Abstract
Vertebrobasilar dolichoectasia (VBD) is an uncommon disease characterized by significant expansion, elongation, and tortuosity of the vertebrobasilar arteries. The exact incidence rate of VBD remains unclear, it is estimated to be 0.05–0.6% of the population. The occurrence of VBD is thought to be due to the interactions of multiple factors, including congenital factors, infections and immune status, and degenerative diseases. The VBD clinical manifestations are complex which exhibits ischemic stroke predominantly, followed by progressive compression of cranial nerves and the brain stem, cerebral hemorrhage, and hydrocephalus. Treatment of VBD itself remains difficult. Currently, there are no precise and effective treatments, and available managements mainly target the complications of VBD. With the development of endovascular interventional technology, it may become an effective treatment for VBD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Uncommon disease
- Vertebrobasilar dolichoectasia
- Microvascular decompression
- Endovascular interventional technique
- Flow diversion
- Operative technique
1 Introduction
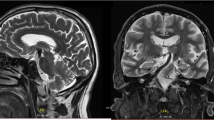
Vertebrobasilar dolichoectasia (VBD) is an uncommon disease characterized by significant expansion, elongation, and tortuosity of the vertebrobasilar arteries (Fig. 10.1a–c). Although there is no current data on the exact incidence of VBD in the general population, angiography and autopsy results suggest that the overall incidence is 0.05–0.6% [1, 2]. A Japanese study revealed that among people undergoing routine magnetic resoance imaging (MRI) and magnetic resonance angiography (MRA) examinations, the asymptomatic VBD incidence rate was 1.3% [3].
Vertebrobasilar dolichoectasia (VBD) was incidentally found in a 68-year-old man, the typical images in view of magnetic resonance imaging (MRI) and digital subtraction angiography (DSA). (a) VBD showed vascular flow voids signal (yellow arrow) on T2-weighted image. (b) Sagittal view on T1-weighted image showed compression (yellow arrow) on the pons. (c) 3D-DSA showed expansion, elongation, and tortuosity of the vertebra-basilar artery (yellow arrow)
Exact etiology of VBD is unknown; however, it is observed in association with several other diseases like atherosclerosis, hypertension, collagen vascular disease, polycystic kidney disease, and sickle cell anemia [4]. An imbalance between matrix metalloproteinase and antiprotease activity within the connective tissue of arterial wall leading to aberrant vascular remodeling and defective connective tissue formation within the wall of arteries is thought to be causative mechanism in development of this disease [4]. VBD is diagnosed incidentally in most of the cases [5]. Symptomatic patients may present with vascular symptoms like episodes of transient ischemic attacks, ischemic strokes, subarachnoid hemorrhages, obstructive hydrocephalus, or with compressive symptoms related to brainstem or cranial nerve compression [5]. The natural clinical history of patients affected by VBD is unfavorable with 7.8 years of median survival [6].
Nowadays, there are no widely accepted quantitative standards for VBD diagnostic imaging. Diagnosis of VBD usually relies on assessment of the patient’s vascular images by clinicians and radiologists. Dilatation can be diagnosed when, at any point, the basilar artery diameter is greater than 4.5 mm [7]. The MRA standards raised by Ubogu define extension by MRA as the length of basilar artery greater than 29.5 mm or the vertical distance from the connection of the basilar artery starting point and a bifurcation point greater than 10 mm. For vertebral arteries, if the length is greater than 23.5 mm, or at any point the vertical distance from the connection of the skull entry point and the basilar artery starting point is greater than 10 mm, it is considered extension [8].
2 Patient Selection and Preoperative Evaluation
Preoperative evaluation including cranial computed tomography or MRI, cerebral computed tomography angiography/MRA/digital subtraction angiography (DSA), clinical presentation, associated syndromes, general status. The clinical symptoms and neuroimaging evaluation are essential factors that determine the optimal treatment planning for each patient. Surgical therapy for VBD is indicated if the patient demonstrates as follows: (1) obvious neurological deficits caused by vascular events (ischemic or hemorrhage) or hydrocephalus; (2) associated syndromes or symptoms: brainstem compression, trigeminal neuralgia, hemifacial spasm, compression of other cranial nerves, patients had previously received medical treatment for years until the treating physician determined that surgical treatment was indicated.
3 Surgical Principles
At present, there is no universally accepted effective treatment and surgery is associated with high morbidity and mortality. For VBD itself, there is currently no precise and effective treatment, and available treatments mainly target selected symptoms or VBD complications. Endovascular flow diverter/stent placement with or without coiling may be effective in selected patients [2].
4 Surgical Therapy for VBD
A variety of treatments are available to control VBD-induced drug-refractory trigeminal neuralgia and hemifacial spasm, including radiofrequency ablation, gamma-knife, percutaneous balloon compression of the trigeminal nerve, and botulinum toxin injection. However, the most effective treatment is microvascular decompression.
The patients were positioned contralaterally after general anesthesia. The dura mater was cut after retrosigmoid craniectomy and then was suspended to release the appropriate amount of cerebrospinal fluid to provide space for operation. Through arachnoid dissection and slight elevation of the cerebellum, the neurovascular conflicts located at the trigeminal nerve root entry zone were carefully observed using an operating microscope [9].
In order to decompress the trigeminal nerve by dolichoectatic artery, two different microvascular decompression techniques were used [9]: interposition technique and transposition technique. In the interposition subgroup, the author introduced the chopped Teflon felt implant into the conflicting neurovascular area between the artery and nervous structures, thereby separating the VBD from the trigeminal nerve (Fig. 10.2a), while in the transposition subgroup, the proximal part of the vertebrobasilar artery was moved ventrally and cranially through the gap between the IX and VII–VIII nerves, and then fixed on the nearby petrous bone wall with biomedical glue (Fig. 10.2b). It is worth noting that the perforating arteries should be protected to avoid secondary damage when suspended and to avoid twisting into angles for the responsible blood vessels [10].
Intraoperative pictures of intervention method and transposition method. (a) Intervention method: the Teflon felt was inserted between the trigeminal nerve and VA. (b) Transposition method: the basilar artery was fixed on the nearby petrous dura mater by using biological glue and a space was created between the trigeminal nerve and the BA. (Zhao et al. Comparison of Microvascular Decompression and Two Isocenters Gamma Knife for the Treatment of Trigeminal Neuralgia Caused by Vertebrobasilar Dolichoectasia. Front Neurol. 2021; 12: 707985)
5 Endovascular Interventional Techniques
Flow diversion technology refers to the placement of stents in aneurysm-bearing arteries in order to reduce blood flow into the aneurysm and form venous stasis, which leads to gradual thrombosis and neointimal coverage, and the normal functioning of the surrounding arteries and perforating arteries is maintained.
The patient was taken to neurointerventional suite and placed under general anesthesia. A complete six-vessel cerebral DSA demonstrated severe dolichoectasia involving the left vertebral and the basilar arteries (Fig. 10.1c): Through a 6 French sheath, a 6F neuron guide catheter was used to catheterize the left vertebral artery. The patient was given 3000 units of heparin intravenous injection. A Marksman catheter (Covidien, CA) along with a Synchro 2 (Stryker, MI) standard microwire was navigated through the left dolichoectatic vertebral artery into the basilar artery [11]. Next, pipeline embolization device (PED) was deployed in a telescoping fashion starting from proximal part of the basilar artery down to the left vertebral artery, essentially reconstructing the entire length of the affected vertebrobasilar system. Six months later, a cerebral angiogram after the PED placement demonstrated good patency of the target vessels and there was thrombosis outside the PED and the cavity of VBD reduced (Fig. 10.3). The patient was continued on aspirin 100 mg and clopidogrel 75 mg daily.
Postoperative findings of endovascular approach. (a) Thrombus around the stent (yellow arrow) were shown in VBD in the axial images of computed tomography. (b) The cavity of the stent (yellow arrow) was shown in VBD in the axial images of MRI. (c) 3D-DSA showed the cavity of VBD (yellow arrow) reduced obviously
The advantage of releasing of stents can reduce blood flow in the aneurysm cavity, increase forward flow, straighten tortuous vessels, and increase blood supply to branching arteries. The use of antiplatelet drugs after stent implantation can reduce thrombosis and the risk of bleeding is lower compared to using antiplatelet drugs alone.
6 Postoperative Complications
After decompression surgery, most patients underwent pain relief, the postoperative complications mainly included hearing loss, taste hypoesthesia and wound infection, dry eyes, hearing loss, numbness, facial palsy, diplopia, and cerebrospinal fluid leakage [9].
After stent implantation, the most common complication in VBD is brain stem infarction, with an incidence rate as high as 22.2% [12], due to occlusion of the branching vessels, but excessive anticoagulation treatment may result in fatal bleeding risks. The combination of stent construction and anticoagulant therapy may lower the risk of artery rupture and bleeding compared to anticoagulant or antiplatelet therapy alone. Stent construction can reduce the cavity of VBD, thereby mitigating the mass effects of VBD, including cranial nerve compression and hydrocephalus, and thus deserves further clinical validation. The key to reducing the incidence of complications is to gradually change the hemodynamics in VBD. In light of this, staging surgery and minimizing the use of coils should be considered to optimize the surgery.
7 Conclusion
VBD is a complex progressive arterial disease whose pathogenesis needs further investigation. It has complex clinical manifestations, poor prognosis, and a median survival of only 7.8 years. VBD in adult likely represent an incidental finding on routine MRI. Operation for VBD was difficult, surgery should be cautious, and the surgery was used to improve the presentation of symptomatic patients and stopped the deterioration of the asymptomatic cases. Currently, there is no standard protocol for the management of VBD. However, when surgery is discussed, the surgeon should always consider his experience and institutional practice, potential operational risk as well as individual management.
References
Vanaclocha V, Herrera JM, Martinez-Gomez D, Rivera-Paz M, Calabuig-Bayo C, Vanaclocha L. Is there a safe and effective way to treat trigeminal neuralgia associated with vertebrobasilar dolichoectasia? Presentation of 8 cases and literature review. World Neurosurg. 2016;96:516–29.
Yuan YJ, Xu K, Luo Q, Yu JL. Research progress on vertebrobasilar dolichoectasia. Int J Med Sci. 2014;11(10):1039–48.
Ikeda K, Nakamura Y, Hirayama T, Sekine T, Nagata R, Kano O, Kawabe K, Kiyozuka T, Tamura M, Iwasaki Y. Cardiovascular risk and neuroradiological profiles in asymptomatic vertebrobasilar dolichoectasia. Cerebrovasc Dis. 2010;30(1):23–8.
Samim M, Goldstein A, Schindler J, Johnson MH. Multimodality imaging of vertebrobasilar dolichoectasia: clinical presentations and imaging spectrum. Radiographics. 2016;36(4):1129–46.
Prasad SN, Singh V, Selvamurugan V, Phadke RV. Vertebrobasilar dolichoectasia with typical radiological features. BMJ Case Rep. 2021;14(2):e239866.
Umana GE, Alberio N, Graziano F, Fricia M, Tomasi SO, Corbino L, Nicoletti GF, Cicero S, Scalia G. Vertebrobasilar dolichoectasia, hypoplastic third ventricle, and related biventricular hydrocephalus: case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2023;84(2):206–11.
Smoker WR, Price MJ, Keyes WD, Corbett JJ, Gentry LR. High-resolution computed tomography of the basilar artery: 1. Normal size and position. AJNR Am J Neuroradiol. 1986;7(1):55–60.
Ubogu EE, Zaidat OO. Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatry. 2004;75(1):22–6.
Zhao Z, Chai S, Wang J, Jiang X, Nie C, Zhao H. Comparison of microvascular decompression and two Isocenters gamma knife for the treatment of trigeminal neuralgia caused by vertebrobasilar dolichoectasia. Front Neurol. 2021;12:707985.
Liu J, Chen Z, Feng T, Jiang B, Yuan Y, Yu Y. Biomedical glue sling technique in microvascular decompression for trigeminal neuralgia caused by atherosclerotic vertebrobasilar artery: a description of operative technique and clinical outcomes. World Neurosurg. 2019;128:e74–80.
Tan LA, Moftakhar R, Lopes DK. Treatment of a ruptured vertebrobasilar fusiform aneurysm using pipeline embolization device. J Cerebrovasc Endovasc Neurosurg. 2013;15(1):30–3.
Wu X, Xu Y, Hong B, Zhao WY, Huang QH, Liu JM. Endovascular reconstruction for treatment of vertebrobasilar dolichoectasia: long-term outcomes. AJNR Am J Neuroradiol. 2013;34(3):583–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zuo, Y., Guo, F. (2023). Incidental Intracranial Arterial Dolichoectasia. In: Turgut, M., Guo, F., Turgut, A.T., Behari, S. (eds) Incidental Findings of the Nervous System. Springer, Cham. https://doi.org/10.1007/978-3-031-42595-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-42595-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42594-3
Online ISBN: 978-3-031-42595-0
eBook Packages: MedicineMedicine (R0)