstriction induced by the release of noradrenaline reduces glucose re-uptake by altering the ability of cells to transport glucose across their membranes, a hallmark of insulin resistance). Insulin resistance, a metabolic disorder characterized by reduced tissue sensitivity to insulin that originates from the effect of a sedentary lifestyle and central obesity, among other environmental factors, in individuals with a significant genetic predisposition, has been proposed as a common pathophysiological mechanism underlying the metabolic syndrome. It is less clear whether it is insulin resistance that leads to a condition of sympathetic overactivity or vice versa. The work of Anderson et al. [54] has documented that systemic infusion of insulin while maintaining constant plasma glucose concentrations results in a marked increase in outflow from sympathetic endings to skeletal muscle vessels. This ability of the SNS to determine or worsen insulin resistance resulted in a further increase in plasma insulin levels, which in turn triggered further sympathetic activation. Furthermore, the work of Masuo et al. [55] documented that in young, nonobese subjects who were normotensive at study inclusion but had a significant increase in BP over 10 years, sympathetic overactivity preceded the onset of hyperinsulinemia; at the onset of the first stages of hypertension, on the other hand, both sympathetic hyperactivity and hyperinsulinemia were present, suggesting that sympathetic hyperactivity may be the primum movens capable of triggering both hypertension and insulin resistance [56].
In fact, in subjects with a family history of hypertension or with white-coat hypertension, an abnormal increase in serum adrenaline and noradrenaline has been shown to be caused by an increased release from the sympathetic nerve endings. Sympathetic overactivity has also been found in different subtypes of hypertensive patients, irrespective of age group, and in particular groups of patients, e.g., in pregnancy hypertension. This suggests that autonomic dysfunction may be a process involved in the genesis of hypertension regardless of the specific context in which the disease develops. In these patients a progressive parasympathetic dysfunction accompanies sympathetic hyperactivity, as evidenced by a gradual reduction in responses to arterial baroreceptor stimulation, leading to reduced vagal modulation and an altered cardiac vagal response, resulting in reduced Heart Rate Variability (HRV), particularly low-frequency fluctuations [57]. A possible substrate for the relationship between autonomic dysfunction and the development and maintenance of hypertension is provided by some evidence in favor of the importance of arterial baroreceptors not only in the short-term control of blood pressure but also in its long-term control. In particular, the resetting of these baroreceptors to higher pressure levels appears to be an important element, and involves hypothalamic regions located antero-ventral to the third ventricle, structures that are sensitive to arterial pressure and volemic changes [58]. As evidence of this autonomic dysfunction in hypertensive patients a reduction in HRV has been shown and in normotensive patients a lower HRV has been correlated with an increased risk of developing hypertension [59]. Furthermore, autonomic dysfunction with sympathetic hyperactivation correlates not only with the development of hypertension but also with the severity of vascular and cardiac damage in hypertensive patients, independent of blood pressure values [57].
Insulin resistance, through the vicious circle triggered by the SNS, can in turn lead to:
-
Impaired glucose metabolism with the development of diabetes mellitus.
-
Alterations in the metabolism of plasma triglycerides and free fatty acids resulting in atherogenic dyslipidemia.
-
Increased blood pressure.
-
Alterations in vascular reactivity.
-
Endothelial dysfunction.
-
Chronic subclinical inflammation.
-
Prothrombotic phenotype.
Thus, insulin resistance is a key pathophysiological factor in the development of vascular risk. Epidemiological data support its role in cerebrovascular pathology, reporting that metabolic syndrome is an independent risk factor for stroke in all ethnic groups and in both sexes, particularly for atherothrombotic stroke. In prospective studies, metabolic syndrome increases the risk of ischemic stroke by about fivefold [60].
The persistence of a condition of sympathetic overactivity is decisive not only in the appearance but also in the progression of atherosclerosis. The evolution of the atherosclerotic plaque and its vulnerability to rupture is determined by several factors that can be modulated by the ANS. A vulnerable plaque is characterized by the presence of a lipid-rich core, a thin fibrous cap, the presence of active inflammatory processes, and active remodeling processes. On the other hand, the vulnerability of a plaque is not predictable when based on its size and degree of stenosis. An autonomic dysfunction, in particular a predominance of SNS activity, can favor the evolution towards plaque instability, facilitating the occurrence of chronic inflammatory processes at the level of the atherosclerotic plaque [61]. In addition, sympathetic hyperactivity can induce an increase in the uptake of LDL cholesterol by endothelial cells, a pivotal process in the induction and progression of atherosclerosis [62, 63]. Carotid atherosclerosis can also enhance autonomic dysfunction secondary to the atherosclerotic process by interfering with carotid baroreceptors and chemoreceptors, directly promoting sympathetic-vagal dysregulation with a prevalence of sympathetic activity and a reduction in vagal tone [59].
In addition to arterial vessels, another target organ of sympathetic overactivity is the heart (as will be discussed later in this chapter).
The role of SNA in the emergence and maintenance of risk factors for ischemic stroke is summarized in Fig. 5.4.
5.4.2 Acute Risk Factors or Triggers
To determine the occurrence of an acute cerebrovascular event on a terrain predisposed to the presence of chronic risk factors, it is necessary that acute risk factors or triggers capable of determining an abrupt increase in short-term risk (days, hours) intervene to be able to explain the occurrence of a cerebral ischemic event at a specific time. The ANS, already chronically unbalanced towards a state of sympathetic hyperactivity due to the presence of chronic risk factors, responds in a dysfunctional manner with a further accentuation of the sympathetic response, which in turn can trigger phenomena such as vasoconstriction, acute prothrombotic state, sudden increase in blood pressure and heart rate, arrhythmias, and endothelial dysfunction capable of triggering the cerebral ischemic event.
The main acute risk factors for ischemic stroke and their relationship to SNA are shown in Table 5.5.
Circadian Rhythm: Several studies have reported a circadian rhythm in the incidence of stroke, both ischemic and hemorrhagic, characterized by a peak between 6 a.m. and noon, and a less evident one between 2 p.m. and 4 p.m. The circadian distribution is not influenced by the etiopathogenetic category of the stroke [64]. Circadian rhythms, generated centrally, are influenced by the activity of the ANS, whose activity is in turn modulated by the CNS according to the different phases of the circadian rhythm. By assessing the variation in HRV at different times of the day, we can see a peak in HRV during the night (prevalence of parasympathetic activity during nighttime rest) and a maximum reduction in the early morning hours, at the transition between sleep and wakefulness. This reduction reflects greater sympathetic activity in this phase, which is reflected in the cardiovascular system. The imbalance towards sympathetic activity, as previously highlighted, leads to unfavorable changes in the cardiovascular system, in blood pressure and heart rate values, as well as in the hormonal profile and inflammatory activation, promoting the development of acute cerebrovascular events [65].
Exercise/Physical Activity: Regular physical activity has been widely shown to be a protective factor for stroke. However, isolated episodes of intense physical activity may lead to an acute increase in sympathetic nervous system activity, resulting in a transient increase in heart rate, blood pressure, and an alteration of the coagulation balance towards a prothrombotic profile [66]. In the hour following intense physical exertion, a significant increase in the risk of stroke, up to 2.3 times greater, has been demonstrated, particularly in subjects who do not habitually practice physical activity [67]. Similar data have emerged about hemorrhagic stroke, and intense physical activity can also be considered a trigger event for this type of event [68].
Acute Psychological Stress: The occurrence of particularly stressful life-events has been recognized as a possible trigger for acute cerebrovascular events through activation of the hypothalamic-pituitary-adrenal axis and the autonomic nervous system. It has been shown that this activation in response to stressful psychological events can lead to an increase in heart rate and blood pressure, release of catecholamines and procoagulant factors, and even transient endothelial dysfunction. Overall, this alteration in homeostasis creates a substrate favoring the occurrence of stroke [69]. In some cases, it is possible to document an acute, nonischemic but stress-induced myocardial dysfunction, probably as expression of an acute and tendentially reversible organ damage caused by a massive release of catecholamines as a result of stressful events: this condition is called Takotsubo syndrome, after the original name of a Japanese octopus trap whose shape resembles the appearance of the left ventricle on ultrasound investigations performed in such cases (this appearance is also known as ‘apical ballooning’). Takotsubo syndrome is a cause of cardioembolism as it is associated with the formation of thrombotic apposition in the left ventricle due to contraction dyssynergy; a 1–5% risk of developing ischemic stroke after a diagnosis of Takotsubo has been reported [70, 71].
Substances: Acute intake of an excessive amount of alcohol induces sympathetic hyperresponsiveness and a decrease in parasympathetic activity. This autonomic dysregulation may lead to an acute alcohol-induced arrhythmogenic substrate and be an exogenous trigger for an acute cerebrovascular event [72]. Cocaine, amphetamine and its derivatives result in indirect stimulation of the ANS through the release of norepinephrine, dopamine and serotonin. Cannabis is also capable of causing a sympathetic-vagal imbalance. The effect of marijuana and its active component THC on the cardiovascular system varies depending on the dose taken, the frequency and duration of intake, and the route of administration. In low to moderate doses, smoked marijuana increases sympathetic activity and reduces parasympathetic activity, leading to tachycardia and hypertension. It may also trigger atrial fibrillation [73]. High doses cause bradycardia and hypotension [74].
Environmental Temperature: Global warming produces not only hotter summers and more frequent heatwaves but also colder winters, especially in more temperate areas where populations are not used to extreme weather conditions. Exposure to excessively cold or warm temperatures can increase the risk of stroke through different mechanisms. In cold weather, the increased risk has been linked mainly to increased blood pressure, peripheral vasoconstriction, increased platelet count and increased blood viscosity caused mainly by increased sympathetic activity. However, the data are still contradictory. In contrast, elevated room temperature may increase the risk of stroke through increased sweating and skin blood flow, dehydration, increased blood viscosity, hemoconcentration and increased plasma cholesterol levels [75].
Infections: Numerous case-control studies have demonstrated an association between a previous systemic – mostly viral—infection, and the occurrence of a stroke, often with a time interval of several days from the onset of the infectious symptoms. The mechanisms by which viral infection can promote the occurrence of a cerebral ischemic event are manifold: activation of the immune system, hypercoagulability, hemodynamic alterations secondary to myocardial involvement, endothelial damage, and destabilization of atherosclerotic plaque [76].
5.4.3 After the Cerebrovascular Event
There is a great deal of evidence of interactions between SNA and stroke, both in the acute phase and in the outcome. The acute attack is known to be associated with a central autonomic alteration responsible for a marked increase in BP and heart rate, also associated with a reduction in parasympathetic activity compared to controls (increase in HF at the frequency spectrum) both in patients with pathology of the large vessel (Large Artery Atherosclerosis—LAA) and in those with lacunar infarction (LAC or SVO, from Small Vessel Occlusion, symptomatic of pathology of the small cerebral vessel). At 7 days, this reduction in parasympathetic function is present only in the first group (LAA), while lacunar ischemia seems to be especially pronounced if the ischemia is at the level of the putamen or thalamus, key structures of the central autonomic network (CAN). Therefore, it can be said that it is, above all, the ischemic (or hemorrhagic) lesion at the supratentorial site that determines a more relevant and lasting reduction in parasympathetic activity and a concomitant increase in sympathetic activity [77]. In turn, the severity of neurological impairment (NIHSS) directly correlates with the degree of impairment of cardiovascular autonomic function, with the fall in parasympathetic tone and baroreceptor sensitivity, as well as the progressive shift towards sympathetic dominance. The resulting autonomic dysfunction increases the risk of cardiovascular complications and worsens the outcome [78]. The parasympathetic deficit, also assessed by Ewing tests and HRV spectral analysis, is always greater in LAA patients (82% of patients) than in LAC patients (63%), who together have a greater parasympathetic deficit than control subjects [79]. Outdated studies show that it is mainly ischemic lesions of the trunk-brain that present a greater functional impairment (HRV) than supratentorial lesions, which show a higher level of plasma catecholamines than the former [80].
It is interesting to underline how the impairment of the SNA, and in particular of the pressor response to the Valsalva test (with a reduced increase in systolic pressure in phase IV), has been shown to characterize the greater evolutivity of the pathology linked to the lacunar lesions or the progression, sometimes observed during hospitalization, of subjects with cerebral small-vessel disease, even at an outcome of 3 months [81].
Autonomic dysfunction is also associated with an increase in the pathological changes observed in the disease of the small cerebral vessels, and in lesions of the white matter, regardless of the form of cognitive impairment. This suggests that dysfunction is a risk factor for cerebral hypoperfusion, which may contribute, in different ways among the various forms of dementia, to induce pathology in the advanced phase of the disease [82].
Demonstrating the role of altered cerebral autoregulation in determining autonomic dysfunction, recent studies seem to show that, after a lacunar infarction, while no significant differences are found in the “overall” vegetative function of patients and control subjects, there is a reduction in cerebral blood flow in the middle cerebral artery (mCBFV) and in systolic blood pressure before, during and after a breath-holding test. The positive correlation found between systolic pressure and mCBFV in patients compared to controls suggests an alteration of parasympathetic drive in this pathology, especially of autonomic control over CBF in the pathophysiological mechanisms of vasodilation induced by hypercapnia caused by deep breathing. Cerebral vascular reactivity is indeed reduced in patients with lacunar infarction due to reduced parasympathetic activity [83].
The pathological sympathetic activation associated with acute cerebral vascular injury (both ischemic and hemorrhagic) has repercussions on autonomic functions, with an increased risk of cardiac arrhythmias, increased levels of circulating catecholamines, myocardial damage, and heart failure (even in the absence of cardiac pathologies), pressure instability and neurogenic pulmonary edema. In fact, the main autonomic dysfunction in the post-stroke period involves the baroreflex dysfunction (BRS) as the probable mechanism underlying the almost invariable occurrence of arterial hypertension (hypertensive crises, pressure lability, altered circadian variability of blood pressure, altered dipping) during the acute phase of stroke (more than 80% in several cases) [84,85,86].
Post-stroke impairment of BRS is associated with worse clinical outcomes and is directly proportional to mortality and morbidity. The site and extent of vascular injury are major determinants of the duration, extent and reversibility of autonomic dysfunction. The anatomo-functional central connections of the baroreceptors include the nucleus of the solitary tract, the ventrolateral bulbar portion, the insula, the medial prefrontal cortex, the cingulate cortex, and finally, the amygdala, hypothalamus and thalamus, and the cerebellum. In particular, the insula is a strategic area for autonomic control of the heart, with parasympathetic regulation located in the left insula and sympathetic regulation located contralaterally.
Significant alteration of BRS has been documented in acute stroke patients with insular lesion, with greater impairment in patients with bilateral insular involvement. Especially lesions in the left insular cortex seem to be associated with sympathetic hyperactivation, and, consequently, with increased circulating levels of catecholamines, baroreceptor dysfunction, cardiac arrhythmias, catecholamine-mediated myocardial damage (myocytolysis), immune dysregulation, and hyperglycemia. In contrast, right insular damage is associated with marked bradycardia and asystole because of reduced sympathetic tone and subsequent predominance of parasympathetic tone [1, 85, 87, 88].
Interindividual differences also depend on hemispheric dominance; an advantage has been observed for left-handed and ambidextrous people, who have a lower rate of sudden death than right-handed people. Functional lateralization is more frequent in men, whereas in women, a pattern of physiological bihemispheric activation is described. In rats, stimulation of the posterior rostral region of the left insula causes an increase in heart rate, while stimulation of the caudal region of the insula causes bradycardia; stimulation of the left insular cortex during the T wave of the cardiac cycle causes arrhythmias, QT prolongation, ST-segment elevation, and asystole. These evidence, based on preclinical research experiences, is, however, complicated by several factors in the clinical setting: first of all, ischemic or hemorrhagic lesions are very rarely limited to the insular region. Moreover, as already mentioned, other brain areas are involved in autonomic control. In general, however, it can be asserted that hemispheric stroke carries an increased risk of cardiac arrhythmias such as atrial fibrillation and sudden death [89, 90].
As mentioned, a massive release of circulating catecholamines, which is possible in conditions of acute stress of any kind, can lead to an acute cardiomyopathy known as Takotsubo syndrome or “apical ballooning,” which has significant hemodynamic repercussions as well as a potential cardioembolism due to the possible formation of intraventricular thrombotic apposition. Of note, Takotsubo can be triggered by the occurrence of a stroke, as a “stressor,” but because of the emboligenic potential, it determines it can also be the cause of a cerebral ischemic event [70].
The arterial hypertension frequently observed during the acute phase of stroke is thought to be an expression of increased sympathetic tone resulting in renin release and arteriolar vasoconstriction. It may result from direct damage to inhibitory/modulatory brain regions or indirect damage from reduced parasympathetic activation, which in turn leads to dysregulation of the baroreceptor reflex. Other contributory factors may be nitric oxide release during ischemic injury, increased intracranial pressure, and compression on the brain stem. In addition, the increase in pressure may be an attempt to compensate for the perfusion deficit that occurs in ischemic conditions, especially when the etiopathogenesis of the stroke is atherothrombotic or lacunar, whereas in cases of cardioembolism, pressures tend to be lower [91,92,93].
Sympathetic hyperactivation is also hypothesized to be the cause of neurogenic pulmonary edema, which is associated with lesions in certain ‘trigger’ areas: the hypothalamus, the bulb, cortical areas A1 and A5, the nucleus of the solitary tract and the postrema area, which are closely linked to the central control of respiration [94].
Vascular lesions in the insula have also been correlated with the immunosuppression that accompanies acute stroke in about a third of cases, and which is characterized by a systemic anti-inflammatory response with increased susceptibility to infections, especially in the respiratory and urinary systems, with a significant impact on 30-day mortality. Stroke in fact alters the balance between sympathetic and parasympathetic connections that regulate the immune system and endocrine organs, part of the hypothalamic-pituitary axis. The early occurrence of infections after acute stroke is in fact associated with hyperactivation of the adrenomedullin axis, documented by elevated circulating levels of catecholamines during the first day after stroke. Clinical severity and extent of the lesion do not seem to determine an increased risk of infection, which would be favored in the case of lesions in the territory of the anterior circulation [95].
Another form of autonomic dysfunction associated with acute stroke is urinary disorders, which affect about one-third of patients and include various forms of incontinence, either transient or persistent. The gastrointestinal tract may also show post-stroke functional alterations, with constipation, fecal incontinence, dysphagia and sialorrhea. Finally, thermoregulation is often altered in the acute phase of stroke, due to damage to both vaso- and sudomotor autonomic fibers, leading mainly to contralateral hyperhidrosis and asymmetry of thermal sensitivity [96,97,98].
5.4.4 Evaluation of Dysautonomia in Patients with Cerebrovascular Risk
In clinical practice, various invasive and noninvasive techniques are used to assess SNA. In fact, it has been carried out with the study of orthostatic PA and HR changes [99], the head-up tilt test [100], the baroreceptor sensitivity study [101], the Valsalva maneuver [100], the complete Ewing battery [102], the plasma catecholamine assay [103], and the SNA assessment [101].
Their in-depth description is beyond the scope of this chapter and is amply illustrated in the reference ones. Here, we will focus on the role of the Heart Rate Variability (HRV) assessment as the most widely used in the evaluation of SNA in patients with cerebrovascular disease. It is in fact a noninvasive test, which can be performed in patients with cerebrovascular risk factors, with stroke in both acute and chronic phases as it does not require the cooperation of the patient. It only requires the recording of an ECG trace. This allows for continuous monitoring in both hospital and outpatient settings using wearable devices. Further advantages of the method are its high reliability and reproducibility as well as its low cost; however, it cannot be applied in patients with heart rhythm disorders such as AF and frequent extrasystoles.
HRV is the variation in time of the interval between two consecutive beats; the regulation is mainly extrinsic, dependent on the activity of the SNA. Specifically, the sympathetic nervous system (SNS) causes an acceleration and the parasympathetic nervous system (SNP) a deceleration of the heartbeat, the result being the balance of the two activities.
The measurement can be easily obtained using pulse oximetry or photoplethysmography, even over long periods of time using wearable devices; however, acquisition of an ECG trace provides more accurate results.
The rhythmic contribution to heart rate variability made by the two systems, SNS and SNP, occurs at different frequencies. In particular, the SNS is associated with low frequencies (0.04–0.15 Hz), and the SNP with high frequencies (0.15–0.4 Hz). This difference makes it possible, by means of an HRV analysis, to distinguish the activity of the two branches of the SNS [104].
Two main categories of variables are obtained from the ECG recording, time domain and frequency domain. The time domain variables can be divided into short-time variability and longtime variability (less than 6/min), both calculated from the RR intervals in the recording. Several parameters can be calculated from the duration of the RR intervals in the recording (Table 5.6). Among the time domain variables, the evaluation of RMSSD is preferable because it is more sensitive and less influenced by respiratory rate, heart rate and duration of recording, and largely reflects parasympathetic activity [105].
The frequency domain variables (Table 5.7) require more complex analyses; however, they allow better discrimination between SNS and SNP activity by identifying activity in the low and high frequencies, which are related to the SNS and SNP, respectively. The LF/HF ratio expresses this relationship. However, the respiratory rate can influence these measurements and should therefore be considered in the interpretation [105].
From a clinical point of view, it should be remembered that a reduction in HRV correlates with the degree of carotid stenosis. In these patients, the real impact of carotid atherosclerotic lesions on SNA is evidenced by the partial recovery of HRV values after long-term treatment with statins [63].
In addition, a reduction in HRV has been associated with an increased risk of AF and in diabetic patients, a reduction in the LF/HF ratio, a marker of predominant sympathetic activity, has been associated with an increase in AF episodes [59].
Although lacking definitive evidence, HRV assessment shows promise in both identifying patients at increased risk of cerebrovascular events in selected populations and in post-event monitoring. Indeed, reduced values of the time and frequency domain parameters are associated with an increased risk of ischemic stroke, particularly in diabetic patients [106]. In addition, low nocturnal SDNN values seem to be predictive of a first ischemic event, independently of traditional vascular risk factors, and therefore their integration into individual patient risk assessment could allow a more personalized therapeutic approach [107]. Low HRV values have been associated with the occurrence of early complications such as in-hospital mortality, post-stroke infections and motor function recovery [108].
From the point of view of the risk of early recurrence, TIA, and minor stroke patients with low SDNN values showed an increased risk of ischemic recurrence at 90 days. These data might suggest the integration of HRV measurement into risk stratification models, allowing a better identification of high-risk patients, both to modulate secondary prevention therapy and to identify a possible target population for new therapeutic trials. High post-event SDNN values have been associated with improved functional recovery at 90 days in patients with ischemic stroke, suggesting a possible contribution of post-stroke dysautonomia in the development of an unfavorable functional outcome [109].
5.4.5 Therapeutic Perspectives
It follows that in the quantification of cerebrovascular risk both the metabolic syndrome and indicators of the state of activation of the sympathetic nervous system such as HRV should be introduced to better define the long-term and short-term risk profile and consequently adjust the type and timing of therapeutic interventions. For example, at HRV assessment, a reduction in the values of the time and frequency domain parameters has been associated with an increased risk of ischemic stroke, particularly in diabetic patients [106]. In addition, low nocturnal SDNN values have been found to be associated with the development of a first ischemic event, independent of traditional vascular risk factors and therefore could be integrated with this in individual patient risk assessment [107]. From the point of view of the risk of early recurrence, patients with TIA and minor stroke with low SDNN values showed an increased risk of ischemic recurrence at 90 days. These data might suggest the integration of HRV measurement into risk stratification models, allowing a better identification of high-risk patients, both to modulate secondary prevention therapy and to identify a possible target population for new therapeutic trials. High values of post-stroke SDNN have been associated with better functional recovery at 90 days in patients with ischemic stroke, suggesting a possible contribution of post-stroke dysautonomia in the development of an unfavorable functional outcome [109]. Low HRV values have also been associated with early complications such as intra-hospital mortality, post-stroke infections and motor function recovery [108].
Although, to date, there is no conclusive data on therapies aimed at modulating the autonomic nervous system, the evidence in favor of a contribution of dysautonomia, with sympathetic hyperactivity, in the development and worsening of vascular risk factors, as well as the deleterious effects of sympathetic hyperreactivity in the acute phase, makes SNP stimulation and SNS blockade interesting therapeutic possibilities.
The effect of SNP stimulation can lead to CNS neuroplasticity, anti-inflammatory, antioxidant and anti-apoptotic effects through activation of the α7nAchR cholinergic receptor. In animal models, both invasive and noninvasive stimulation of the vagus nerve (VNS) has been shown to [59, 110]:
-
Reduce systemic inflammation.
-
Reducing appetite and body weight.
-
Significantly reduce the volume of the ischemic core.
If these are confirmed in humans, vagal stimulation may be an interesting therapeutic strategy for both the prevention and acute treatment of ischemic stroke.
Two randomized pilot studies showed the benefit of VNS combined with rehabilitation in the recovery of moderate to severe upper limb motor deficits. The same favorable effect on motor recovery was obtained by transcutaneous stimulation, making the procedure easier and more tolerable [111]. However, evidence on efficacy is preliminary and needs to be confirmed, as does the safety of stimulation, particularly regarding possible cardiological side effects [110].
References
Al-Qudah ZA, Yacoub HA, Souayah N. Disorders of the autonomic nervous system after hemispheric cerebrovascular disorders: an update. J Vasc Interv Neurol. 2015;8(4):43–52.
Benarroch E, Biaggioni I. Central autonomic control primer on the autonomic nervous system. San Diego, CA: Academic Press; 2012. p. 9–12.
CB Knowledge. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Ann Rev Neurosci. 2002;25:433–69.
Cechetto DF. Central representation of visceral function. Fed Proc. 1987;46:17–23.
Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol. 1998;54:149.
Cheyuo C, Jacob A, Wu R, Zhou M, Coppa GF, Wang P. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31:1187–95.
Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238.
Kaplan NM. Management of hypertensive emergencies. Lancet. 1994;344:1335.
Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1:507–10.
Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:1598–614.
Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–92.
Wagner EM, Traystman RJ. Hydrostatic determinants of cerebral perfusion. Crit Care Med. 1986;14:484–90.
Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–9. https://doi.org/10.1161/01.STR.26.6.1014.
Birch AA, Dirnhuber MJ, Hartley-Davies R, Iannotti F, Neil-Dwyer G. Assessment of autoregulation by means of periodic changes in blood pressure. Stroke. 1995;26:834–7. https://doi.org/10.1161/01.STR.26.5.834.
Claassen JAHR, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications and autoregulation. Physiol Rev. 2021;101:1487–559.
Goadsby PJ. Autonomic nervous system control of the cerebral circulation. In: Buijs RM, Swaab DF, editors. Handbook of Clinical Neurology, Vol. 117 (3rd series) Autonomic Nervous System. Elsevier B.V.: Amsterdam; 2013.
Giovannitti JA Jr, Thoms SM, Crawford. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62(1):31–8.
Dacey RG, Duling BR. Effect of norepinephrine on penetrating arterioles of rat cerebral cortex. Am J Phys. 1984;246:H380–5.
Rosendorff C, Mitchell G, Mitchell D. Adrenergic innervation affecting local cerebral blood flow. In: Owman C, Edvinsson L, editors. Neurogenic control of brain circulation: Werner-Gren center international symposium series, vol. 30. Oxford: Pergamon; 1977. p. 455–64.
Sandor P. Nervous control of the cerebrovascular system: doubts and facts. Neurochem Int. 1999;35:237–59.
Sercombe R, Hardebo JE, Kahrstrom J, Seylaz J. Amine-induced responses of pial and penetrating cerebral arteries: evidence for heterogeneous responses. J Cereb Blood Flow Metab. 1990;10:808–18.
Edvinsson L, Owman C, Sjoberg NO. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 1976;115:377–93.
Willie CK, Tzeng Y-C, Fisher JA, Ainsli PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592(5):841–59.
ter Laan M, et al. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth. 2013;111:361–7. https://doi.org/10.1093/bja/aet122.
Faraci FM, et al. Cerebral circulation: effects of sympathetic nerves and protective mechanisms during hypertension. Circ Res. 1987;61:102–6.
Marcus ML, Heistad DD. Effects of sympathetic nerves on cerebral blood flow in awake dogs. Am J Physiol Heart Circ Physiol. 1979;236:H549–53. https://doi.org/10.1152/ajpheart.1979.236.4.H549).
Micieli G, et al. Intracerebral vascular changes induced by cold pressor test: a model of sympathetic activation. Neurol Res. 1994;15:163–7.
Roatta S, et al. Effect of generalised sympathetic activation by cold pressor test on cerebral haemodynamics in healthy humans. J Auton Nerv Syst. 1998;71:159–66.
Lewis NC, Smith KJ, Bain AR, Wildfong KW, Numan T, Ainslie PN. Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (Lond). 2015;129:169–78.
Tymko MM, Richards CA, Skow RJ, Ingram-Cotton NC, Howatt MK, Day TA. The effects odf superimposed tilt and lower body negative pressure on anterior and posterior cerebral circulation. Physiol Rep. 2016;4(17):e12957. https://doi.org/10.14814/phy2.12957.
Tzeng YC, Willie CK, Atkinson G, Lucas SJ, Wong A, Ainslie PN. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension. 2010;56(2):268–73.
D’Alecy LG, Rose CJ. Parasympathetic cholinergic control of cerebral blood flow in dogs. Circ Res. 1977;41:324–31. https://doi.org/10.1161/01.res.41.3.324.
Hamner JW, et al. Cholinergic control of the cerebral vasculature in humans. J Physiol. 2012;590:6343–52. https://doi.org/10.1113/jphysiol.2012.245100.
Faraci FM, Brian JE Jr. Nitric oxide and the cerebral circulation. Stroke. 1994;25:692–703. https://doi.org/10.1161/01.STR.25.3.692.
Sakas DE, et al. Trigeminovascular fibers increase blood flow in cortical gray matter by axon reflex-like mechanisms during acute severe hypertension or seizures. Proc Natl Acad Sci U S A. 1989;86:1401–5. https://doi.org/10.1073/pnas.86.4.1401.
Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 2002;12:358–70.
Iadecola C. Hypertension and dementia. Hypertension. 2014;64:03–5.
Federico A, Di Donato I, Bianchi S, Di Palma C, Taglia I, et al. Hereditary cerebral small vessel diseases: a review. J Neurol Sci. 2012;322:25–30.
Brassard P, Tymko MM, Ainslie PN. Sympathetic control of the brain circulation: appreciating the complexities to better understand the controversy. Auton Neurosci Bas Clin. 2017;207:37–47. https://doi.org/10.1016/j.autneu.2017.05.003.
Uryga A, Nasr N, Kasprowicz M, Budohoski K, Sykora M, Smielewski P, Burzyńska M, Czosnyka M. Relationship between baroreflex and cerebral autoregulation in patients with cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Front Neurol. 2022;12:740338.
Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001.
Micieli G, Cavallini A. The autonomic nervous system and ischemic stroke: a reciprocal interdependence. Clin Auton Res. 2008;18:308–17.
Jefferson G. Isolated oculomotor palsy caused by intra- cranial aneurysm. Proc R Soc Med. 1947;40(8):419–32.
Martin TJ. Horner syndrome: a clinical review. ACS Chem Neurosci. 2018;9(2):177–86.
Drexler I, Traenka C, von Hessling A, Gensicke H. Internal carotid artery dissection and asymmetrical facial flushing: the harlequin sign. Stroke. 2014;45(5):e78–80.
Oppenheimer S. Striking reciprocity. Clin Auton Res. 2008;18(6):296–7.
Guan L, Collet J-P, Mazowita G, Claydon VE. Autonomic nervous system and stress to predict secondary ischemic events after transient ischemic attack or minor Stroke: possible implications of heart rate variability. Front Neurol. 2018;9:90.
Curtis BM, O’Keefe JH. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc. 2002;77(1):45–54.
Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17(5):350–64.
Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition, and health. J. Wiley: Chichester; 1988. p. 629–49.
Suvarnaa B, Suvarnaa A, Phillips R, Justerc R-P, McDermott B, Sarnyaia Z. Health risk behaviours and allostatic load: a systematic review. Neurosci Biobehav Rev. 2020;108:694–711.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome. Circulation. 2009;120:1640–5.
Licht CMM, de Geus EJC, Penninx BWJH. Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(6):2484–93.
Anderson EA, Hoffmann RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–52.
Masuo K, Mikami H, Ogihara T, Tuck ML. Sympathetic nerve hyperactivity precedes hyperinsulinemia and blood pressure elevation in a young, nonobese Japanese population. Am J Hypertens. 1997;10:77–83.
Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, Reid J, Van Zwieten PA. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–20.
Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114(11):1804–14.
Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10(5):457–66.
Carandina A, Lazzeri G, Villa D, et al. Targeting the autonomic nervous system for risk stratification, outcome prediction and neuromodulation in ischemic Stroke. Int J Mol Sci. 2021;22(5):2357.
Zhang F, Liu L, Zhang C, Ji S, Mei Z, Li T. Association of metabolic syndrome and its components with risk of stroke recurrence and mortality: a meta-analysis. Neurology. 2021;97(7):e695–705.
Ulleryd MA, Prahl U, Bö Rsbo J, et al. The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS One. 2017;12(4):e0174974.
Chistiakov DA, Ashwell KW, Orekhov AN, Bobryshev YV. Innervation of the arterial wall and its modification in atherosclerosis. Auton Neurosci Basic Clin. 2015;193:7–11.
Rupprecht S, Finn S, Hoyer D, Guether A, Witte OW, Schultze T, Schwab M. Association between systemic inflammation, carotid arteriosclerosis, and autonomic dysfunction. Transl Stroke Res. 2020;11(1):50–9.
Fodor DM, Marta MM, Perju-Dumbravă L. Implications of circadian rhythm in Stroke occurrence: certainties and possibilities. Brain Sci. 2021;11(7):865.
Riganello F, Prada V, Soddu A, di Perri C, Sannita WG. Circadian rhythms and measures of CNS/autonomic interaction. Int J Environ Res Public Health. 2019;16(13):2336.
Do LC, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34(10):2475–81.
Mostofsky E, Laier E, Levitan EB, Rosamond WD, Schlaug G, Mittleman MA. Physical activity and onset of acute ischemic stroke: the stroke onset study. Am J Epidemiol. 2011;173(3):330–6.
Anderson C, Ni Mhurchu C, Scott D, Bennett D, Jamrozik K, Hankey G. Triggers of subarachnoid hemorrhage: role of physical exertion, smoking, and alcohol in the Australasian cooperative research on subarachnoid hemorrhage study (ACROSS). Stroke. 2003;34(7):1771–6.
Guiraud V, Touzé E, Rouillon F, Godefroy O, Mas J-L. Stressful life events as triggers of ischemic stroke: a case-crossover study. Int J Stroke. 2013;8(5):300–7.
Ranieri M, Finsterer J, Bedini G, Parati EA, Bersano A. Takotsubo syndrome: clinical features, pathogenesis, treatment, and relationship with cerebrovascular diseases. Curr Neurol Neurosci Rep. 2018;18(5):20. https://doi.org/10.1007/s11910-018-0833-7.
Templin C, Ghadri J, Diekmann J, Napp C, Bataiosu D, Jaguszewski M, Cammann V, Sarcon A, Geyer V, Neuman C, Seifert B, Hellermann J. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. NEJM. 2015;373(10):929–38.
Brunner S, Winter R, Werzer C, von Stülpnagel L, Clasen I, Hameder A, Stöver A, Graw M, Bauer A, Sinner MF. Impact of acute ethanol intake on cardiac autonomic regulation. Sci Rep. 2021;11(1):13255.
Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7(1):45–59.
Fisher BAC, Ghuran A, Vadamalai V, Antonios TF. Cardiovascular complications induced by cannabis smoking: a case report and review of the literature. Emerg Med J. 2005;22(9):679–80.
Lavados PM, Olavarría VV, Hoffmeister L, Ambient Temperature and Stroke Risk. Evidence supporting a short-term effect at a population level from acute environmental exposures. Stroke. 2018;49:255–61.
Bahouth MN, Venkatesan A. Acute viral illnesses and ischemic Stroke pathophysiological considerations in the era of the COVID-19 pandemic. Stroke. 2021;52:1885–94.
Chen P-L, Kuo TBJ, Hang CCH. Parasymapthetic activity correlates with early outcome in patients with large artery atherosclerotic stroke. J Neurol Sci. 2012;314(1–2):57–61.
Hilz MJ, Moeller S, Akhundova A, Marthol H, Pauli E, De Fina P, Schwab S. High NIHSS values predict impairment of cardiovascular autonomic control. Stroke. 2011;42(6):1528–33.
Xiong L, Leung HW, Chen XY, Leung WH, Soo OY, Wong KS. Autonomic dysfunction in different subtypes of post-acute ischemic stroke. J Neurol Sci. 2014;337(1-2):141–6.
Meglic B, Kobal J, Psredkar J, Pogacnik T. Autonomic nervous system function in patients with acute brainstem stroke. Cerebrovasc Dis. 2001;11(1):2–8.
Ha SY, Park KM, Kim SE, Lee BI, Shin KJ. Autonomic function in progressive lacunar infarction. Acta Neurol Scand. 2018;138:1–9.
Hase Y, Polvikoski TM, Firbank MJ, Craggs LJL, Hawthorne E, Platten C, Stevenson W, Deramecourt V, Ballard C, Kenny RA, Perry RH, Ince P, Carare RO, Allan LM, Hosburgh L, Kalaria RN. Small vessel disease pathological changes in neurodegenerative and vascular dementias concomitant with autonomic dysfunction. Brain Pathol. 2020;30(1):191–202.
Intarhakham K, Suwanpraset K, Muengtaweepongsa S. Correlation between parasymnathetic activity and reduced cerebrovascular reactivity in patients with lacunar infarct. Curr Neurovasc Res. 2017;14(1):65–70.
Zygmunt A, Stanczyk J. Methods of evaluation of auto- nomic nervous system function. Arch Med Sci. 2010;6(1):11–8.
Sykora M, Diedler J, Rupp A, Turcani P, Steiner T. Impaired baroreceptor reflex sensitivity in acute stroke is associated with insular involvement, but not with carotid atherosclerosis. Stroke. 2009;40:737–42.
Acampa M, Lazzerini PE, Martini G. Atrial cardiopathy and sympatho-vagal imbalance in cryptogenic stroke: pathogenic mechanisms and effects on electrocardiographic markers. Front Neurol. 2018;9:1–10.
Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllyä VV. Abnormal heart rate variability as a manifestation of autonomic dysfunction in hemispheric brain infarction. Stroke1996;27:2059–2063.
Kolin A, Norris JW. Myocardial damage from acute cerebral lesions. Stroke. 1984;15:990–3.
Togha M, Sjarifpour A, Asharf H, Moghadam M, Sahraian MA. Electrocardiographic abnormalities in acute cerebrovascular events in patients with/without cardiovascular disease. Ann Indian Acad Neurol. 2013;16:66–71.
Abboud H, Berroir S, Labreuche J, et al. On behalf of the GENIC investigators. Insular involvement in brain infarction increases risk for cardiac arrhythmias and death. Ann Neurol. 2006;59:691–9.
Meyer S, Strittmatter M, Fischer C, Georg T, Schmitz B. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004;15:357–61.
Orlandi G, Fanucchi S, Strata G, Pataleo L, Landucci Pellegrini L, Prontera C, Martini A, Murri L. Transient autonomic nervous system dysfunction during hyper- acute stroke. Acta Neurol Scand. 2000;102:317–21.
Marcheselli S, Cavallini A, Tosi P, Quaglini S, Micieli G. Impaired blood pressure increase in acute cardioembolic stroke. J Hypertens. 2006;24(9):1849–56.
Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012;16(2):212.
Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–103.
Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–66.
Gelber DA, Good DC, Laven LJ, Verhulst SJ. Causes of urinary incontinence after acute hemispheric strokes. Stroke. 1993;24:378–82.
Ullman T, Reding M. Gastrointestinal dysfunction in stroke. Semin Neurol. 1996;16:269–75.
Naver HK, Blomstrand C, Wallin BG. Reduced heart rate variability after right-sided stroke. Stroke. 1996;27(2):247–51.
Ha SY, Park KM, Park J, Kim SE, Lee BI, Shin KJ. Autonomic function testing in progressive lacunar infarction et al. Acta Neurol Scand 2018;138(1):32–40.
Tang S, Xiong L, Fan Y, Mok VCT, Wong KS, Leung TW. Stroke outcome prediction by blood pressure variability, heart rate variability, and baroreflex sensitivity. Stroke. 2020;51(4):1317–20.
Xiong L, Leung HHW, Chen XY, Han JH, Leung TWH, Soo YOY, Chan AYY, Lau AYL, Wong LKS, et al. Int J Stroke. 2013;8(8):645–51.
Feibel JH, Hardy PM, Campbell RG, Goldstein MN, Joynt RJ. Prognostic value of the stress response following stroke. JAMA. 1977;238(13):1374–6.
Acharya RU, Joseph PK, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. 2006;44(12):1031–51.
Singh N, Moneghetti KJ, Christle JW, Hadley D, Plews D, Froelicher V. Heart rate variability: an old metric with new meaning in the era of using mHealth technologies for health and exercise training guidance. part one: physiology and methods. Arrhythm Electrophysiol Rev. 2018;7(3):193–8.
Fyfe-Johnson AL, Muller CJ, Alonso A, et al. Heart rate variability and incident Stroke: the atherosclerosis Risk in communities study. Stroke. 2016;47(6):1452–8.
Binici Z, Mouridsen MR, Køber L, Sajadieh A. Decreased nighttime heart rate variability is associated with increased stroke risk. Stroke. 2011;42(11):3196–201.
Lees T, Shad-Kaneez F, Simpson AM, Nassif NT, Lin Y, Lal S. Heart rate variability as a biomarker for predicting stroke, post-stroke complications and functionality. Biomark Insights. 2018;13:1177271918786931.
Li C, Meng X, Pan Y, Li Z, Wang M, Wang Y. The association between heart rate variability and 90-Day prognosis in patients with transient ischemic attack and minor Stroke. Front Neurol. 2021;12:861.
Zhao M, Gun L, Wang Y. The association of autonomic nervous system function with ischemic stroke and treatment strategies. Front Neurol. 2020;10:141.
Capone F, Miccinilli S, Pellegrino G, et al. Transcutaneous Vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after Stroke. Neural Plast. 2017;2017:1–6.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Micieli, G., Canavero, I., Mazzacane, F., Cavallini, A. (2023). Autonomic Nervous System and Cerebrovascular Diseases. In: Micieli, G., Hilz, M., Cortelli, P. (eds) Autonomic Disorders in Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-031-43036-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-43036-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43035-0
Online ISBN: 978-3-031-43036-7
eBook Packages: MedicineMedicine (R0)