Abstract
Hypoxia-inducible factor 1 (HIF-1) is a major player in the oxygen sensor system as well as a transcription factor. HIF-1 is also associated in the pathogenesis of many brain diseases including Alzheimer’s disease (AD), epilepsy and stroke. HIF-1 regulates the expression of many genes such as those involved in glycolysis, erythropoiesis, angiogenesis and proliferation in hypoxic condition. Despite several studies, the mechanism through which HIF-1 confers neuroprotection remains unclear, one of them is modulating metabolic profiles and inflammatory pathways. Characterization of the neuroprotective role of HIF-1 may be through its stabilization and the regulation of target genes that aid in the early adaptation to the oxidative stressors. It is interesting to note that mounting data from recent years point to an additional crucial regulatory role for hypoxia-inducible factors (HIFs) in inflammation. HIFs in immune cells regulate the production of glycolytic energy as well as innate immunity, pro-inflammatory gene expression, and mediates activation of pro-survival pathways. The present review highlights the contribution of HIF-1 to neuroprotection where inflammation is the crucial factor in the pathogenesis contributing to neural death. The potential mechanisms that contribute to neuroprotection as a result of the downstream targets of HIF-1α are discussed. Such mechanisms include those mediated through IL-10, an anti-inflammatory molecule involved in activating pro-survival signaling mechanisms via AKT/ERK and JAK/STAT pathways.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 HIF-1 in Hypoxia and Inflammation
Under various pathologies and metabolic conditions, hypoxia and inflammation appear to have a complex interaction [1, 2]. The formation of hypoxic signaling intermediates are known to accompany inflammatory conditions, which in turn initiate inflammatory responses by activating cytokines and inflammatory cells [3]. In the recent years HIF-1 has been described to be a significant modulator of hypoxia signaling in inflammation. HIF-1 is a heterodimeric protein complex that plays a significant role in responding to low concentrations of oxygen or hypoxia and is constitutively expressed as a subunit-β and an oxygen-dependent subunit-α. During normoxic conditions, HIF-1α is synthesized and degraded by the ubiquitin-proteasome system [2]. Oxygen-independent mechanisms can also regulate HIF-1 transcription and translation under normoxia during altered metabolic states. Thus, HIF-1 is a crucial transcriptional factor that regulates thousands of genes for maintaining cellular homeostasis. This process is crucial for the survival and function of immune cells by regulating gene transcription. It has been observed that the blood levels of inflammatory cytokines such IL-1, IL-6, and tumor necrosis factor alpha (TNF-α) are elevated in hypoxia conditions [4]. Intriguingly, HIF-1 stability has shown to regulate the production of inflammatory cytokines like TNF-α and as a result inflammation and hypoxia signaling augment one another through a positive feedback loop [5,6,7].
Mechanism and Role of HIF-1α in Pathophysiology
HIF-1α may be the major contributor behind beneficial and deleterious effects throughout the emergence of the most important dysfunctionality connected to neurodegeneration. Depending on the degree of hypoxia, HIF-1α has been shown to play a dual role as a “protective transcription factor” or a “killing factor” (when linked to p53) [8]. Once HIF-1α is stabilized, the HIF-1 complex is subsequently moved into the nucleus where it acts as a transcriptional activator for over thousands of genes [9]. The activation of HIF-1α during moderate hypoxia can enhance tolerance to a more severe hypoxic lesion later on and thus enable the adaptive changes needed for a quicker and better recovery of the afflicted tissue. HIF-1 functions as an endogenous biological defense mechanism that confers protection against global cerebral ischemia or potential fatal damages under ischemic conditions [10]. Additionally, it has been shown that HIF-1 is up-regulated in a variety of disorders, including several neurological diseases, as a brain neuroprotective response element against stresses including reactive oxygen species (ROS) and inflammation [11].
HIF-1α and Inflammation in Neurological Disorders
Inflammation is known to exacerbate disease related pathologies by releasing pro-inflammatory cytokines IL-1β, IL-6 and triggering ERK1/2/AKT, JNK/MAPK and JAK/STAT signaling pathways and thus resulting in severe neural dysfunctionality. Neurological disorders such as Alzheimer’s disease (AD), epilepsy, amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD) and stroke are characterized by progressive loss of neural functions. During pathogenesis of neurological disorders, the inflammatory cytokines, including as macrophage-derived TNF-α and IL-1β, upregulate HIF-1 through mechanisms that inhibit prolyl hydroxylase (PHD) enzymes and increase HIF-1 stability and transcriptional activity [12,13,14]. Additionally, HIF-1 may inhibit the production of pro-inflammatory cytokine receptors, which reduces the dangers of severe neuroinflammation during ischemic insult [15]. HIF-1α-mediated down regulation of pro-inflammatory cytokines such as IL-6 and TNF-α has been recently described to act through IL-10-mediated attenuation of NLRP3 inflammasome or via IL-10/JAK1/STAT3-mediated transcriptional attenuation [16, 17]. IL-10-mediated immune regulation occurs through the downregulation of pro-inflammatory cytokines. IL-10-mediated inflammatory pathways have been studied for its implications in the design of targeted approaches aiming at controlling deleterious inflammation in the brain [18]. Additionally, IL-10 receptor activation has been shown to specifically activate the JAK1-STAT3-mediated downregulation of pro-inflammatory cytokines [19, 20]. Further, IL-10/JAK1/STAT3 pathway has been described as the negative regulator of inflammation that controls both the degree and duration of inflammation [21, 22].
2 Inhibition of NLRP3 Inflammasome by HIF-1α
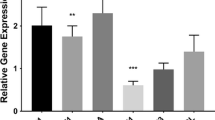
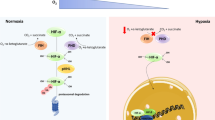
Activation of the NLRP3 inflammasome plays a crucial role in the outcome of various brain diseases and injury such as Alzheimer’s and traumatic brain injury [23]. The authors describe that the contribution of NLRP3 inflammasome is associated with cellular damage and increased inflammatory responses following traumatic brain injury. Furthermore, blocking or inhibiting the activation of the NLRP3 inflammasome may have substantial potential to salvage tissue damage during traumatic brain injury. The binding of HIF-1α to IL-10 promoter has been reported to be involved in HIF-1α-mediated IL-10 expression and its role in modulation of cell metabolism and inflammatory responses. Reports have shown that HIF-1α stabilization elicits a neuroprotective response through modification of inflammatory pathways via modulation of cytokine regulation. Figure 6.1 shows a proposed scheme of the mechanisms of how HIF-1α mediates downstream inflammatory pathways. Once HIF-1α is stabilized, it acts to activate the JAK-STAT3 pathways and/or inhibit the NLRP3 inflammasome, and thus resulting in neuroprotection. Diet-induced stabilization of HIF-1α and upregulation of IL-10 in rodent brain under normoxic ketotic conditions has been shown to play a role in the upregulation of the IL-10 and downregulation IL-6 and TNF-α [17, 24, 25, 26], whereby HIF-1α transcriptionally regulates IL-10 levels by direct binding to hypoxia responsive elements (HREs) on the IL-10 promoter [24, 27]. Recent studies bring together ketosis-mediated stabilization of HIF-1α as a potential neuroprotective phenotype in mice and rats in an oxygen-independent manner via IL-10-mediated activation of JAK1-STAT3, AKT/ERK pathways [17, 28].
Neuroprotective Phenotype & HIF-1α. Working model of IL-10-mediated JAK1-STAT3 pathway activation following HIF-1α stabilization through a metabolic induced inhibition of prolyl hydroxylase (PHD). Accumulation of HIF-1α as a result of an altered metabolic state activates IL-10, whereby resulting in an upregulation of pro-survival pathways involving JAK1-STAT3, ERK/AKT and inhibition of NLRP3 inflammasome
References
Semenza GL (2004) Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology 19:176–182
Semenza GL (2011) Oxygen sensing, homeostasis, and disease. N Engl J Med 365(6):537–547
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364(7):656–665
Crifo B, Taylor CT (2016) Crosstalk between toll-like receptors and hypoxia-dependent pathways in health and disease. J Investig Med 64(2):369–375
Shin DH, Li SH, Yang SW, Lee BL, Lee MK, M.K., Park J.W. (2009) Inhibitor of nuclear factor-kappaB alpha derepresses hypoxia-inducible factor-1 during moderate hypoxia by sequestering factor inhibiting hypoxia-inducible factor from hypoxia-inducible factor 1alpha. FEBS J 276(13):3470–3480
Fong GH, Takeda K (2008) Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ 15:635–641
Masson N, Ratcliffe PJ (2003) HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O2 levels. J Cell Sci 116:3041–3049
Piret JP, Mottet D, Raes M, Michiels C (2002) Is HIF-1 a pro- or an anti-apoptotic protein? Biochem Pharmacol 64(5–6):889–892
Sharp FR, Bernaudin M (2004) HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5(6):437–448
Chen S, Sang N (2016) Hypoxia-inducible Factor-1: a critical player in the survival strategy of cells. J Cell Biochem 117(2):267–278
Bhatia D, Ardekani MS, Shi Q, Movafagh S (2017) Chapter 21: Hypoxia and its emerging therapeutics in neurodegenerative, inflammatory and renal diseases. In: Hypoxia and human diseases. INTECH. https://doi.org/10.5772/66089
Dehne N, Brune B (2009) HIF-1 in the inflammatory microenvironment. Exp Cell Res 315:1791–1797
Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W (1999) Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood 94:1561–1567
Zhou J, Fandrey J, Schumann J, Tiegs G, Brune B (2003) NO and TNF-alpha released from activated macrophages stabilize HIF-1alpha in resting tubular LLC-PK1 cells. Am J Physiol Cell Physiol 284:C439–C446
Xing J, Lu J (2016) HIF-1 alpha activation attenuates IL-6 and TNF-alpha pathways in hippocampus of rats following transient global ischemia. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 39:511–520
Gurung P, Li B, Subbarao Malireddi RK et al (2015) Chronic TLR stimulation controls NLRP3 Inflammasome activation through IL-10 mediated regulation of NLRP3 expression and Caspase-8 activation. Sci Rep 5:14488
Sethuraman A, Rao P, Pranay A, Xu K, LaManna JC, Puchowicz MA (2021) Chronic ketosis modulates HIF1α-mediated inflammatory response in rat brain. Adv Exp Med Biol 1269:3–7
Lobo-Silva D, Carriche GM, Castro AG et al (2016) Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflammation 13(1):297
Hwang CJ, Yun HM, Jung YY et al (2015) Reducing effect of IL-32alpha in the development of stroke through blocking of NF-kappaB, but enhancement of STAT3 pathways. Mol Neurobiol 51(2):648–660
Verma R, Balakrishnan L, Sharma K et al (2016) A network map of Interleukin-10 signaling pathway. J Cell Commun Signal 10(1):61–67
Riley JK, Takeda K, Akira S et al (1999) Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem 274(23):16513–16521
Hutchins AP, Diez D, Miranda-Saavedra D (2013) The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics 12(6):489–498
Ismael S, Ahmed HA, Adris T, Parveen K, Thakor P, Ishrat T (2021) The NLRP3 inflammasome: a potential therapeutic target for traumatic brain injury. Neural Regen Res 16(1):49–57. PMID: 32788447
Puchowicz MA, Zechel JL, Valerio J et al (2008) Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab 28(12):1907–1916
Xu K, Ye L, Sharma K et al (2017) Diet-induced ketosis protects against focal cerebral ischemia in mouse. Adv Exp Med Biol 977:205–213
Zhang Y, Xu K, Kerwin T et al (2018) Impact of aging on metabolic changes in the Ketotic rat brain: glucose, oxidative and 4-HNE metabolism. Adv Exp Med Biol 1072:21–25
Cai Z, Luo W, Zhan H et al (2013) Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci 110:17462–17467
Garcia JM, Stillings SA, Leclerc JL et al (2017) Role of Interleukin-10 in acute brain injuries. Front Neurol 8:244
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Puchowicz, M.A., Parveen, K., Sethuraman, A., Ishrat, T., Xu, K., LaManna, J. (2023). Pro-survival Phenotype of HIF-1α: Neuroprotection Through Inflammatory Mechanisms. In: Scholkmann, F., LaManna, J., Wolf, U. (eds) Oxygen Transport to Tissue XLIV. ISOTT 2022. Advances in Experimental Medicine and Biology, vol 1438. Springer, Cham. https://doi.org/10.1007/978-3-031-42003-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-42003-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42002-3
Online ISBN: 978-3-031-42003-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)