Abstract
Diamond/Al-alloy composite, with its features of high thermal conductivity and low density, is one kind of recently developed lightweight heat dissipation material. In this paper, a novel method of semi-solid powder forming (SSPF), a fabrication method that exploits the unique behavior of a liquid-solid mixture, is used to fabricate the diamond/Al-alloy composite. The Al-alloy matrix is designed as a bi-metal system, including an Al-alloy powder with higher melting temperature acting as solid flexible skeleton to support the diamond, and another with lower melting temperature acting as liquid filler to fill pores between powder gaps. The density, electrical conductivity, thermal conductivity, and microstructure of the composites were studied. Results show that the interface wettability and plastic deformation are encouraged while the sintering temperature is increased from 753 K to 833 K, which helps to increase the relative density of the composite from 95.8% to 99.8%. Besides, the thermophysical properties of the composite are significantly influenced by the intrinsic conductivity of reinforcement and matrix. It is helpful to reduce the residual pores by deforming and sintering in a liquid-solid co-existent state during the SSPF process. Moreover, the densification mechanism is summarized as four stages: volume packed density reaching peak; alloy powder softening and deforming; alloy powder partial melting and filling into powder gap; cooling and densification.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
With the development of high integration and high power of semiconductor devices, packaging materials with higher thermal conductivity are needed to improve the heat dissipation efficiency of devices. To reduce thermal stress damage caused by thermal mismatch, it is required for the semiconductor materials and packaging materials to have comparable thermal expansion coefficients [1, 2]. Diamond/Al-based composites have received increasing attention and have been thoroughly researched by several academics due to their outstanding high thermal conductivity and low expansion. Diamond/Al-based composites can be manufactured using a variety of techniques, such as spark plasma sintering (SPS), gas-assisted pressure infiltration (GPI), and vacuum hot pressing (VHP). These materials typically have good thermal conductivity, a low thermal expansion coefficient, high hardness, and bending strength [3,4,5,6].
Utilized as a near-net forming process, semi-solid powder forming (SSPF) can successfully promote the uniformity of particles and regulate the performance and microstructure of composites [7, 8]. The SSPF is an advanced manufacturing process for diamond/Al-based composites, which has good component adjustability and formability for functional components [9,10,11]. However, the thermal shrinkage stress or external forces generated in the solidification process will affect the microstructure and usually cause the formation of defects like pores, hot cracking, etc., which can result in waste products or serious degradation of performance. However, the microstructure and thermophysical properties of diamond/Al-alloy composite fabricated by the SSPF process are rarely reported, and the densification mechanism is not clear. Therefore, it is necessary to investigate the relationship between microstructure and properties of the composite, which is significant to understanding the densification mechanism and reducing the pores and cracks during fabricating.
In this paper, in order to study the densification mechanism of the composite during the SSPF process, the SPS technology was used to sintering the diamond/Al-alloy composite. The Al-alloy matrix is designed as a bi-metal system, including two kinds of Al-alloy powder with different melting points, so as to acquire a liquid-solid co-existent state during the sintering stage. The thermophysical properties of the composite are measured and calculated. Moreover, the microstructure of the composite is observed and analyzed, which is helpful to understand the densification mechanism of the SSPF process and promote the properties of the composite.
2 Raw Materials and Experimental Procedure
2.1 Materials and Methods
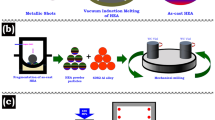
In the present study, as matrix material, two kinds of gas-atomized powder, Al6063 alloy powder (shown in Fig. 1(a), whose thermal conductivity (λ) is 218 W/(m·K)) and AlSi10Mg alloy powder (shown in Fig. 1(b), whose λ is 113 W/(m·K)) are mixed. In the bi-metal system of the mixture, the Al6063 allloy powder with higher melting temperature acting as solid flexible skeleton to support the diamond, and another with lower melting temperature acting as liquid filler to fill pores between powder gaps. The particle size distribution and the chemical composition of the two aluminum alloys powder are listed in Tables 1 and 2. AlSi10Mg alloy powder has a low melting point and wide liquid-solid interval (from 823 K to 869 K), which will turn into a liquid phase during sintering. As shown in Fig. 1(c), II b-type synthetic diamond powder (whose mean particle diameter range is 50 μm, provided by Zhongyuan Jingangshi Co. LTD) is employed as a filler material and the thermal conductivity of the synthetic diamond is estimated to be about 2000 W/(m·K).
Due to some experimental constraints and research objectives behavior, in the present study, all the composite samples are used with 46.6 wt.% of the diamond. The diamond is dispersed into the bi-metallic matrix composed of 26.8 wt.% Al6063 and 26.6 wt.% AlSi10Mg alloy powder, and the composites with the sintering temperature at 753 K and 833 K are named D/Bi-M-753 and D/Bi-M-833, respectively. In addition, two comparisons are designed, one of which is named Bi-M-753 which is composed of 50.2 wt.% Al6063 and 49.8 wt.% AlSi10Mg alloy powder without diamond. The other is named D/Mono-M-803 which is composed of 53.4 wt.% AlSi10Mg alloy powder and 46.6 wt.% diamond particles.
2.2 Powder Mixing Method and Fabrication Process
Before sintering, the aluminum alloy powder is carried out on the YXQM-1L equipment with a rotation speed of 100 rpm for 4 h to get the bi-metallic mixtures. And then, the diamond particles are mixed with the different Al-alloy powder mentioned above by a mechanical stirring method. The SPS unit used is model LABOX-600, which is composed of an electric power source and a pressure system, and composite fabrication is carried out after powder mixtures and placed in a graphite die-set. To make one disk-shaped composite specimen with gauge dimensions of 2.5 mm thick and Φ12.7 mm in diameter, about 0.7 –1.0 g of the powder mixture is placed carefully in the above-described custom-made die-set, and consolidation is carried out. During the forming process, the powder mixture is pressed at a pressure of 45 MPa through the upper and lower punches, and then spark plasma sintering is carried out at various die temperatures of 753 K, 803K, and 833 K, respectively, and a maximum heating rate of 1.5 K/s.
2.3 Test of Density, Relative Density, and Thermophysical Properties
Disk-shaped composites thus fabricated are examined by scanning electron microscopy (SEM) using a JSM-6010 microscope. Diamond/Al alloy composite disks are graphite-coated and their thermal diffusivities are measured by a laser flash technique using a Netzsch LFA-467 thermal constants analyzer. The achieved real density of the composite disks fabricated is measured in the Archimedes method for calculating thermal conductivity and relativity density (the ratio of real density to theoretical density).
3 Results and Discussion
3.1 Correlation between Densification and Thermophysical Properties
The real density and relative density of the diamond particle dispersed bi-metallic or mono-metallic matrix composites are depicted in Fig. 2. The real density of the D/Bi-M-753 composite is higher than that of the Bi-M-753 composite, which conforms to the addition rule of composite materials. The higher the relative density of the composite, the lower porosity in the composite, which is affected by the content of the reinforcement and the sintering temperature. The relative density of the D/Bi-M-753 composite is lower than that of the Bi-M-753 composite, which is caused by the addition of diamond particles. As the sintering temperature increases, the relative density increases from 95.8% of the D/Bi-M-753 composite to 99.8% of the D/Bi-M-833 composite. This indicates that a suitable sintering temperature is beneficial to improve the densification degree of the composite. That is because the AlSi10Mg alloy powder melts and the matrix is in a state of solid-liquid co-existent while sintering at 833 K. which promotes the liquid phase to fill the pores between the powder and diamond particles, thus improving the relative density of the composite.
The electrical conductivity of a composite is determined by the intrinsic electrical conductivity of both aluminum alloy and diamond, which is also affected by forming defects such as pores and cracks. As shown in Fig. 3(a), the electrical conductivity of the Bi-M-753 composite reaches 28.1 MS /m, which is higher than that of 10.0 MS/m of the D/Bi-M-753 composite. Moreover, all the composites with diamond have similar electrical conductivity, and the average value is about one-third of the electrical conductivity value of the Bi-M-753 composite. The electrical conductivity is related to the number and transport capacity of free electrons in the material. At the atomic level of diamond, due to strong covalent bonds existing between carbon atoms, the number of free electrons is very small thus the intrinsic conductivity value is low. Therefore, the electrical conductivity of the composite is lower with the diamond particles dispersed in the Al-alloy matrix. In addition, the electrical conductivity of the D/Bi-M-753 composite and the D/Mono-M-803 composite is slightly lower than that of the D/Bi-M-833 composite. Considering that it is due to the scattering of free electrons by defects and interfaces that enhance the electrical impedance. It is also indicated that the densification degree of the composite is somewhat relevant to the electrical conductivity, however, more accurate effects need in-depth quantitative research.
Unlike the very low intrinsic electrical conductivity of diamond, its thermal conductivity is much higher than that of aluminum alloy matrix. As depicted in Fig. 3(b), the thermal conductivity of the Bi-M-753 composite is lower than that of the D/Bi-M-753 composite but higher than that of the D/Mono-M-803 composite. In addition, the thermal conductivity of the D/Bi-M-833 composite is higher than that of the D/Bi-M-753 composite. It is indicated that the thermal conductivity of the composite will increase by increasing the content of the diamond or decreasing the volume fraction of the AlSi10Mg alloy powder. It is reasonable to conclude that the thermal conductivity of composite is directly affected by the intrinsic thermal conductivity of reinforcement and matrix. In general, the main heat transfer medium for metal-matrix composites is phonons (lattice vibrations in the material). Compared to some reported studies [8,9,10,11], it is thought that the contribution ratio of diamond for thermal conductivity in this study has not reached the expected level even to the D/Bi-M-833 composite. It is inferred that is related to the micro-defects and the interfacial compound. Because they have a lower velocity of phonon than the matrix, which increases the acoustic mismatch between diamond and aluminum, thus inhibiting the thermal conductivity of the composites.
3.2 Microstructure
As shown in Fig. 4, the microstructure morphology is observed by SEM technology to investigate the relationship between microstructure and densification of the composite. Figure 4(a) and (e) are scanning micrographs taken from cross sections of Bi-M-753 composite, as the yellow contour shows, Al6063 alloy powder retains the original powder outline after sintering due to its high melting point and low alloying element content. However, the original AlSi10Mg alloy powder outline disappears and the silicon phase is segregated with a spherical morphology in the matrix (as yellow arrow shown in Fig. 4(e)), due to the AlSi10Mg alloy powder being deformed and melted under high temperature and pressure. As the yellow arrow shown in Fig. 4(b) and (f), it is found that some porosities are present on the interface between the diamond particle and Al-alloy matrix in the D/Bi-M-753 composite. However, few pores or cracks are observed on the interface between the diamond particle and the Al-alloy matrix in the D/Bi-M-833 composite (as shown in Fig. 4(c) and (g)) and the D/Mono-M-803 composite (as shown in Fig. 4(d) and (h)). Meanwhile, it also can be seen that some single diamond particles are evenly distributed in the Al-alloy matrix, while others are composed of two or more diamond particles in clusters, in which some porosities are found in the gaps of the clusters (as yellow circle shown in Fig. 4(f) and (g)). Combined with the analysis of the relative density in Fig. 2, it is essential to eliminate the porosities to improve the relative density. As a good solution, the SSPF method is helpful to encourage interface wettability and plastic deformation, thus the pores and cracks will be reduced.
3.3 Densification Mechanism During the SSPF Process
In the study, the powder mixture was cleaned, mixed, dried, and sintered to fabricate a diamond/bi-metallic composite by the SSPF method. According to the discussion of the relationship between the microstructure and densification of the composite, the densification mechanism is summarized. As shown in Fig. 5, firstly, the powder mixture is put into a graphite die and compressed from loose packing to dense packing when the pressure and temperature rise. At the second stage end, the alloy powder is deformed and the mixture reaches the maximum volume packed density. With the coupling effect of temperature and pressure, the Al6063 alloy powder gets more plastic deformation, and part of the AlSi10Mg alloy powder melts into a liquid and fills the particle gaps. Finally, some micro-pores and micro-cracks may be generated in the interface of diamond and aluminum, due to the solidification shrinkage and thermal residual stress during the consolidation stage.
4 Conclusion
In this paper, the thermophysical properties and microstructure of the diamond/Al-alloy composite are investigated including real density, relativity density, electrical conductivity, thermal conductivity, thermal diffusivity, and SEM image. In addition, it is summarized the densification mechanism of the diamond/Al-alloy composite during the SSPF process. According to the analyses of results, the following conclusions can be drawn:
-
(a)
The interface wettability and plastic deformation are encouraged while the sintering temperature at 833 K, which helps to increase the relative density of diamond/Al-alloy composites from 95.8% to 99.8%. Therefore, the sintering temperature is important to reduce residual pores and de-bonding in the interface of the diamond and aluminum alloy.
-
(b)
The intrinsic conductivity of reinforcement or matrix plays a fundamental role in the thermophysical properties of the composite. However, there is still a large room for improvement of thermal conductivity, which is mainly related to the existence of interface micro-defects.
-
(c)
The densification mechanism during the SSPF process is summarized as four stages: volume packing density reaching peak; alloy powder softening and deforming; alloy powder partial melting and filling into powder gaps; cooling and densification.
At present, there are shortcomings in the process, resulting in unsatisfactory thermal conductivity. As a prospect, it is important to study how to eliminate the micro-defects on the interface between diamond and matrix. It is planned a quantitative study of the internal micro-defects of the composite by using 3D tomography (CT) technology.
References
Zhang, L., et al.: Aluminum/graphene composites with enhanced heat-dissipation properties by in-situ reduction of graphene oxide on aluminum particles. J. Alloy. Compd. 748, 854–860 (2018)
Yu, K., Li, C., Yang, J., Cai, Z.Y.: Production and properties of a 90%Si-Al Alloy for electronic packaging applications. Mater. Sci. Forum 610–613, 542–545 (2009)
Mizuuchi, K., et al.: Thermal conductivity of diamond particle dispersed aluminum matrix composites fabricated in solid–liquid co-existent state by SPS. Compos. B Eng. 42, 1029–1034 (2011)
Zhang, H.L., Wu, J.H., Zhang, Y., Li, J.W., Wei, X.T., Sun, Y.H.: Mechanical properties of diamond/Al composites with Ti-coated diamond particles produced by gas-assisted pressure infiltration. Mater. Sci. Eng., A Struct. Mater. Prop. Microstruct. Process. 626, 362–368 (2015)
Wu, Y.F., Kim, G.Y., Anderson, I.E., Lograsso, T.A.: Fabrication of Al6061 composite with high SiC particle loading by semi-solid powder processing. Acta Mater. 58(13), 4398–4405 (2010)
Jia, Q.J., Liu, J.Y., Li, Y.X., Wang, W.S.: Microstructure and properties of electronic packaging box with high silicon aluminum-base alloy by semi-solid thixoforming. Trans. Nonferrous Metals Soc. China 23(1), 80–85 (2013)
Guo, M.H., Liu, J.Y., Li, Y.X.: Microstructure and properties of SiCp/Al electronic packaging shell produced by liquid–solid separation. Trans. Nonferrous Metals Soc. China 24(4), 1039–1045 (2014)
Zhou, H.Y., et al.: Improvement of thermal conductivity of diamond/Al composites by optimization of liquid-solid separation process. J. Mater. Process. Technol. 297, 117267 (2021)
Mizuuchi, K., et al.: Thermal properties of Al/Diamond composites fabricated in continuous solid-liquid co-existent state by SPS. Mater. Sci. Forum 706–709, 1967–1972 (2012)
Xin, L., et al.: Enhanced stability of the Diamond/Al composites by W coatings prepared by the magnetron sputtering method. J. Alloy. Compd. 763, 305–313 (2018)
Tan, Z.Q., Ji, G., Addad, A., Li, Z.Q., Silvain, J.F., Zhang, D.: Tailoring interfacial bonding states of highly thermal performance diamond/Al composites: Spark plasma sintering vs. vacuum hot pressing. Compos. A Appl. Sci. Manuf. 91, 9–19 (2016)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Zhang, W., Li, Y., Du, H., Lang, L. (2024). An Experimental Study of the Densification Mechanism in Semi-solid Powder Forming of Diamond/Al-Alloy Matrix Composite. In: Mocellin, K., Bouchard, PO., Bigot, R., Balan, T. (eds) Proceedings of the 14th International Conference on the Technology of Plasticity - Current Trends in the Technology of Plasticity. ICTP 2023. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-41341-4_28
Download citation
DOI: https://doi.org/10.1007/978-3-031-41341-4_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-41340-7
Online ISBN: 978-3-031-41341-4
eBook Packages: EngineeringEngineering (R0)