Abstract
A simple and reliable technique of glow discharge ignited at 700 Pa in a mixture of methane with hydrogen (5:1) was used to synthesize 15 min 2D carbon nanosheets with a thickness of about 15 nm and size up to a few μm, as well as composite nanostructures that incorporate copper nanoparticles with a diameter of 15 to 40 nm into the carbon nanosheets. The presence of the copper species was ensured by incorporating a copper anode into the setup. The nanostructures were found in a space between the graphite sample mounted on a graphite cathode and in the dents and craters left after the preliminary stage of ion cleaning of the surfaces. As a result, it was concluded that the necessity of screening the area where the synthesis takes place by a design structure cuts off the flux of ions extracted from plasma but leaves the flux of neutral species containing the carbon and copper precursors.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Nowadays, nanostructures with various morphology [1] and chemical composition [2] were applied in several branches of science and industry because of their outstanding properties acquired at the transition of the materials from the traditional 3D morphology. A synergistic effect originated from the high chemical activity of CuO nanoparticles, and an array of carbon nanosheets with a huge surface-to-volume ratio resulted in the development of a high-performance electrochemical biosensor for glucose detection [3]. Complex hierarchical structures composed of a Pt nanostructured layer and carbon nanowalls with an optional addition of an Au layer were formed on the surface of the SiO2/Ti sample. Adding gold changed the current-voltage relations from the diode-like to the graphene-like [4]. A net of 2D carbon nanostructures was obtained in RF magnetron discharge ignited in an argon atmosphere with a mixture of hydrogen and methane. The nanowalls grown with the addition of the mixture show the increase of the electric field by three orders of magnitude concerning the walls grown just in argon atmosphere, thus making them suitable for application in field emission devices [5]. A surface structure with controlled optical properties was obtained by Pierpaoli et al. when doping the carbon nanowalls with boron; the doping is considered the main factor in reaching the transparency of the structures, while the increase in the growth temperature improves the electrical conductivity [6]. The morphology of 2D carbon nanostructures is vital for their optical and electrical properties, such as reflectance, transmittance, absorbance, conductivity, and Hall coefficient [7]. Wearable and highly sensitive pressure sensors were developed on a base of a composite 3D microstructure made of carbon nanowalls embedded in a polydimethylsiloxane matrix [8]. The compressive strength of the typical maze-like structure of carbon nanowalls can reach the value of 50 MPa with Young’s modulus of about 28 GPa, which is much higher than for other pyrolytic graphite and graphene-based materials [9]. The mechanical strength and capability to withstand the action of aggressive media are considered the main factors limiting the energy [10] and space [11] applications of nanostructures.
2 Literature Review
To meet the specifications developed for the successful implementation of the nanostructures, many production methods have been developed, including chemical, mechanical [12], and plasma techniques [13]. The later are conditioned by the flexible control of plasma fluxes [14] and the effect caused by the ions on the growing surface layer [15]. Electrodeless methods of plasma synthesis of nanostructures are recognized for the absence of contamination of the nanostructures caused by the materials sputtered from the electrodes [16]. Many techniques have been developed for the last decade based on setups that utilize single-type plasma sources, explained by the relative cost-effectiveness and simplicity [17]. 2D carbon network was grown on a surface of silicon samples in the atmosphere of Ar/H2/C2H2 mixture (1050/25/1 sccm) at the gas pressure of 100 Pa when 300 W of capacitively coupled plasma (CCP) discharge power was applied for 10 min [18]. The sample temperature was 700 ℃), promoting the growth of relatively thick (500 nm) nanowalls suitable for supercapacitor applications. Inductively coupled plasma (ICP) was used by Shoukat et al. to enhance the CVD process of synthesis of carbon nanowalls on silicon, glass, and pyrex substrates coated with Ni catalyst at the temperature of 650 ℃ and argon pressure of 2.18 Pa [19]. Chemical vapor deposition, assisted with microwave plasma (MPECVD), was implemented by Kwan et al. to produce a gas sensor with carbon nanowalls engaged as the sensitive element [20]. For the growth of the carbon nanostructures, the mixture of methane and hydrogen at a ratio of 2:1 at the total gas pressure of 65 Pa and the microwave power of 1300 W was applied for 10 min at the substrate temperature of 600 ℃. At that, the average height of the nanowalls reached the value of 1 μm at the distance between them of about 200–300 nm. MPECVD was also implemented at 1300 W to grow carbon 2D nanostructures in the mixture of CH4 and hydrogen H2 gases (1:1) at 7 Pa for 10 min for acetone sensing [21]. The ratio of the carbon precursor gas (methane, acetylene, etc.) and discharge power are considered the main control parameters. In the experiments conducted by Batryshev et al., radio-frequency (RF) capacitively coupled plasma (CCP) discharge was ignited to obtain an array of carbon nanowalls on a silicon substrate with nickel catalyst deposited on the substrate surface [22]. For the fixed Ar flow (7 sccm), methane CH4 was introduced into the chamber at the flows of 0.7 to 1 sccm, and the synthesis time varied from 10 to 30 min. At the fixed gas pressure of 250 Pa and substrate temperature of 430 ℃, the discharge power was changed independently in the range of 8 to 15 W. It was shown that low power (1–7 W) results in formation of nanotubes; intermediate power (8–15 W) leads to formation of the carbon nanowalls; high power (20–25 W) allows obtaining the multi-layered graphene, while further increasing of the CCP discharge power forms nanoclusters from the nanowalls. At that, the increase in the methane flow rate improves the conditions of the nanowall formation when applied to the low and intermediate power modes. Pulsed filtered cathodic vacuum arc deposition (PFCVAD) was employed by Tüzemena et al. at the pressure of 0.35 Pa to grow the carbon nanowalls that exhibited low reflectance in visible light and are considered for optical applications [23].
However, the characterization and implementation of the developed structures show insufficiency of the existing setups. Thus, bombardment of a substrate surface with energetic ions is the characteristic property of plasma discharges. Implementing large energies like 500–1000 eV leads to drastic thickening of 2D structures, thus turning them into a net of 3D structures, which still can have advanced properties [24]. That is why more sophisticated methods were developed. A review of the growth methods is carried out by Vesel et al., and the problem of developing a reliable method of production of carbon nanowalls is stressed [25]. One of the approaches relies on the combination of different types of plasma sources. Thus, carbon nanowalls were successfully synthesized in a setup that incorporates capacitively coupled (100 MHz) and surface wave (2.45 GHz) sources to generate CH4/H2 (2:1) plasma and an inductive energy storage circuit to feed a sample holder with high-voltage pulses (~1 μs) to sustain a temperature of 650 ℃ of Si sample at the total pressure of 1 Pa in the chamber [26]. For the growth time of 290 s, the nanowalls with a height of 500 nm were obtained with a strong relation of the wall-to-wall distance to the substrate bias voltage. In the experiment, for the input voltage increase from 90 to 150 V, which corresponds to the output voltage of about 500 V and change of power from 60 to 100 W, the average wall-to-wall distance increased from 200 to 700 nm, thus proving the substrate bias being a reliable tool to control the density of 2D carbon nanostructures on the sample surface. It can be concluded from the results that the intensive ion bombardment prevents the nucleation of the nanostructures. Despite the successful implementation, the methods imply an increase in the cost of the equipment.
Another approach is improving the simple methods concerning the modification of the preliminary stages, such as deposition of additional layers or use of catalyst, implementation of screens to remove the negative effect of ion bombardment, change of gases, etc. The effect of a substrate’s chemical composition and morphology on the formation of carbon nanowalls was studied by Yerlanuly et al. [27]. It was observed that the height of the carbon nanowalls decreases at the increase of thickness of Al foil. For porous Al2O3 membrane, the wider pore size resulted in a perfect reproduction of the membrane surface by the carbon nanowalls. Copper foil is widely applied as a catalyst in synthesizing 2D carbon nanostructures because its presence favors the nucleation at the atomic steps of the Cu(111) surface [28]. Despite the comprehensive utilization of hydrogen in the processes of synthesis of carbon nanosheets, which is conditioned by the ability of this gas to create radicals from the carbon precursor gas, nitrogen was also successfully implemented in electron-cyclotron resonance (ECR) plasma discharge [29].
In the present paper, the utilization of a conventional glow discharge over a graphite cathode with the implementation of a copper anode is described, and the effect of the discharge configuration and material application is discussed. The work is considered a further development of the research [30] to design an optimal configuration of a simple and reliable plasma-based setup for producing carbon nanostructures.
3 Research Methodology
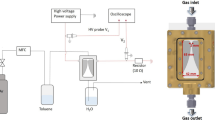
To carry out the research, a stainless-steel vacuum chamber was used. A setup comprising a disk-shaped graphite cathode with a diameter of 35 mm and a thickness of 6 mm and a copper anode with a diameter of 15 mm and 5 mm was designed. The anode was grounded, as well as the vacuum chamber walls. The distance between the electrodes was set to 30 mm. Graphite sample with a diameter of 8 mm and a height of 5 mm was arranged in the center of the cathode. The chamber was filled with a mixture of methane CH4 and hydrogen H2 at a ratio of 5:1 at a total gas pressure of 700 Pa. A schematic of the setup is shown in Fig. 1.
The discharge was sustained at the voltage between the electrodes of 900 V and a discharge current of 0.1 A. At that, the temperature of the sample reached the value of 650 ℃. Before achieving the stable mode of the glow discharge, unipolar arcs were generated on the surface of the sample, resulting in craters on the sample surface. After the plasma treatment in the stable operation mode for 15 min, the sample was cooled in the chamber and then studied using scanning electron microscopy (SEM) (Fig. 2).
4 Results and Discussion
SEM images obtained for the surfaces of the sample and cathode exposed to the direct action of plasma showed no traces of nanostructures. However, the surfaces of the sample and cathode that contacted each other, i.e., the bottom part of the sample and the part of the cathode covered by the sample, revealed the presence of the nanostructures found in the dent of the surfaces.
SEM images of the surface of the graphite cathode located under the sample are shown in Fig. 3. A general view (Fig. 3, a) of nanostructures found under the edge of the sample in the space that was screened from the plasma exposes the film-like 2D carbon nanostructures and copper microparticles, which are incorporated into a net-like folded microstructure with a thickness of the carbon sheet of about 13.5 nm (Fig. 3, c). In contrast, the surface of the cathode exposed to the direct flux of plasma particles (not covered by the sample) resembles the bulky nanostructures usually observed after the treatment by direct ion flux in ECR plasma setups with a lot of copper droplets probably generated either under the action of the unipolar arcs or as a result of nucleation of the material sputtered from the anode (Fig. 3, d). At the same time, 2D carbon nanostructures are also found in a crater left on the cathode near the edge of the sample (Fig. 3, e) with the thickness of the nanostructures of 15.5 nm and size of about 5 μm (Fig. 3, f).
SEM image of a surface area of the graphite cathode located under the sample that was processed for 15 min in methane-hydrogen (5:1) plasma (700 Pa, 900 V, and 0.1 A): a – general view of nanostructures under the edge of the sample, which exposes film-like 2D carbon nanostructures and copper microparticles; b – magnified view of the nanostructures; c – thickness of the nanostructures of 13.5 nm; d – the surface of the cathode exposed to the direct flux of plasma particles (not covered by the sample); e – 2D carbon nanostructures found in a crater left on the cathode near the edge of the sample; b – a magnified view that reveals the thickness of 15.5 nm of 2D nanostructures.
SEM images of the bottom surface of the edge of the graphite sample, which is contacted with the cathode surface, are shown in Fig. 4.
In this part, the nanostructures differ slightly from those found on the cathode counterpart of the growth area. The size of 2D carbon nanostructures is smaller, and they are sparser, as it is shown in Fig. 4, a. At the same time, the structures are folded with large flat parts of about 1 μm in width and clearly distinguished edges (Fig. 4, b). Moreover, the nanosheets with sizes of about 2 μm (Fig. 4, c) were densely covered (~300 particles/μm2) with the array of copper nanoparticles of 15 to 40 nm in diameter (Fig. 4, d).
SEM images of the nanostructures found on the edge of the bottom part of the sample treated for 15 min in methane-hydrogen (5:1) plasma (700 Pa, 900 V, and 0.1 A): a – general view; b – a magnified view, which shows the folded structure of the whole 2D nanosheet with the size of the fragments of about 1 μm; c – enlarged view of 2D carbon nanostructures with a size of about 2 μm covered with copper nanoparticles; d – a magnified view showing the diameters of the copper nanoparticles of 15 to about 40 nm.
These findings allow suggesting the following mechanism of formation of the nanostructures. The surfaces of the sample and cathode exposed to the glow discharge plasma undergo the action of plasma ions accelerated in the cathode DC sheath up to the energies of 900 eV, which far exceed the threshold where the sputtering equals the deposition rate. Thus, the intense ion bombardment supposedly inhibits the nanostructure growth. However, for the dents and craters on the surface of the negatively biased elements of the setup structure, the shadowing is the factor that cuts off the ion flux, thus leaving only the flux of copper- and carbon-containing neutrals and radicals from plasma. The latter flux can penetrate a few millimeters into the space between the cathode and sample, where the conditions benefit the nanostructure growth.
5 Conclusions
The simple and reliable glow discharge plasma ignition technique proved to be a perspective tool for synthesizing 2D nanostructures. Some limitations caused by the severe ion bombardment should be removed to expand the productivity of the process. For 15 min, dense arrays of 2D carbon nanosheets were grown on the surfaces screened by the details of the surface relief, such as dents and craters left after the preliminary stage of the ion cleaning conducted over the surfaces before the synthesis. At that, the addition of copper anode provided the growth region with a flux of catalyst, which allowed the formation of a composite material comprised of copper nanoparticles incorporated into the carbon sheet. The structure can be considered for further catalytic applications since the copper particles are protected from oxidation with the carbon layer. In the future, the proposed synthesis method is planned to update with a set of graphitic sheets located on the cathode and separated from each other by a few mm spaces to screen the nanostructure growth area from the ion flux.
References
Baranov, O., Košiček, M., Filipič, G., Cvelbar, U.: A deterministic approach to the thermal synthesis and growth of 1D metal oxide nanostructures. Appl. Surface Sci. 566, 150619 (2021)
Guo, B., et al.: Single-crystalline metal oxide nanostructures synthesized by plasma-enhanced thermal oxidation. Nanomaterials 9(10), 1405 (2019)
Zhai, Z., et al.: Rational construction of 3D-networked carbon nanowalls/diamond supporting CuO architecture for high-performance electrochemical biosensors. Small 15, 1901527 (2019)
Bita, B., et al.: On the structural, morphological, and electrical properties of carbon nanowalls obtained by plasma-enhanced chemical vapor deposition. Hindawi J. Nanomater. 2020, 8814459 (2020)
Guzmán-Olivos, F., Espinoza-González, R., Fuenzalida, V., Morell, G.: Field emission properties of carbon nanowalls prepared by RF magnetron sputtering. Appl. Phys. A 125, 354 (2019)
Pierpaoli, M., et al.: Tailoring electro/optical properties of transparent boron-doped carbon nanowalls grown on quartz. Materials 12, 547 (2019)
Yerlanuly, Y., et al.: Physical properties of carbon nanowalls synthesized by the ICP‑PECVD method vs. the growth time. Sci. Rep. 11, 19287 (2021)
Zhou, X., Zhang, Y., Yang, J., Li, J., Luo, S., Wei, D.: Flexible and highly sensitive pressure sensors based on microstructured carbon nanowalls electrodes. Nanomaterials 9, 496 (2019)
Ghodke, S., et al.: Mechanical properties of maze-like carbon nanowalls synthesized by the radial injection plasma enhanced chemical vapor deposition method. Mater. Sci. Eng. A 862, 144428 (2023)
Ruzaikin, V., Lukashov, I.: Experimental method of ammonia decomposition study based on thermal-hydraulic approach. Results Eng. 15, 100600 (2022)
Levchenko, I., et al.: Diversity of physical processes: challenges and opportunities for space electric propulsion. Appl. Sci. 12(21), 11143 (2022). https://doi.org/10.3390/app122111143
Gnytko, O., Kuznetsova, A.: Theoretical research of the chip removal process in milling of the closed profile slots. Arch. Mater. Sci. Eng. 113(2), 69–76 (2022)
Breus, A., Abashin, S., Lukashov, I., Serdiuk, O.: Catalytic growth of carbon nanostructures in glow discharge. In: Ivanov, V., Trojanowska, J., Pavlenko, I., Rauch, E., Peraković, D. (eds.) DSMIE 2022. LNME, pp. 375–383. Springer, Cham (2022). https://doi.org/10.1007/978-3-031-06025-0_37
Baranov, O., Romanov, M., Fang, J., Cvelbar, U., Ostrikov, K.: Control of ion density distribution by magnetic traps for plasma electrons. J. Appl. Phys. 112(7), 073302 (2012)
Baranov, O., Fang, J., Rider, A., Kumar, S., Ostrikov, K.: Effect of ion current density on the properties of vacuum arc-deposited TiN coatings. IEEE Trans. Plasma Sci. 41(12), 3640–3644 (2013)
Baranov, O., Filipič, G., Cvelbar, U.: Towards a highly-controllable synthesis of copper oxide nanowires in radio-frequency reactive plasma: fast saturation at the targeted size. Plasma Sources Sci. Technol. 28, 084002 (2019)
Breus, A., Abashin, S., Lukashov, I., Serdiuk, O.: Anodic growth of copper oxide nanostructures in glow discharge. Arch. Mater. Sci. Eng. 114(1), 24–33 (2022)
Guerra, A., et al.: ZnO/Carbon nanowalls shell/core nanostructures as electrodes for supercapacitors. Appl. Surface Sci. 481, 926–932 (2019)
Shoukat, R., Khan, M.I.: Synthesis of nanostructured based carbon nanowalls at low temperature using inductively coupled plasma chemical vapor deposition (ICP-CVD). Microsyst. Technol. 25, 4439–4444 (2019)
Kwon, S., et al.: Room temperature gas sensor application of carbon nanowalls using electrical resistance change by surface adsorption of toxic gases. Mater. Res. Bull. 141, 111377 (2021)
Choi, H., Kwon, S.H., Kang, H., Kim, J.H., Choi, W.: Zinc-oxide-deposited carbon nanowalls for acetone sensing. Thin Solid Films 700, 137887 (2020)
Batryshev, D., Yerlanuly, Y., Alpysbaeva, B., Nemkaeva, R., Ramazanov, T., Gabdullin, M.: Obtaining of carbon nanowalls in the plasma of radio-frequency discharge. Appl. Surface Sci. 503, 144119 (2020)
Tüzemena, E.Ş, et al.: Formation of carbon nanowalls by pulsed filtered cathodic vacuum arc deposition. Diam. Relat. Materials 93, 200–207 (2019)
Çelikel, O., Kavak, H.: Catalyst-free carbon nanowalls grown on glass and silicon substrates by ECR-MPCVD method. Diam. Relat. Mater. 120, 108610 (2021)
Vesel, A., Zaplotnik, R., Primc, G., Mozetič, M.: Synthesis of vertically oriented graphene sheets or carbon nanowalls—Review and challenges. Materials 12, 2968 (2019)
Ichikawa, T., Shimizu, N., Ishikawa, K., Hiramatsu, M., Hori, M.: Synthesis of isolated carbon nanowalls via high-voltage nanosecond pulses in conjunction with CH4/H2 plasma enhanced chemical vapor deposition. Carbon 161, 403–412 (2020)
Yerlanuly, Y., et al.: Synthesis of carbon nanowalls on the surface of nanoporous alumina membranes by RI-PECVD method. Appl. Surface Sci. 523, 146533 (2020)
Wang, M., et al.: Controllable electrodeposition of ordered carbon nanowalls on Cu(111) substrates. Mater. Today 57, 75–83 (2022)
Kar, R., Tripathy, S.P., Keskar, N., Sinha, S.: Effect of processing gas compositions on growth of carbon nanowalls by ECR-CVD process. Mater. Res. Express 6, 065029 (2019)
Breus, A., Abashin, S., Serdiuk, O.: Carbon nanostructure growth: new application of magnetron discharge. J. Achievements Mater. Manuf. Eng. 109(1), 17–25 (2021)
Acknowledgment
The research was partially sponsored by the NATO Science for Peace and Security Programme under grant id. G5814 project NOOSE. A. Breus and S. Abashin acknowledge the support from the project funded by the National Research Foundation of Ukraine under grant agreement No. 2020.02/0119.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Breus, A., Abashin, S., Serdiuk, O. (2024). Formation of 2D Carbon Nanosheets and Carbon-Shelled Copper Nanoparticles in Glow Discharge. In: Tonkonogyi, V., Ivanov, V., Trojanowska, J., Oborskyi, G., Pavlenko, I. (eds) Advanced Manufacturing Processes V. InterPartner 2023. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-42778-7_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-42778-7_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42777-0
Online ISBN: 978-3-031-42778-7
eBook Packages: EngineeringEngineering (R0)