Abstract
Clostridioides difficile is ubiquitous and is found in humans, animals and in variety of environments. The substantial overlap of ribotypes between all three main reservoirs suggests the extensive transmissions. Here we give the overview of European studies investigating farm, companion and wild animals, food and environments including water, soil, sediment, wastewater treatment plants, biogas plants, air, and households. Studies in Europe are more numerous especially in last couple of years, but are still fragmented in terms of countries, animal species, or type of environment covered. Soil seem to be the habitat of divergent unusual lineages of C. difficile. But the most important aspect of animals and environment is their role in C. difficile transmissions and their potential as a source for human infection is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Clostridioides (Clostridium) difficile is regarded mainly as an important human pathogen. Because it can colonize his natural niche, the gut, only in the absence of established gut microbiota, it seem that his natural multiplying hosts are young animals and children. As an anaerobic spore-forming bacterium, it will be transmitted from the gut into different environments. C. difficile is hence ubiquitous and can be found in humans, animals, and the environment with a great variety of transmission routes between them.
Several reviews suggest a common reservoir of the bacterium in the environment, food, and animals. In addition, the latest genomic sequencing techniques have revealed cross-transmission of C. difficile between animals and humans (Rodriguez et al. 2016; Rupnik 2007, 2010; Weese 2010; Otten et al. 2010; Hensgens et al. 2012; Rodriguez-Palacios et al. 2013; Warriner et al. 2016; Lim et al. 2020; Rivas et al. 2020; Weese 2020). Here we give the overview of studies performed to date in Europe.
2 C. difficile in Farm Animals: European Studies
Looking back to the early research on C. difficile, the presence of these bacteria in farm animals first gained attention in the 1970s. The first reference in the literature describing C. difficile in farm animals (rabbit, horse, and cow dung) and in the environment (hay, sand, and river mud) in Europe dates from 1974 (Hafiz 1974). Thereafter, other authors in different European geographic areas also confirmed the presence of C. difficile and infection in hares (France) (Dabard et al. 1979), pigs (UK) (Lysons et al. 1980; Jones and Hunter 1983), goats (UK) (Hunter et al. 1981; Borriello et al. 1983), ducks, geese, rabbits, and chickens (UK) (Borriello et al. 1983). The first report of C. difficile in cattle in Europe was published in 2008 in which bacterial toxins were found in biological samples from calves (Pirs et al. 2008).
Over the last 20 years, several studies have investigated not only the presence and the prevalence of C. difficile in different farm animal species but also the pathogenic potential of the bacterium in these animals. In addition to the interest in C. difficile as an infectious agent in livestock animals and the economic losses that it can generate, the main objective of research groups worldwide has been to demonstrate the existence of an animal reservoir and to elucidate the relationships between potential reservoirs and C. difficile infection in humans, through the genetic similarities between strains. Hence, many studies also report the potential for zoonotic spread (Table 1).
2.1 C. difficile in Pigs and Cattle
Pigs are the farm animals that have been most commonly studied in Europe in the context of infection by C. difficile, followed by cattle (Fig. 1). In cattle, the described prevalence (up to 33%) is much lower than that in pigs (up to 96%) and studies have reported between 90 and 100% toxigenic strains circulating in both types of animal farms. In cattle, several studies have addressed the possibility of age and breeding effect on C. difficile colonization in animals and therefore different types of production systems have been investigated, including production farms, fattening farms, or dairy farms (Koene et al. 2012; Romano et al. 2012a; Zidaric et al. 2012; Rodriguez et al. 2017). A recent study also suggests that the presence of C. difficile PCR ribotype 033 on different farms studied may be a direct result of inter-farm trade of calves (Bandelj et al. 2018). However, in pigs, these possible differences between types of breed have not been addressed in the literature. Only two studies report the prevalence of C. difficile on free-range pigs, but the results of the study revealed the C. difficile prevalence in this population similar to the prevalence found in intensively raised animals (Álvarez-Pérez et al. 2013, 2018).
2.2 C. difficile in Other Less Commonly Studied Farm Animals in Europe
Poultry seem to be a natural host as colonized birds are asymptomatic, the prevalence in young animals is very high, and the diversity of ribotypes within a farm is very high. Still, not many studies in Europe have explored this species on farms. Also, goats and sheep were only recently studied in respect to C. difficile. A mean prevalence of 8.6% was reported in sheep, 5.8% in goats, and 33.1% in poultry (Table 1).
As interest has increased regarding the possible zoonotic transmission of C. difficile in recent years, new studies have investigated the prevalence and epidemiology of the bacterium in animal production types that are less commonly addressed than cattle, pigs, or poultry. An investigation conducted in Italy reported a C. difficile prevalence of 3% for rabbits raised in industrial holdings for food production (Drigo et al. 2015).
2.3 Factors Associated with C. difficile Colonization in Farm Animals
Several factors, including animal species, age, microbiota, breeding effect, and seasonality have been associated with C. difficile colonization in farm animals (Fig. 2) and likely apply also for other animals. It is possible that C. difficile is better adapted to some animal hosts than to others. The reported prevalence varies strongly between different species and studies (Rodriguez et al. 2016; Table 1). Also, laboratory diagnosis of C. difficile infection in animals and the performance of commercially available methods may vary depending on the animal species (Carvalho et al. 2022).
Age is the best studied among factors associated with C. difficile carriage in farm animals. All of the studies conducted in various European countries (Álvarez-Pérez et al. 2009; Schneeberg et al. 2013a; Bandelj et al. 2018) have shown high colonization rates in newborn animals that are either considerably reduced or eliminated in adult animals. In pig production, a C. difficile prevalence of 77% of piglet litter samples and 21% of sow samples was reported (Stein et al. 2017). This reduction in infection prevalence with age has two important consequences. First, the risk of foodborne transmission from contaminated animal products during harvest is greatly reduced. Second, Clostridioides difficile infection (CDI) in adult animals is very rare; therefore, C. difficile is currently not considered a common health problem in adult farm animals.
Regarding gut microbiota composition, in Europe, some studies have evaluated changes in the intestinal microbiota with C. difficile colonization in poultry (Skraban et al. 2013), calves (Redding et al. 2021), and pigs (Proctor et al. 2021). In poultry, differences in the presence of Enterococcus cecorum, Lactobacillus gallinarum, Moniliella sp., and Trichosporon asahii were detected among C. difficile-positive and C. difficile-negative animals. Interestingly, Acidaminococcus intestini, identified for the first time as a part of the poultry intestinal microbiota in this study, was detected in high abundance in animals not colonized by C. difficile. In dairy calves, positive animals showed increased levels of Ruminococcus, Lachnoclostridium, Butyricicoccus, and Clostridium sensu stricto 2 compared to C. difficile-negative animals. In pigs, the Bacteroides, Fusobacterium, Enterobacteriaceae, and Sutterella groups were dominant in younger animals, and their abundance decreased with age. Prevotella was the dominant group in older piglets, which is negatively associated with the abundance of C. difficile in young piglets. Further studies may lead to the identification of several bacterial populations that can potentially protect hosts from CDI.
2.4 Infection vs. Carriage of C. difficile in Farm Animals
In farms, C. difficile shows a similar prevalence among animals with or without diarrhoea (Pirs et al. 2008; Álvarez-Pérez et al. 2009; Koene et al. 2012; Schneeberg et al. 2013a; Rodriguez et al. 2017; Stein et al. 2017; Bandelj et al. 2018; Mertens et al. 2022), which may indicate that the bacterium is not the main causal agent of disease, but instead, an opportunistic pathogen that worsens the clinical status and outcome of affected animals. In a recent study in Spain, more than 80% of faecal samples obtained from diarrhoeic piglets showed mixed infections, including Clostridium perfringens (C. perfringens), C. difficile, species A rotavirus, species C rotavirus, and porcine epidemic diarrhoea virus (Monteagudo et al. 2022). In piglets, C. difficile causes important economic losses in farms due to both diarrhoea and premature death as well as delays in growth and reduced weight gain (Songer 2000; Squire and Riley 2013). There are a few reports of C. difficile infection in pigs in Europe, including one study that reported an outbreak in periparturient sows in a large outdoor production unit in Croatia (Kiss and Bilkei 2005) and one case-report study of typhlocolitis and diarrhoea in piglets in Ireland (McElroy et al. 2016). In calves and poultry, C. difficile has also been proposed as a possible cause of diarrhoea, enteritis, and death (Hammitt et al. 2008; Cooper et al. 2013), although there is no evidence of outbreaks due to the bacterium in these animal species. A review of these data indicates that the incidence, clinical relevance, and pathogenesis of CDI in farm animals in Europe have not yet been elucidated.
2.5 Farm Animals and Colonization with Different C. difficile PCR Ribotypes
A great variety of C. difficile PCR ribotypes has been reported in different farm animals in Europe. Comparative international study with 12 participating European and non-European countries that included 112 strains from 13 species including farm animals has distributed strains into 50 PCR ribotypes. Some ribotypes were found across all tested species (014, 078) while some others are more likely to be associated with a given animal species (033 with cattle) (Janezic et al. 2012).
An interesting aspect is also ribotype variability within the farm. At pig farms a single PCR ribotype will be present. In cattle the variability will be greater although the number of detected types is still modest. In contrast, in poultry and rabbit farms the reported variability is very high and from 12 to 16 PCR ribotypes are found per single farm (Zidaric et al. 2008; Drigo et al. 2015).
PCR ribotype 078 is the only one that has been repeatedly reported in swine throughout different European countries and is described in several studies as the dominant type irrespective of age or diarrhoeal status (Koene et al. 2012; Rodriguez et al. 2012; Schneeberg et al. 2013a; McElroy et al. 2016; Stein et al. 2017; Krutova et al. 2018; Moloney et al. 2021). The remaining PCR ribotypes isolated from pig farms constitute a long list and include ribotypes 002, 011, 014, 015, 023, 033, 045, 126, 150, and 193; however, they have only been reported in specific studies (Avbersek et al. 2009; Hopman et al. 2011; Keessen et al. 2011b; Koene et al. 2012; Rodriguez et al. 2012; Schneeberg et al. 2013a; Noren et al. 2014; McElroy et al. 2016; Stein et al. 2017; Krutova et al. 2018).
In cattle, an even greater variety of PCR ribotypes has been isolated. PCR ribotype 078 has also been commonly detected in cattle farms in different countries in Europe (Hoffer et al. 2010; Rodriguez et al. 2012; Zidaric et al. 2012; Schneeberg et al. 2013b; Romano et al. 2018; Blasi et al. 2021). In contrast to pig farms, where isolates within the farm are clonal, at least one study on veal calves farm did not detect clonal dissemination (Zidaric et al. 2012). Calves were mostly colonized already upon the arrival to farm and two of all detected ribotypes (078 and 126) were persisting from the beginning to the last stages of the production cycle. Another PCR ribotype, 033, seems to be cattle-associated and has been described in five different studies conducted in Belgium, Germany, Switzerland, and Slovenia. Recent studies on family dairy farms revealed that the prevalence of C. difficile ribotype 033 increased linearly with the number of calves, with a close genetic relationship between farms (Bandelj et al. 2018), and that this ribotype together with ribotype 126 is more prevalent in cattle farms using digestate as a product of biogas plants (Masarikova et al. 2020). Other PCR ribotypes frequently associated with these animals are types 012 and 002, which were described in Belgium, the Netherlands, and Slovenia (Avbersek et al. 2009; Koene et al. 2012; Rodriguez et al. 2012; Zidaric et al. 2012). Other types like 015 and 020 were also isolated in specific studies (Rodriguez et al. 2017). The percentage of toxigenic strains in cattle varies between 70 and 100%, but no association between diarrhoeal status and colonization with specific PCR ribotypes has been established.
For other small ruminants such as goats and sheep, as well as poultry or rabbits, the presence of specific PCR ribotypes has not been widely described in part because there are only a few studies in Europe describing the presence of C. difficile in these animal species, and the few available studies describe a large variety composed of different types, and in other cases the studies have not carried out ribotyping characterization (Zidaric et al. 2008; Indra et al. 2009; Koene et al. 2012; Romano et al. 2012a; Avbersek et al. 2014; Candel-Pérez et al. 2021; Marcos et al. 2021) A recent study in Italy identified PCR ribotype 614 in sheep and various PCR ribotypes, such as 003, 014, and 078, among others, in rabbits (Barbanti and Spigaglia 2020) (Table 1).
2.6 Antimicrobial Susceptibility of C. difficile Isolates Isolated from Farm Animals
Drug resistance in C. difficile strains is usually associated with specific antibiotics, especially quinolones, erythromycin, and clindamycin, and with specific PCR ribotypes. In pig and cattle production, different studies have reported resistances to fluoroquinolones, ciprofloxacin, and erythromycin, especially among isolates of PCR ribotype 078 (Keessen et al. 2013; Pelaez et al. 2013), but also among PCR ribotypes 012 and 033 (Bandelj et al. 2017). Barbanti and Spigaglia (2020) reported the presence of multidrug resistant strains (to erythromycin, clindamycin, and moxifloxacin/rifampicin) in pigs and rabbits. In pork and cattle industry, the use of fluoroquinolones has also been related with the isolation of multiple antibiotic-resistant strains (Zidaric et al. 2012).
For C. difficile isolates from small ruminants, the limited available data in the literature reported antibiotic susceptibility to vancomycin, metronidazole, and moxifloxacin of all isolates obtained from goats and sheep and a possible relationship between PCR ribotype 045 and resistance to fluoroquinolones, beta-lactams, lincosamides, and macrolides (Avbersek et al. 2014).
Susceptibility to several other drugs, including antibiotics typically used for the treatment of CDI in humans like metronidazole, vancomycin or rifampicin, completely inhibited C. difficile growth (Pirs et al. 2013), which reflects no major differences in antibiotic susceptibilities between animal and human strains. In a previous study comparing human and animal isolates, the prevalence of multidrug resistant isolates, especially to erythromycin, clindamycin, and metronidazole, was found to be higher in clinical isolates (73%) than in animal isolates (30%). Resistance to erythromycin, clindamycin, or moxifloxacin was the most frequent among the animal isolates, while only 10% and 1.6% of these animal isolates showed resistance to metronidazole and rifampicin, respectively (Barbanti and Spigaglia 2020).
3 C. difficile in Companion Animals in Europe
Dogs and cats are the most studied companion animals. Taking the European studies involving dogs and cats together, the overall prevalence for C. difficile in cats is slightly lower than in dogs, but studies including cats are scarce.
In eight European studies including cats from veterinary clinics or shelters, the C. difficile prevalence ranged from 0 to 30% (2%, Al Saif and Brazier 1996; 15.7%, Koene et al. 2012; 3.7%, Schneeberg et al. 2012; 8%, Weber et al. 1989; 2.5%, Rabold et al. 2018; 16.4%, Alves et al. 2023) (Table 1). Both studies marking the prevalence borders included only a small number of 37 and 20 cats, respectively (Álvarez-Pérez et al. 2017 ; Borriello et al. 1983). A larger study on cats living in households yielded a prevalence of 2.5% (10 of 403) while another study in a more clinical setting yielded a prevalence of 16.4% (23 of 140) (Rabold et al. 2018; Alves et al. 2023).
More information is available in respect to dogs in Europe. The reported prevalence rates in the different studies range from 1.45% in dogs of a control group (1 of 74) up to 100% in puppies of one litter at certain time-points (Perrin et al. 1993; Álvarez-Pérez et al. 2015). Other reports describe C. difficile carriage rates of 3.4%–26% for dogs in different study settings (Table 1). A Germany study investigated 437 dogs in household settings and detected a carriage rate of 3.4% (15 of 437) (Rabold et al. 2018). A positivity rate of the same range 4.9% (11 of 225) was reported from Denmark where dog faecal deposits in public gardens were collected (Bjöersdorff et al. 2021). A Portuguese study with sampling from veterinary clinics and collected laboratory samples reported a prevalence of 26% (87 of 335) (Alves et al. 2023). A canine case-control study at a referral veterinary hospital in Scotland revealed 18.7% (61 of 327) (Albuquerque et al. 2021). Interestingly not only faecal samples were investigated; 24% (6 of 25) dog paws in household setting in Slovenia (Janezic et al. 2018) and nasal discharge from 4 (19%) dogs in Belgium (Rodriguez et al. 2019a) were positive for C. difficile reflecting the extraintestinal and environmental presence.
15 European studies reported PCR ribotypes in dogs and only five considered cats. Ribotypes 009, 010, 014/020, 039, and 106 are common in dogs and cats across Europe. The most frequently reported ribotypes in cats are 010, 039 or 039/2, 014 or 014/020 and 106 (Koene et al. 2012; Schneeberg et al. 2012; Álvarez-Pérez et al. 2017; Rabold et al. 2018; Alves et al. 2023). The most frequently described ribotypes in dogs are 009, 010, 012, 014, 014/020, 020, 023, 039, 056, 078, 106 (Table 1).
Factors most likely associated with C. difficile colonization in dogs and cats are age, enteric disease, antibiotic treatment, and hospitalization.
A plausible association of age and carriage rate in dogs (puppies and older animals) was reported. In puppies high prevalence up to 100% was noted in the time from 2 to 6 weeks after birth. The carriage rate in puppies markedly decreased with age and reached 3.1 and 0% at the end of the observation time (Perrin et al. 1993; Álvarez-Pérez et al. 2015). Additionally, Álvarez-Pérez et al. (2017) reported that carriage was significantly linked with age over 7 years investigating 105 dogs from 17 veterinary clinics. Rabold et al. (2018) recognized an association of C. difficile detection and treatment with antibiotics or proton pump inhibitors in small companion animals. Additionally, dogs and cats tended to be C. difficile-positive more often when the owner suffered from a chronic disease or diarrhoea (Rabold et al. 2018). A study conducted at a referral veterinary hospital in Scotland also found antibiotic treatment to be a risk factor for C. difficile carriage increasing with the length of treatment (Albuquerque et al. 2021), while other investigations could not find an association with antibiotic administration (Finsterwalder et al. 2022; Alves et al. 2023).
Despite some case reports of C. difficile infection in dogs and cats, an association with diarrhoea was not obvious in a number of studies. Regarding the available data from Europe, it seems that C. difficile does not cause disease in dogs and cats beyond single cases as similar percentages are isolated from symptomatic and healthy animals and no statistical correlation was detectable (Weber et al. 1989; Wetterwik et al. 2013; Duijvestijn et al. 2016; Albuquerque et al. 2021; Finsterwalder et al. 2022; Alves et al. 2023). Interestingly some studies with sampling scenarios involving veterinary clinics or hospitals showed higher prevalence (Albuquerque et al. 2021; Finsterwalder et al. 2022; Alves et al. 2023) than household or public park sampling scenarios (Rabold et al. 2018; Bjöersdorff et al. 2021). However, dogs and cats can harbour C. difficile strains with virulence potential (Table 1) and with exception of the longitudinal studies conducted in puppies the duration of C. difficile shedding was scarcely addressed. It is not clear whether a C. difficile carriage can be a result of a longer lasting colonization or is just connected with a short transient passage. Recently interspecies transmission of toxigenic C. difficile was reported involving a 10-month-old infant and the family dog, both with diarrhoea and without other diagnosis. The dog was reported with recurrent diarrhoea indicating a longer lasting carriage or infection (Rodríguez-Pallares et al. 2022).
In respect to antibiotic resistance, metronidazole-resistant C. difficile strains were isolated from dogs with recorded application of metronidazole (Wetterwik et al. 2013; Orden et al. 2017a) or suspected metronidazole treatment as it is commonly used for Giardia spp. infections in Italian dogs (Spigaglia et al. 2015). Metronidazole resistant isolates were also observed in Austria, Italy, Spain, and Portugal (Andrés-Lasheras et al. 2018; Barbanti and Spigaglia 2020; Finsterwalder et al. 2022; Alves et al. 2023). Recently, research on metronidazole resistance discovered a plasmid-mediated metronidazole resistance in European RT010 from humans and animals and RT020 strains from humans.
Resistance to clindamycin, erythromycin, and moxifloxacin is frequently detected while tetracycline and rifampicin resistance is rarely reported. Multidrug resistant isolates (MDR) isolates are not very frequent but geographically widespread, the resistance pattern clindamycin, erythromycin, and metronidazole was repeatedly noticed in dogs (Andrés-Lasheras et al. 2018; Barbanti and Spigaglia 2020; Bjöersdorff et al. 2021; Finsterwalder et al. 2022; Alves et al. 2023).
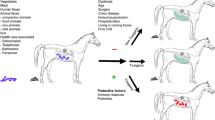
4 C. difficile in Horses in Europe
In contrast to other companion animals, horses are reported to develop C. difficile enteric disease. Foals and adult horses could be affected and outbreaks as well as sporadic cases were described. Antibiotic treatment and hospitalization have been depicted as important risk factors. C. difficile rates in horses with enteric disease were 5–63% in different studies. Healthy horses may harbour C. difficile as well; reported prevalence was ranging between 0 and 10% (reviewed in Diab et al. 2013). More recent European studies reported 0 and 1.5% in healthy and non-hospitalized horses, respectively (Kecerova et al. 2019; Schoster et al. 2019). Horses with colic and horses with diarrhoea had prevalence rates of 19% (cumulative, in three samplings) and 6.6%, respectively (Schoster et al. 2019). In a group of hospitalized horses, prevalence was 21.3% (Kecerova et al. 2019). A Swedish study found higher carriage rates of 29% in healthy foals younger than 14 days. Additionally, soil samples from stud farms contained C. difficile more frequent than soil samples from farms with mature horses. It was concluded that strains from the environment and healthy foals can serve as reservoir (Baverud et al. 2003). European studies report C. difficile in horses from Czechia, Switzerland, Slovenia, Italy, the Netherlands, and Belgium with carriage rates from 0 to 33.3% (Table 1) showing a remarkably high diversity of detected ribotypes (Avbersek et al. 2009; Koene et al. 2012; Ossiprandi et al. 2010; Rodriguez et al. 2014a, 2015; Kecerova et al. 2019; Schoster et al. 2019). Only three of these studies contain information on antibiotic resistance. In the first study conducted in Sweden, the resistance of 52 strains isolated from horses and their close environments was investigated for 10 different antibiotics. All of these strains were resistant to trimethoprim/sulphamethoxazole and bacitracin, but susceptible to metronidazole and fusidic acid. A total of 14 C. difficile strains, all of them isolated from hospitalized horses, were resistant to erythromycin and rifampicin (Baverud et al. 2003). As all of these strains were isolated from horses previously treated with erythromycin alone or in combination with rifampicin, authors suggest that erythromycin treatment probably selects the spread of this resistant pattern (Baverud et al. 2004). In a further study conducted in Belgium, antibiotic resistance was tested from ten strains isolated from hospitalized horses. All isolates displayed resistance to clindamycin and ceftiofur. Ceftiofur is one of the most commonly used antibiotics in the equine clinic (Rodriguez et al. 2014a). A Czech study investigated 18 isolates, whereof all were resistant to enrofloxacin, eight were resistant to tetracycline, five to clindamycin, and one to erythromycin and clindamycin (Kecerova et al. 2019).
5 C. difficile in Wild Animals in Europe
Limited data are available in Europe regarding the presence of C. difficile in wild animals outside of their direct or indirect relationships with livestock. In Slovenia, a study found C. difficile in barn swallows in an area identified as a barn swallow congregation point during the autumn migration of the species across Europe. The authors found an overall prevalence of 4% (4.6% (7/152) in juvenile birds and 0/23 in adults). PCR ribotypes 078, 002, and 014 were identified among a large variety of new types. The conclusions of this study focus on the possible role of barn swallows in the national and international dissemination of the bacterium (Bandelj et al. 2014). Another study also conducted in Slovenia investigated the carriage of C. difficile in migrating passerine birds by sampling cloacal specimens from animals during migration (Bandelj et al. 2011). However, in this study, none of the samples yielded a positive result for the presence of the bacterium. In the same country, a recent study described a C. difficile prevalence of 18% (4/22) in captative wild animals, including Eurasian collared dove, Tawny owl, Eurasian eagle-owl, and black stork (Zlender et al. 2022).
In Spain, the faecal shedding of C. difficile by 40 zoo animal species was investigated (Álvarez-Pérez et al. 2014). The bacterium was found with an infection prevalence of 3.5% in samples from the chimpanzee (Pan troglodytes troglodytes), dwarf goat (Capra hircus), Iberian ibex (Capra pyrenaica hispanica), and plains zebra. All isolates displayed resistance to the fluoroquinolones ciprofloxacin, enrofloxacin, and levofloxacin and belonged to PCR ribotypes 078, 039, and 110. The distribution of these PCR ribotypes typically found in farm or companion animals and humans may be explained by the close contact of zoo animals with humans and their environment as well as by continuous contact between these animals and droppings of other wild animals such as birds, which may aid in the dissemination of these common C. difficile strains. Also, in Spain, C. difficile was detected in two wild boars (prevalence of 1%) foraging in urban and peri-urban areas (Darwich et al. 2021).
In a clinical case study conducted in a zoo in Denmark, C. difficile was reported as a cause of Asian elephant enterocolitis. Molecular differences between the isolates obtained from three different elephants were not detected; thus, it was suggested that the same clone caused the outbreak. The origin of the contamination was not elucidated. The elephants were fed large quantities of broccoli, and authors hypothesized that sulforaphane, which is present in this vegetable, could have caused dysbiosis and subsequently led to CDI (Bojesen et al. 2006). However, because the same clone was present in all of the affected elephants, it is also possible that the broccoli itself was contaminated with toxigenic C. difficile; therefore, the broccoli could have been the source of contamination.
C. difficile was also investigated in zooplankton populations and associated environments at five sampling stations in the Gulf of Naples, Italy. The bacterium was detected in zooplankton samples but not in marine sediments. Many types were characterized including PCR ribotypes 009 and 066. These results demonstrated for the first time that C. difficile is also well adapted to aquatic marine populations that were not previously studied, which suggests that the bacterium could be transmitted through the ingestion of raw or undercooked seafood (Pasquale et al. 2011).
6 Transmissions Between Animals and Environment
Clostridium difficile colonizes the intestinal tract of animals, which then excrete the bacterial spores in the faeces. In this way, animals can serve as source of environmental contamination or as vectors in direct and indirect transmission. Environmental contamination will include manure and farm waste recycling (as fertilizers or biogas substrates), soil contamination (pastures), water contamination, or aerial contamination and some examples will be described in Sect. 7.
To assess the direct or indirect transmission of C. difficile by vermin in pig farms, samples of house mice, drain flies, lesser houseflies, yellow mealworms, house sparrows, and bird droppings were investigated. C. difficile prevalence ranging between 4 and 100% was reported, and PCR ribotype 078 was identified in each type of sampling. The authors concluded that vermin could be important sources of C. difficile contamination in farms (Burt et al. 2012). Similarly, a recent study conducted in north-eastern Spain reported the presence of C. difficile in pest species including rodents and pigeons in pig farms and the associated environment. Most of the characterized isolates were identified as the susceptible metronidazole and vancomycin strains, PCR ribotypes 078 and 126, which were also isolated from pigs. This study also confirmed the cross-transmission of bacterium between wild animals and production animals in farms, although the impact of this phenomenon on the epidemiology of C. difficile was not well established (Andrés-Lasheras et al. 2017). C. difficile was also detected in flies at dairy farms (Bandelj et al. 2016). In the Netherlands, a recent study reported the presence of C. difficile in rodents and insectivores in 3.2% of 347 animals tested, with a total of 13 different PCR ribotypes identified (Krijger et al. 2019). Another study also conducted in the Netherlands reported that house mice carried C. difficile with a prevalence of 35%. The authors also found that more than one third of the positive mice were colonized with C. difficile ribotypes associated with human infection (Burt et al. 2018).
In respect of dogs and cats and their role in transmission of C. difficile between companion animals and environment in Europe, nearly nothing is known, but two studies comprise interesting information. Occurrence of the same strain (Multi-locus variable number tandem repeat analysis (MLVA) and ribotype) in dogs and a cat indicating direct or indirect transmission was described in animal shelters in Germany (Schneeberg et al. 2012). Orden et al. (2017b) investigated recreational sandboxes for children and dogs within the Madrid region (Spain). Two of the most frequent ribotypes (009 and 106) were also reported in independent study in Madrid dogs (Álvarez-Pérez et al. 2017). A recent study also investigated the prevalence of C. difficile on shoe soles of veterinarians, veterinary support staff, and veterinary students at the Veterinary Faculty Campus. The prevalence found ranged from 86.7% in samples from veterinarians and 100% in samples from support staff and students. PCR ribotype 010 was the most prevalent while other common types found were identified as ribotypes 010 and 014/020. In the study, the authors highlighted the role of students’ shoes as potential vectors for the spread of the bacterium (Wojtacka et al. 2021).
7 C. difficile in Food in Europe
Foodborne zoonotic pathogens are transmitted via the consumption of contaminated food and drinking water. The possible foodborne transmission of C. difficile was reported for the first time in 1983 in Europe (Borriello et al. 1983). However, currently, the importance of C. difficile as a zoonotic disease remains largely unknown.
Food contamination routes can be various. Apparently healthy animals can carry C. difficile spores through the slaughter stage and introduce a potential risk of meat contamination during processing. Vegetables would be contaminated by manure spread or irrigation with contaminated water. Root vegetables could carry C. difficile spores often present in soil irrespective of fertilizing.
7.1 Detection of Contaminated Meats in Retail Markets
The evidence that carcass contamination occurs inside the slaughterhouse reinforces the hypothesis of the potential risk of foodborne infections linked to the ingestion of foods contaminated with C. difficile spores. A recent study in Turkey reported a high prevalence of the bacterium in cattle (33.6% (83/247)) and sheep (25.3% (78/308)) carcass samples (Hampikyan et al. 2018). In Europe, meats have been found contaminated with C. difficile with a frequency ranging from 2.3 to 7.5%, and the main PCR ribotypes identified were 078, 001, 012, 014, 015, 045, 053, 078, and 087 (Bouttier et al. 2010; Jobstl et al. 2010; De Boer et al. 2009; Rodriguez et al. 2014b; Tkalec et al. 2020) (Table 2). Nevertheless, other surveys have failed to find C. difficile in meat samples (Indra et al. 2009; Hoffer et al. 2010; De Boer et al. 2009). Some recent studies have isolated the bacterium in edible chicken giblets, gizzard samples, liver, and other chicken meats at slaughterhouse (Candel-Pérez et al. 2021). Similarly, a national food surveillance for C. difficile in Slovenia detected the presence of the bacteria in beef, pork, and poultry, with a prevalence ranging from 3.8 to 5% (Tkalec et al. 2020). The reason for the lower variety of PCR ribotypes in meat samples is not clear considering the high variety of types found in farm animal faecal samples. One possible explanation is that there are differences in the sporulation frequencies and susceptibilities to external agents among the different PCR ribotypes (Zidaric et al. 2012). This feature may contribute to the survival of only some PCR ribotypes to the final stages of the meat supply chain (i.e. distribution in retail markets). Furthermore, it is noteworthy that animals may not be the sole origin of C. difficile contamination via meat and that other sources could involve contamination during processing or in retail markets.
7.2 C. difficile in Foods Other than Meats in Europe
In Europe, only a couple of studies have addressed the presence of C. difficile in foods other than meat, such as seafood and vegetables. The prevalence reported for seafood ranges from 5.9% to more than 50% of samples showing positive results (Pasquale et al. 2011; Pasquale et al. 2012; Agnoletti et al. 2019; Tkalec et al. 2020); while the prevalence described for vegetables is slightly lower, ranging between 1.9 and 26.7% (Eckert et al. 2013; Tkalec et al. 2019, 2020; Scholtzek et al. 2022). A recent study in Slovenia points to potatoes as the vegetable most frequently contaminated by C. difficile (prevalence of 28%), followed by ginger (prevalence of 6.7%) and leaf vegetables (prevalence of 9.4%) (Tkalec et al. 2019). Also, in Germany, C. difficile was found in potatoes and salads with a prevalence of 26.7% and 1.9%, respectively (Scholtzek et al. 2022). A large study on C. difficile in potatoes in 12 European countries found a prevalence of 22.4% (33/147) and identified a total of 38 different ribotypes (Tkalec et al. 2022). Furthermore, several PCR ribotypes have been detected in these types of samples including PCR ribotypes 011/049, 014/020, 078, 001, and 015, among others, and most of these PCR ribotypes have also been associated with CDI in humans in European hospitals (Bauer et al. 2011; Agnoletti et al. 2019).
8 Studies on C. difficile in Environment in European Countries
Although the first large study including samples from non-hospital environment was done in Europe, the reports on C. difficile in environmental sources in European countries were scarce. However, in recent 5 years, the number of environmental studies increased and they often include also comparisons with animal or clinically relevant strains on genomic level (Table 3). Tested environments include water, soil, wastewater treatment plants (WWTP), biogas plants, air, sediment, manure, silage/hay, sandboxes, surfaces in public places, and households.
Unsurprisingly, WWTPs seem to be the environment with very high positivity rate and C. difficile is often detected in all tested samples either from inlet water, sewage, or effluent (Kotila et al. 2013; Steyer et al. 2015; Romano et al. 2012b; Moradigaravand et al. 2018; Cizek et al. 2022). A single study, using non-culturing method, reported positivity rate lower than 100% (Romanazzi et al. 2016). Another report from Germany also had positivity rate lower than 100% and in this case C. difficile was detected in all WWTPs associated samples except in effluent (Blau and Gallert 2023).
Rivers and sediments also have variable proportions of C. difficile-positive samples, from 41.7 to 87.5% in river samples and from none to 61.9% in sediment samples (Table 3) (Zidaric et al. 2010; Hargreaves et al. 2013; Numberger et al. 2019; Cizek et al. 2022).
Prevalence of C. difficile seems to be somewhat lower in soil. Most studies on different soil types (farm associated, domestic gardens, fields, populated areas) reported positivity rates between 30 and 50% (Janezic et al. 2016; Rodriguez et al. 2019b; Janezic et al. 2020; Marcos et al. 2021) but this can depend on soil type (Table 3). As an example, the overall prevalence in more than 500 soil samples in Sweden was 4%. While soil from public environments (parks, playgrounds, gardens, cultivated fields) showed the 4% positivity, samples from pastures and paddocks in stables with only mature horses were positive only in 1% and in stud farms at 11% (Baverud et al. 2003). Spores were detected significantly more often during winter soil sampling than during the summer sampling (Rodriguez et al. 2019b). Importantly, a long-term C. difficile persistence of almost 3 years in a single field after manure application was described (Frentrup et al. 2021).
Sandboxes, here specified as environments different than soil, showed slightly different positivity rate if they were used by children (9 positive of 20) or designated for dogs (12 positive of 20) (Orden et al. 2017b).
Another example of unequal distribution within the given environment are biogas plants. In Germany, eight plants with different substrate use (single predominate substrate which was either grass silage or cattle manure) were sampled (Froschle et al. 2015). C. difficile that was most frequently detected of all clostridia tested (44.8% of samples), followed by C. novyi (3.9% of samples); other tested species were not detected (C. botulinum, C. chauvoei, C. haemolyticum, C. septicum). Animal substrates were more likely to contain C. difficile than plant substrates (10/17; 58.8% vs. 2/44; 4.5%). Because all settings use mixed substrates (animal and plant, with predominance of one) the positivity of digested sludge was 22 of 42 samples (52.4%) and in digestion products 35 of 51 samples (68.6%).
Two European studies have detected C. difficile in air. A single study has investigated airborne spore transmission within and around a pig production farm with known high C. difficile prevalence (Keessen et al. 2011a). C. difficile was detected in all farm units except in the pregnant sow unit. The detected airborne C. difficile colony counts ranged from 2 to 625 CFU/m3. At farrowing unit pens with piglets of different age were sampled and the C. difficile spores detected in the air decreased with piglet age being highest in pens with neonatal and up to 2 weeks old piglets. Air exhausts at roofs of four different units resulted in spore counts from 6 to 120 CFU/m3, two of four air samples at 20 m distance downwind were positive while air samples up to 140 m distance were all negative. Frentrup et al. (2021) sampled the air during the manure application on the field and detected C. difficile at the distance of 20 m from the tractor, but not at 50 m or 100 m.
Strain typing was done in most of the studies (Table 3). Variety of detected ribotypes within a single environment is very large, but PCR ribotypes detected almost in every study were 014 and 010. Soil, in particular in rural but not urban areas, was shown to be natural environment for very distinctive and divergent lineages of C. difficile strains (Janezic et al. 2016). These divergent strains from cryptic clades CI-III most likely represent individual species (Knight et al. 2021). They can possess atypical toxin genes for toxin A or B and plasmid encoded binary toxin (Riedel et al. 2017; Ramírez-Vargas et al. 2018; Williamson et al. 2022). Occasionally they are detected also in patients (Janezic et al. 2015; Ducarmon et al. 2022).
Antibiotic resistance was tested in several studies (Table 3) and mainly to only few selected antibiotics. Environmental isolates are resistant to similar antibiotics as human isolates. Interestingly, nontoxic environmental strains could be more resistant than toxigenic environmental strains (Janezic et al. 2016).
9 Importance of Animals, Food, and Environment for Human Infection
The transmission of C. difficile from animal and environmental source occurs via the faecal-oral route through either direct or indirect contact with contaminated surfaces (e.g. water, foods, or faeces) or when spores are ingested. Furthermore, close contact with colonized animals may also be involved in the epidemiology of C. difficile in humans. Potential of airborne transmissions from farms and during manure application was shown (Keessen et al. 2011a; Frentrup et al. 2021). Another interesting option for spore transmissions between settings are shoes. In the households, a higher proportion of shoes in comparison to dog paws was positive on C. difficile spores (Janezic et al. 2018). Potato as one of the mostly eaten vegetable in Europe was shown to be often contaminated with C. difficile and is probably an example how spores are transmitted transnationally (Tkalec et al. 2020, 2022).
A certain proportion of C. difficile strains is very likely constantly transmitted between humans, animals, and the environment as partial overlap of ribotypes isolated from humans to those found in food, animals, or environment is well documented. A comparison of PCR ribotypes isolated in a single country during 3 year period from humans, animals, and environment showed that 11 of total 90 PCR ribotypes were shared between all three reservoirs (Janezic et al. 2012). Strains within a given ribotype still represent very heterogeneous group and whole genome sequence level is needed for identity confirmation. This was initially done in two studies, one on ribotype 078 strains in Netherlands and other on ribotype 014 strains in Australia (Knight et al. 2016; Knetsch et al. 2014). Although in both studies, identity between pig and human strains was proven, the proportion of such shared strains within the studied ribotype was very low. The recent C. difficile studies on animal and environmental strains often include also whole genome sequence comparisons and have confirmed also shared sequence types (STs) between humans, animals, and environment (Table 3).
To date, no direct infection originating from food, animal, or environmental source was described. Single study in Finland aimed at linking environmental samples from sewage and tap water to a large gastroenteritis outbreak associated with sewage contaminated drinking water (Kotila et al. 2013). Authors claimed to report for the first time that ‘waterborne transmission of C. difficile spores was possible and a potential cause of CDI during outbreak’. However, only limited number of samples was obtained either from environment or from patients (9 strains from 19 CDI patients). Only one patient and one tap water isolate showed same PCR ribotype (014). As this is the one of the most prevalent PCR ribotypes in humans, some animals, and most environments, only whole genome sequencing could confirm the true association and identity of both strains.
Impact and prevention of C. difficile foodborne transmission is an emerging issue in C. difficile field. The verified presence of C. difficile in food begets the question about the risks for consumers. If the gut microbiota is normal, intestinal colonization may be transient (i.e. in the sense that shedding can result from short-term successful bacterial colonization or from intestinal passage of the ingested dormant spores) and can occur without associated pathology. Even if the spore numbers in foods are typically low, ingestion of a small dose in combination with an altered gut microbiota may be able to trigger infection.
The spores of C. difficile are heat resistant and can survive gentle cooking of foods (70 °C) but cannot survive the same range of high temperatures as the spores of other clostridial species (Rodriguez-Palacios and Lejeune 2011). Therefore, thermal treatment (85 °C for 10 min) may be the best strategy for reducing the risk of foodborne transmission. Furthermore, thermal treatment is an easy household practice that should be emphasized because it is also useful for eliminating other pathogens present in foods. Under this scenario, special attention must be given to the presence of C. difficile in raw foods consumed directly (e.g. raw meats or fish consumed without thermal treatment), biological products (e.g. fruits or vegetables, normally grown with the help of organic fertilizers), or traditional food products in developing countries which are sometimes prepared without the appropriate hygienic procedures. In these cases, the prevalence and counts of spores may have greater importance than is currently recognized and may present an important potential risk of foodborne infection, especially in populations with gastrointestinal perturbations.
Conclusions
C. difficile reservoirs other than humans and hospitals are becoming increasingly recognized. Following the results of numerous studies in recent years on the niche and transmission of C. difficile between humans, animals, the environment and food, the bacterium is widespread in the environment, animals, and foods and should now be considered as a zoonotic pathogen. In addition, new genomic sequencing technologies have revealed the presence of clones or identical strains of C. difficile that cluster in the same lineage in the different niches discussed in this chapter. Therefore, a comprehensive ‘One Health’ approach is needed in future surveillance and control studies of C. difficile infections.
References
Abay S, Ahmed EF, Aydin F et al (2022) Presence of Clostridioides difficile in cattle feces, carcasses, and slaughterhouses: molecular characterization and antibacterial susceptibility of the recovered isolates. Anaerobe 75:102575
Agnoletti F, Arcangeli G, Barbanti F et al (2019) Survey, characterization and antimicrobial susceptibility of Clostridium difficile from marine bivalve shellfish of North Adriatic Sea. Int J Food Microbiol 298:74–80
Albuquerque C, Pagnossin D, Landsgaard K, Simpson J, Brown D, Irvine J, Candlish D, Ridyard AE, Douce G, Millins C (2021) The duration of antibiotic treatment is associated with carriage of toxigenic and non-toxigenic strains of Clostridioides difficile in dogs. PLoS One 16(5):e0245949. https://doi.org/10.1371/journal.pone.0245949
Al Saif N, Brazier JS (1996) The distribution of Clostridium difficile in the environment of South Wales. J Med Microbiol 45:133–137
Al-Saif NM, O’Neill GL, Magee JT et al (1998) PCR-ribotyping and pyrolysis mass spectrometry fingerprinting of environmental and hospital isolates of Clostridium difficile. J Med Microbiol 47:117–1121
Álvarez-Pérez S, Blanco JL, Bouza E et al (2009) Prevalence of Clostridium difficile in diarrhoeic and non-diarrhoeic piglets. Vet Microbiol 137:302–305
Álvarez-Pérez S, Blanco JL, Pelaez T et al (2013) High prevalence of the epidemic Clostridium difficile PCR ribotype 078 in Iberian free-range pigs. Res Vet Sci 95:358–361
Álvarez-Pérez S, Blanco JL, Martinez-Nevado E et al (2014) Shedding of Clostridium difficile PCR-ribotype 078 by zoo animals, and report of an unstable metronidazole-resistant isolate from a zebra foal (Equus quagga burchellii). Vet Microbiol 169:218–222
Álvarez-Pérez S, Blanco JL, Peláez T et al (2015) Faecal shedding of antimicrobial-resistant Clostridium difficile strains by dogs. J Small Anim Pract 56:190–195
Álvarez-Pérez S, Blanco JL, Harmanus C et al (2017) Prevalence and characteristics of Clostridium perfringens and Clostridium difficile in dogs and cats attended in diverse veterinary clinics from the Madrid region. Anaerobe 48:47–55
Álvarez-Pérez S, Blanco JL, Astorga RJ et al (2018) Distribution and tracking of Clostridium difficile and Clostridium perfringens in a free-range pig abattoir and processing plant. Food Res Int 113:456–464
Alves F, Castro R, Pinto M, Nunes A, Pomba C, Oliveira M, Silveira L, Gomes JP, Oleastro M (2023) Molecular epidemiology of Clostridioides difficile in companion animals: Genetic overlap with human strains and public health concerns. Front Public Health 10:1070258. https://doi.org/10.3389/fpubh.2022.1070258
Andrés-Lasheras S, Bolea R, Mainar-Jaime RC et al (2017) Presence of Clostridium difficile in pig faecal samples and wild animal species associated with pig farms. J Appl Microbiol 122:462–472
Andrés-Lasheras S, Martín-Burriel I, Mainar-Jaime RC, Morales M, Kuijper E, Blanco JL, Chirino-Trejo M, Bolea R (2018) Preliminary studies on isolates of Clostridium difficile from dogs and exotic pets. BMC Vet Res 14(1):77. https://doi.org/10.1186/s12917-018-1402-7
Avbersek J, Janezic S, Pate M et al (2009) Diversity of Clostridium difficile in pigs and other animals in Slovenia. Anaerobe 15:252–255
Avbersek J, Pirs T, Pate M et al (2014) Clostridium difficile in goats and sheep in Slovenia: characterisation of strains and evidence of age-related shedding. Anaerobe 15:252–255
Bandelj P, Trilar T, Raenik J et al (2011) Zero prevalence of Clostridium difficile in wild passerine birds in Europe. FEMS Microbiol Lett 321:183–185
Bandelj P, Trilar T, Blagus R et al (2014) Prevalence and molecular characterization of Clostridium difficile isolated from European Barn Swallows (Hirundo rustica) during migration. BMC Vet Res 10:40
Bandelj P, Blagus R, Briski F et al (2016) Identification of risk factors influencing Clostridium difficile prevalence in middle-size dairy farms. Vet Res 47:41
Bandelj P, Golob M, Ocepek M et al (2017) Antimicrobial susceptibility patterns of Clostridium difficile isolates from family dairy farms. Zoonoses Public Health 64:213–221
Bandelj P, Harmanus C, Blagus R et al (2018) Quantification of Clostridioides (Clostridium) difficile in feces of calves of different age and determination of predominant Clostridioides difficile ribotype 033 relatedness and transmission between family dairy farms using multilocus-variable-number tandem-repeat analysis. BMC Vet Res 14(1):298
Barbanti F, Spigaglia P (2020) Microbial characteristics of human and animal isolates of Clostridioides difficile in Italy : Results of the Istituto Superiore di Sanità in the years 2006-2016. Amaerobe 61:102136
Bauer MP, Notermans DW, van Benthem BH et al (2011) Clostridium difficile infection in Europe: a hospital based survey. Lancet 377:63–73
Baverud V, Gustafsson A, Franklin A et al (2003) Clostridium difficile: prevalence in horses and environment, and antimicrobial susceptibility. Equine Vet J 35:465–471
Baverud V, Gustafsson A, Franklin A et al (2004) Clostridium difficile diarrhea: infection control in horses. Vet Clin N Am Equine Pract 20:615–630
Bjöersdorff OG, Lindberg S, Kiil K, Persson S, Guardabassi L, Damborg P (2021) Dogs are carriers of Clostridioides difficile lineages associated with human community-acquired infections. Anaerobe 67:102317. https://doi.org/10.1016/j.anaerobe.2020.102317
Blasi F, Lovito C, Albini E et al (2021) Clostridioides difficile in calves in central Italy : prevalence, molecular typing, antimicrobial susceptibility and associations with antibiotic administration. Animals (Basel) 11(2):515
Blau K, Gallert C (2023) Prevalence, antimicrobial resistance and toxin-encoding genes of Clostridioides difficile from environmental sources contaminated by feces. Antibiotics (Basel, Switzerland) 12(1):162. https://doi.org/10.3390/antibiotics12010162
Bojesen AM, Olsen KE, Bectelsen MF (2006) Fatal enterocolitis in Asian elephants (Elephas maximus) caused by Clostridium difficile. Vet Microbiol 116:329–335
Borriello SP, Honour P, Turner T et al (1983) Household pets as a potential reservoir for Clostridium difficile infection. J Clin Pathol 36:84–87
Bouttier S, Barc MC, Felix B et al (2010) Clostridium difficile in ground meat, France. Emerg Infect Dis 16:733–735
Burt SA, Siemeling L, Kuijper EJ et al (2012) Vermin on pig farms are vectors of Clostridium difficile PCR-ribotypes 078 and 045. Vet Microbiol 160:256–258
Burt SA, Meijer K, Burggraaff P et al (2018) Wild mice in and around the city of Utrecht, the Netherlands, are carriers of Clostridium difficile but not ESBL-producing Enterobacteriaceae, Salmonella spp. or MRSA. Lett Appl Microbiol 67(5):513–549
Candel-Pérez C, Santaella-Pascual J, Ros-Berruezo G et al (2021) Occurrence of Clostridioides (Clostridium) difficile in poultry giblets at slaughter and in retail pork and poultry meat in Southeastern Spain. J Food Prot 84(2):310–314
Carvalho GM, Ramos CP, Lobato FCF et al (2022) Laboratory diagnosis of Clostridioides (Clostridium) difficile infection in domestic animals : a short review. Anaerobe 75:102574
Cizek A, Masarikova M, Mares J, Brajerova M, Krutova M (2022) Detection of plasmid-mediated resistance to metronidazole in Clostridioides difficile from river water. Microbiology spectrum 10(4):e0080622. https://doi.org/10.1128/spectrum.00806-22
Cooper KK, Songer JG, Uzal FA (2013) Diagnosing clostridial enteric disease in poultry. J Vet Diagn Invest 25:314–327
Dabard J, Dubos F, Martinet L et al (1979) Experimental reproduction of neonatal diarrhea in young gnotobiotic hares simultaneously associated with Clostridium difficile and other Clostridium strains. Infect Immun 24:7–11
Darwich L, Seminati C, Lopez-Olvera JR et al (2021) Detection of Beta-Lactam-Resistant Escherichia coli and toxigenic Clostridium difficile strains in wild boars foraging in an anthropization gradient. Animal (Basel) 11(6):1585
De Boer E, Zwartkruis-Nahuis A, Heuvelink A et al (2009) Clostridium difficile PCR-ribotype 078 toxinotype V found in diarrhoeal pigs identical to isolates from affected humans. Environ Microbiol 144:561–511
Diab SS, Songer G, Uzal FA (2013) Clostridium difficile infection in horses: a review. Vet Microbiol 167:42–49
Drigo I, Mazzolini E, Bacchin C et al (2015) Molecular characterization and antimicrobial susceptibility of Clostridium difficile isolated from rabbits raised for meat production. Vet Microbiol 181:303–307
Ducarmon QR, van der Bruggen T, Harmanus C, Sanders IMJG, Daenen LGM, Fluit AC, Vossen RHAM, Kloet SL, Kuijper EJ, Smits WK (2022) Clostridioides difficile infection with isolates of cryptic clade C-II: a genomic analysis of polymerase chain reaction ribotype 151. Clin Microbiol Infect. Advance online publication. https://doi.org/10.1016/j.cmi.2022.12.003
Duijvestijn M, Mughini-Gras L, Schuurman N, Schijf W, Wagenaar JA, Egberink H (2016) Enteropathogen infections in canine puppies: (Co-)occurrence, clinical relevance and risk factors. Vet Microbiol 15:115–122. https://doi.org/10.1016/j.vetmic.2016.09.006
Eckert C, Burghoffer B, Barbut F et al (2013) Contamination of ready to eat raw vegetables with Clostridium difficile in France. J Med Microbiol 62:1435–1438
Finsterwalder SK, Loncaric I, Cabal A, Szostak MP, Barf LM, Marz M, Allerberger F, Burgener IA, Tichy A, Feßler AT, Schwarz S, Monecke S, Ehricht R, Ruppitsch W, Spergser J, Künzel F (2022) Dogs as carriers of virulent and resistant genotypes of Clostridioides difficile. Zoonoses Public Health 69(6):673–681. https://doi.org/10.1111/zph.1295
Frentrup M, Thiel N, Junker V, Behrens W, Münch S, Siller P, Kabelitz T, Faust M, Indra A, Baumgartner S, Schepanski K, Amon T, Roesler U, Funk R, Nübel U (2021) Agricultural fertilization with poultry manure results in persistent environmental contamination with the pathogen Clostridioides difficile. Environ Microbiol 23(12):7591–7602. https://doi.org/10.1111/1462-2920.15601
Froschle B, Messelhäusser U, Höller C et al (2015) Fate of Clostridium botulinum and incidence of pathogenic clostridia in biogas processes. J Appl Microbiol 119:936–947
Hafiz S (1974) Clostridium difficile and its toxins. Ph.D. Thesis. Department of Microbiology, University of Leeds, UK
Hammitt MC, Bueschel DM, Keel MK et al (2008) A possible role for Clostridium difficile in the etiology of calf enteritis. Vet Microbiol 127:343–352
Hampikyan H, Bingol EB, Muratoglu K et al (2018) The prevalence of C. difficile in cattle and sheep carcasses and the antibiotic susceptibility of isolates. Meat Sci 139:120–124
Hargreaves KR, Colvin HV, Patel KV et al (2013) Genetically diverse Clostridium difficile strains harboring abundant prophages in an estuarine environment. Appl Environ Microbiol 79:6236–6243
Heise J, Witt P, Maneck C et al (2021) Prevalence and phylogenetic relationship of Clostridioides difficile strains in fresh poultry meat samples processed in different cutting plants. Int J Food Microbiol 339:109032
Hensgens MP, Keessen EC, Squire MM et al (2012) Clostridium difficile infection in the community: a zoonotic disease? Clin Microbiol Infect 18:635–645
Hoffer E, Haechler H, Frei R et al (2010) Low occurrence of Clostridium difficile in faecal samples of healthy calves and pigs at slaughter and in minced meat in Switzerland. J Food Prot 73:973–975
Hopman NEM, Oorburg D, Sanders I et al (2011) High occurrence of various Clostridium difficile PCR-ribotypes in pigs arriving at the slaughterhouse. Vet Q 31:179–181
Hunter D, Bellhouse R, Baker K (1981) Clostridium difficile isolated from a goat. Vet Rec 109:291–292
Indra A, Lassing H, Baliko N et al (2009) Clostridium difficile: a new zoonotic agent? Wein Klin Wochensr 121:91–95
Janezic S, Ocepek M, Zidaric V et al (2012) Clostridium difficile genotypes other than ribotype 078 that are prevalent among human, animal and environmental isolates. BMC Microbiol 12:48
Janezic S, Marín M, Martín A, Rupnik M (2015) A new type of toxin A-negative, toxin B-positive Clostridium difficile strain lacking a complete tcdA gene. J Clin Microbiol 53(2):692–695. https://doi.org/10.1128/JCM.02211-14
Janezic S, Potocnik M, Zidaric V et al (2016) Highly divergent Clostridium difficile strains isolated from the environment. PLoS One 11:e0167101
Janezic S, Mlakar S, Rupnik M (2018) Dissemination of Clostridium difficile spores between environment and households: Dog paws and shoes. Zoonoses Public Health. 65(6):669–674. https://doi.org/10.1111/zph.12475
Janezic S, Smrke J, Rupnik M (2020) Isolation of Clostridioides difficile from different outdoor sites in the domestic environment. Anaerobe 62:102183. https://doi.org/10.1016/j.anaerobe.2020.102183
Jobstl M, Heuberger S, Indra A et al (2010) Clostridium difficile in raw products of animal origin. Int J Food Microbiol 138:172–175
Jones MA, Hunter D (1983) Isolation of Clostridium difficile from pigs. Vet Rec 112:253
Kecerova Z, Cizek A, Nyc O, Krutova M (2019) Clostridium difficile isolates derived from Czech horses are resistant to enrofloxacin; cluster to clades 1 and 5 and ribotype 033 predominates. Anaerobe 56:17–21. https://doi.org/10.1016/j.anaerobe.2019.01.005
Keessen EC, Donswijk CJ, Hol SP et al (2011a) Aerial dissemination of Clostridium difficile on a pig farm and its environment. Environ Res 111:1027–1032
Keessen EC, van den Berkt AJ, Haasjes NH et al (2011b) The relation between farm specific factors and prevalence of C. difficile in slaughter pigs. Vet Microbiol 154:130–134
Keessen EC, Hensgens MP, Spigaglia P et al (2013) Antimicrobial susceptibility profiles of human and piglet Clostridium difficile PCR-ribotype 078. Antimicrob Resist Infect Control 2:14
Kiss D, Bilkei G (2005) A new periparturient disease in Eastern Europe, Clostridium difficile causes postparturient sow losses. Theriogenology 63:17–23
Knetsch CW, Connor TR, Mutreja A et al (2014) Whole genome sequencing reveals potential spread of Clostridium difficile between humans and farm animals in the Netherlands, 2002 to 2011. Euro Surveill 19:20954
Knight DR, Squire MM, Collins DA et al (2016) Genome analysis of Clostridium difficile PCR ribotype 014 lineage in Australian pigs and humans reveals a diverse genetic repertoire and signatures of long-range interspecies transmission. Front Microbiol 7:2138
Knight DR, Imwattana K, Kullin B, Guerrero-Araya E, Paredes-Sabja D, Didelot X, Dingle KE, Eyre DW, Rodríguez C, Riley TV (2021) Major genetic discontinuity and novel toxigenic species in Clostridioides difficile taxonomy. eLife 10:e64325. https://doi.org/10.7554/eLife.64325
Koene MGJ, Mevius D, Wagenaar JA et al (2012) Clostridium difficile in Dutch animals: their presence, characteristics and similarities with human isolates. Clin Microbiol Infect 18:778–784
Kotila SM, Pitkänen T, Brazier J et al (2013) Clostridium difficile contamination of public tap water distribution system during a waterborne outbreak in Finland. Scand J Public Health 41:541–545
Krijger IM, Meerburg BG, Harmanus C et al (2019) Clostridium difficile in wild rodents and insectivores in the Netherlands. Lett Appl Microbiol 69(1):35–40
Krutova M, Zouharova M, Matejkova J et al (2018) The emergence of Clostridium difficile PCR-ribotype 078 in piglets in the Czech Republic clusters with Clostridium difficile PCR ribotype 078 isolates from Germany, Japan and Taiwan. Int J Med Microbiol 308(7):770–775
Lim SC, Knight DR, Riley TV (2020) Clostridium difficile and one health. Clin Microbiol Infect 26(7):857–863
Lysons RJ, Hall GA, Lemcke RM et al (1980) Studies of organisms possibly implicated in swine dysentery. In: Proceedings of the 6th International Pig Veterinary Society
Marcos P, Whyte P, Rogers T et al (2021) The prevalence of Clostridioides difficile on farms, in abattoirs and in retail foods in Ireland. Food Microbiol 98:10378. https://doi.org/10.1016/j.fm.2021.103781
Marcos P, Whyte P, Burgess C, Ekhlas D, Bolton D (2022) Detection and genomic characterisation of Clostridioides difficile from spinach fields. Pathogens (Basel, Switzerland) 11(11):1310. https://doi.org/10.3390/pathogens11111310
Masarikova M, Simkova I, Plesko M et al (2020) The colonisation of calves in Czech large-scale dairy farms by clonally-related Clostridioides difficile of the sequence type 11 represented by ribotypes 033 and 126. Microorganisms 8(6):901
McElroy MC, Hill M, Moloney G et al (2016) Typhlocolitis associated with C. difficile PCR-ribotypes 078 and 110 in neonatal piglets from a commercial Irish pig herd. Ir Vet J 69:10
Mertens N, TheuB T, Köchling M et al (2022) Pathogens detected in 205 German farms with porcine neonatal diarrhea in 2017. Vet Sci 9(2):44
Moloney G, Eyre DW, Aogáin MM et al (2021) Human and porcine transmission of Clostridioides difficile ribotype 078, Europe. Emerg Infect Dis 27(9):2294–2300
Monteagudo LV, Benito AA, Lázaro-Gaspar S et al (2022) Occurrence of rotavirus A genotypes and other enteric pathogens in diarrheic suckling piglets from Spanish swine farms. Animals (Basel) 12(3):251
Moradigaravand D, Gouliouris T, Ludden C, Reuter S, Jamrozy D, Blane B, Naydenova P, Judge K, Aliyu SH, Hadjirin NF, Holmes MA, Török E, Brown NM, Parkhill J, Peacock S (2018) Genomic survey of Clostridium difficile reservoirs in the East of England implicates environmental contamination of wastewater treatment plants by clinical lineages. Microb Genom 4(3):e000162. https://doi.org/10.1099/mgen.0.000162
Noren T, Johansson K, Unemo M (2014) Clostridium difficile PCR-ribotype 046 is common among neonatal pigs and humans in Sweden. Clin Microbiol Infect 20:O2–O6
Numberger D, Riedel T, McEwen G, Nübel U, Frentrup M, Schober I, Bunk B, Spröer C, Overmann J, Grossart HP, Greenwood AD (2019) Genomic analysis of three Clostridioides difficile isolates from urban water sources. Anaerobe 56:22–26. https://doi.org/10.1016/j.anaerobe.2019.01.002
Orden C, Blanco JL, Álvarez-Pérez S et al (2017a) Isolation of Clostridium difficile from dogs with digestive disorders, including stable metronidazole-resistant strains. Anaerobe 43:78–81
Orden C, Neila C, Blanco JL et al (2017b) Recreational sandboxes for children and dogs can be a source of epidemic ribotypes of Clostridium difficile. Zoonoses Public Health 65(1):88–95
Ossiprandi MC, Buttrini M, Bottarelli E et al (2010) Preliminary molecular analysis of Clostridium difficile isolates from healthy horses in northern Italy. Comp Immunol Microbiol Infect Dis 33:e25–e29
Otten AM, Reid-Smith RJ, Fazil A et al (2010) Disease transmission model for community-associated Clostridium difficile infection. Epidemiol Infect 138:907–914
Pasquale V, Romano VJ, Rupnik M et al (2011) Isolation and characterization of Clostridium difficile from shellfish and marine environments. Folia Microbiol (Praha) 56:431–437
Pasquale V, Romano VJ, Rupnik M et al (2012) Occurrence of toxigenic Clostridium difficile in edible bivalve molluscs. Food Microbiol 31:309–312
Pelaez T, Alcala L, Blanco JL et al (2013) Characterization of swine isolates of Clostridium difficile in Spain: a potential source of epidemic multidrug resistant strains? Anaerobe 22:45–49
Perrin J, Buogo C, Gallusser A et al (1993) Intestinal carriage of Clostridium difficile in neonate dogs. Zentralbl Veterinarmed B 40:222–226
Pirs T, Ocepek M, Rupnik M (2008) Isolation of Clostridium difficile from food animals in Slovenia. J Med Microbiol 57:790–792
Pirs T, Avbersek J, Zdouc I et al (2013) Antimicrobial susceptibility of animal and human isolates of C. difficile by broth microdilution. J Med Microbiol 62:1478–1485
Proctor A, Cornick NA, Wang C et al (2021) Neonatal piglets are protected from Clostridioides difficile infection by age-dependent increase in intestinal microbial diversity. Microbiol Spectr 9(2)
Rabold D, Espelage W, Abu Sin M, Eckmanns T, Schneeberg A, Neubauer H, Möbius N, Hille K, Wieler LH, Seyboldt C, Lübke-Becker A (2018) The zoonotic potential of Clostridium difficile from small companion animals and their owners. PLoS One 13(2):e0193411. https://doi.org/10.1371/journal.pone.0193411
Ramírez-Vargas G, López-Ureña D, Badilla A, Orozco-Aguilar J, Murillo T, Rojas P, Riedel T, Overmann J, González G, Chaves-Olarte E, Quesada-Gómez C, Rodríguez C (2018) Novel Clade C-I Clostridium difficile strains escape diagnostic tests, differ in pathogenicity potential and carry toxins on extrachromosomal elements. Sci Rep 8(1):13951. https://doi.org/10.1038/s41598-018-32390-6
Redding L, Huang E, Ryave J et al (2021) Clostridioides difficile on dairy farms and potential risk to dairy farm workers. Anaerobe 69:102353
Riedel T, Wittmann J, Bunk B, Schober I, Spröer C, Gronow S, Overmann J (2017) A Clostridioides difficile bacteriophage genome encodes functional binary toxin-associated genes. J Biotechnol 250:23–28. https://doi.org/10.1016/j.jbiotec.2017.02.017
Rivas L, Dupont PY, Gilpin BJ et al (2020) Isolation and characterization of Clostridium difficile from a small survey of wastewater, food and animals in New Zealand. Lett Appl Microbiol 70(1):29–35
Rodriguez C, Taminiau B, Van Broeck J et al (2012) Clostridium difficile in young farm animals and slaughter animals in Belgium. Anaerobe 18:621–625
Rodriguez C, Avesani V, Van Broeck J et al (2013) Presence of Clostridium difficile in pigs and cattle intestinal contents and carcass contamination at slaughterhouse in Belgium. Int J Food Microbiol 166:256–262
Rodriguez C, Taminiau B, Brévers B et al (2014a) Carriage and acquisition rates of Clostridium difficile in hospitalized horses, including molecular characterization, multilocus sequence typing and antimicrobial susceptibility of bacterial isolates. Vet Microbiol 172:309–317
Rodriguez C, Taminiau B, Avesani V et al (2014b) Multilocus sequence typing analysis and antibiotic resistance of Clostridium difficile strains, including molecular characterization, multilocus sequence typing and antimicrobial susceptibility of bacterial isolates. Vet Microbiol 172:309–317
Rodriguez C, Taminiau B, Brévers B et al (2015) Faecal microbiota characterisation of horses using 16 rdna barcoded pyrosequencing, and carriage rate of clostridium difficile at hospital admission. BMC Microbiol 15:181
Rodriguez C, Taminiau B, Van Broeck J et al (2016) Clostridium difficile in food and animals: a comprehensive review. Adv Exp Med Biol 4:65–92
Rodriguez C, Hakimi DE, Vanleyssem R et al (2017) Clostridium difficile in beef cattle farms, farmers and their environment : assessing the spread of the bacterium. Vet Microbiol 210:183–187
Rodriguez C, Taminiau B, Bouchafa L, Romijn S, Van Broeck J, Delmée M, Clercx C, Daube G (2019a) Clostridium difficile beyond stools: dog nasal discharge as a possible new vector of bacterial transmission. Heliyon 5(5):e01629. https://doi.org/10.1016/j.heliyon.2019.e01629
Rodriguez C, Bouchafa L, Soumillion K, Ngyuvula E, Taminiau B, Van Broeck J, Delmée M, Daube G (2019b) Seasonality of Clostridium difficile in the natural environment. Transbound Emerg Dis 66(6):2440–2449. https://doi.org/10.1111/tbed.13301
Rodriguez-Palacios A, Lejeune JT (2011) Moist-heat resistance, spore aging, and superdormancy in Clostridium difficile. Appl Environ Microbiol 77:3085–3091
Rodriguez-Palacios A, Borgmann S, Kline TR et al (2013) Clostridium difficile in foods and animals: history and measures to reduce exposure. Anim Health Res Rev 14:11–29
Rodríguez-Pallares S, Fernández-Palacios P, Jurado-Tarifa E, Arroyo F, Rodríguez-Iglesias MA, Galán-Sánchez F (2022) Transmission of toxigenic Clostridioides difficile between a pet dog with diarrhea and a 10-month-old infant. Anaerobe 74:102519. https://doi.org/10.1016/j.anaerobe.2022.102519
Romanazzi V, Bonetta S, Fornasero S et al (2016) Assessing Methanobrevibacter smithii and Clostridium difficile as not conventional faecal indicators in effluents of a wastewater treatment plant integrated with sludge anaerobic digestion. J Environ Manag 184:170–177
Romano V, Albanese F, Dumontet S et al (2012a) Prevalence and genotypic characterization of Clostridium difficile from ruminants in Switzerland. Zoonoses Public Health 59:545–548
Romano V, Pasquale V, Krovacekb K et al (2012b) Toxigenic Clostridium difficile PCR ribotypes from wastewater treatment plants in Southern Switzerland. Appl Environ Microbiol 78:6643–6646
Romano V, Pasqualea V, Lemee L et al (2018) Clostridioides difficile in the environment, food, animals and humans in southern Italy : Occurrence and genetic relatedness. Comp Immunol Microbiol Infect Dis 59:41–46. https://doi.org/10.1016/j.cimid.2018.08.006
Rupnik M (2007) Is Clostridium difficile-associated infection a potentially zoonotic and foodborne disease? Clin Microbiol Infect 13:457–459
Rupnik M (2010) Clostridium difficile: (re)emergence of zoonotic potential. Clin Infect Dis 51:583–584
Schneeberg A, Rupnik M, Neubauer H et al (2012) Prevalence and distribution of Clostridium difficile PCR ribotypes in cats and dogs from animal shelters in Thuringia, Germany. Anaerobe 18:484–488
Schneeberg A, Neubauer H, Schmoock G et al (2013a) Clostridium difficile genotypes in piglet populations in Germany. J Clin Microbiol 51:3796–3803
Schneeberg A, Neubauer H, Schomoock G et al (2013b) Presence of Clostridium difficile PCR ribotype clusters related to 033, 078 and 045 in diarrhoeic calves in Germany. J Med Microbiol 62:1190–1198. Congress 1980, Copenhagen, p 231
Scholtzek AD, Heise J, Witt P et al (2022) Contamination of home-grown and retail vegetables with Clostridioides difficile. Anaerobe 74:102512
Schoster A, Kunz T, Lauper M, Graubner C, Schmitt S, Weese JS (2019) Prevalence of Clostridium difficile and Clostridium perfringens in Swiss horses with and without gastrointestinal disease and microbiota composition in relation to Clostridium difficile shedding. Vet Microbiol 239:108433. https://doi.org/10.1016/j.vetmic.2019.108433
Skraban J, Dzeroski S, Zenko B et al (2013) Changes of poultry faecal microbiota associated with Clostridium difficile colonisation. Vet Microbiol 165:416–424
Songer JG (2000) Infection of neonatal swine with Clostridium difficile. J Swine Health Prod 4:185–189
Spigaglia P, Drigo I, Barbanti F et al (2015) Antibiotic resistance patterns and PCR-ribotyping of Clostridium difficile strains isolated from swine and dogs in Italy. Anaerobe 31:42–46
Squire MM, Riley TV (2013) Clostridium difficile infection in human and piglets: a “One health” opportunity. Curr Top Microbiol Immunol 365:299–314
Stein K, Egan S, Lynch H et al (2017) PCR-distribution of Clostridium difficile in Irish pigs. Anaerobe 48:237–241
Steyer A, Gutiérrez-Aguirre I, Rački N et al (2015) The detection rate of enteric viruses and Clostridium difficile in a waste water treatment plant effluent. Food Environ Virol 7:164–172
Tkalec V, Janezic S, Skok B et al (2019) High Clostridium difficile contamination rates of domestic and imported potatoes compared to some other vegetables in Slovenia. Food Microbiol 78:194–200
Tkalec V, Jamnikar-Ciglenecki U, Rupnik M et al (2020) Clostridioides difficile in national food surveillance, Slovenia, 2015 to 2017. Euro Surveill 25(16):1900479
Tkalec V, Viprey V, Davis G et al (2022) Clostridioides difficile positivity rate and PCR ribotype distribution on retail potatoes in 12 European countries, January to June 2018. Euro Surveill 27(15):2100417
Tramuta C, Spigaglia P, Barbanti F, Bianchi DM, Boteva C, Di Blasio A, Zoppi S, Zaccaria T, Proroga YTR, Chiavacci L, Dondo A, Decastelli L (2021) Comparison of Clostridioides difficile strains from animals and humans: First results after introduction of C. difficile molecular typing and characterization at the Istituto Zooprofilattico Sperimentale of Piemonte, Liguria e Valle d’Aosta, Italy. Comp Immunol Microbiol Infect Dis 75:101623. https://doi.org/10.1016/j.cimid.2021.101623
Von Abercron SMM, Karlsson F, Wigh GT et al (2009) Low occurrence of Clostridium difficile in retail ground meat in Sweden. J Food Prot 72:1732–1734
Warriner K, Xu C, Habash M et al (2016) Dissemination of Clostridium difficile in food and the environment: significant sources of C. difficile community acquired infection? J Appl Microbiol 122:542–553
Weber A, Kroth P, Heil G (1989) The occurrence of Clostridium difficile in fecal samples of dogs and cats. Zentralbl Veterinarmed B 36:568–576
Weese JS (2010) Clostridium difficile in food—innocent bystander or serious threat? Clin Microbiol Infect 16:3–10
Weese JS (2020) Clostridium (Clostridioides) difficile in animals. J Vet Diagn Invest 32(2):213–221
Wetterwik KJ, Trowald-Wigh G, Fernström LL et al (2013) Clostridium difficile in faeces from healthy dogs and dogs with diarrhea. Acta Vet Scand 55:23
Williamson CHD, Stone NE, Nunnally AE, Roe CC, Vazquez AJ, Lucero SA, Hornstra H, Wagner DM, Keim P, Rupnik M, Janezic S, Sahl JW (2022) Identification of novel, cryptic Clostridioides species isolates from environmental samples collected from diverse geographical locations. Microb Genom 8(2):000742. https://doi.org/10.1099/mgen.0.000742
Wojtacka J, Wysoc B, Kocuvan A et al (2021) High contamination rates of shoes of veterinarians, veterinary support staff and veterinary students with Clostridioides difficile spores. Transbound Emerg Dis 00:1–9
Zidaric V, Zemljic M, Janezic S et al (2008) High diversity of Clostridium difficile genotypes isolated from a single poultry farm producing replacement laying hens. Anaerobe 14:325–327
Zidaric V, Beigot S, Lapajne S et al (2010) The occurrence and high diversity of Clostridium difficile genotypes in rivers. Anaerobe 16(4):371–375
Zidaric V, Pardon B, Dos Vultos T et al (2012) Different antibiotic resistance and sporulation properties within multiclonal Clostridium difficile PCR ribotypes 078, 126, and 033 in a single calf farm. Appl Environ Microbiol 78:8515–8522
Zlender T, Golob Z, Rupnik M (2022) Low Clostridioides difficile positivity rate in wild animal shelter in Slovenia. Anaerobe 77:102643
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rodriguez-Diaz, C., Seyboldt, C., Rupnik, M. (2024). Non-human Clostridioides difficile Reservoirs and Sources: Animals, Food, Environment. In: Mastrantonio, P., Rupnik, M. (eds) Updates on Clostridioides difficile in Europe. Advances in Experimental Medicine and Biology(), vol 1435. Springer, Cham. https://doi.org/10.1007/978-3-031-42108-2_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-42108-2_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42107-5
Online ISBN: 978-3-031-42108-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)