Abstract
In the last two decades, an exhaustive amount of research has shown that melatonin is a critical pleiotropic molecule, controlling several developmental and stress-related responses in plants. In this chapter, we discuss the current and potential uses of melatonin during pre-fertilization reproductive stages, with particular emphasis on its involvement in regulating flowering and flower development as well as adaptation of reproductive stages to environmental stresses. Recent evidence indicates that melatonin delays the transition of floral meristem and, thereby, flowering time. It has been proposed that it plays a protective role during the development of flowers particularly male gametophyte development through its antioxidant activity. Recent studies also show that melatonin functions in the production of volatiles in flowers and the induction of parthenocarpy through cooperation with other phytohormones. Finally, melatonin can alleviate the effects of various abiotic stresses during flowering, including high temperature, chilling, and drought. The encouraging results obtained from the various studies point towards diverse roles of melatonin during pre-fertilization reproductive stages and also highlight the enormous potential of melatonin in improving plant performance under stressful environmental conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
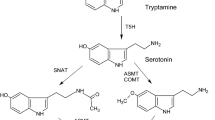
Melatonin is an indoleamine (N-acetyl-5-methoxytryptamine) that was for the very first time isolated from the bovine pineal gland (Lerner et al. 1958). The exclusivity of the animal origin of melatonin changed after its discovery in the unicellular dinoflagellate Gonyaulax polyedra (Poeggeler et al. 1991). Soon, in 1995 the first reports of the presence of melatonin in plants came simultaneously from two independent groups (Dubbels et al. 1995; Hattori et al. 1995). Since then, melatonin has been shown to have pleiotropic effects on several aspects of plant growth. Melatonin regulates circadian rhythms, promotes cell enlargement and root development, delays flowering, delays senescence and improves crop quality, and increases fruit yield (Arnao and Hernandez-Ruiz 2015, 2020, 2021; Back 2021; Sun et al. 2021; Ahn et al. 2021). Melatonin is well-known to have antioxidative effects as it directly or indirectly scavenges reactive oxygen species (ROS) and reactive nitrogen species (RNS) and attenuates oxidative stress in cells, tissues, and organisms (Zhang et al. 2015). Several studies have confirmed that melatonin helps plants in alleviating the negative effects of various kinds of biotic and abiotic stress (Zhang et al. 2014, 2015; Arnao and Hernandez-Ruiz 2014, 2015, 2018; Li et al. 2015, 2021; Nawaz et al. 2015; Chen and Li 2017; Cao et al. 2018; Ahammed et al. 2019; Huang et al. 2019; Siddiqui et al. 2020). Recently, the first putative plant melatonin receptor was identified in Arabidopsis indicating that melatonin could be a phytohormone (Arnao and Hernandez-Ruiz 2020).
Reproduction is the basis for sustenance of any species. In higher plants, the flower is the basic unit of sexual reproduction. The initiation of flowering, growth, and development of sex organs in a flower, interaction between gametophytes, and fertilization are all regulated by complex signaling networks. It is a well-established fact that phytohormones regulate reproductive processes. In recent years, there are growing pieces of evidence that phytohormones like auxin, and gibberellins are indispensable for the development of sex organs. For instance, Gibberellins regulate early stamen development while auxin plays a role in anther dehiscence, pollen maturation, and filament elongation (Song et al. 2013). Multiple studies in the last two decades have shown that melatonin exhibits many hormone-like activities. Although its roles in various plant biological processes are known for a long time, information on its involvement in reproductive development has been quite recent. In this chapter, we explore the myriad roles of melatonin during flowering, pre-fertilization reproductive processes, stress tolerance during reproductive stages, and the molecular mechanisms behind its many functions.
2 Physiological Roles of Melatonin During Pre-fertilization Reproductive Stages
An increasing repository of work has shown that phytomelatonin plays a crucial role in the regulation of various aspects of plant growth and development. Several important roles have been attributed to phytomelatonin like enhancement of plant antioxidant enzyme activity, improvement of plant tolerance to various biotic and abiotic stresses, synchronization of plant resistance, and improving fruit yield and crop quality (Zhang et al. 2015; Li et al. 2015, 2021; Cao et al. 2018; Arnao and Hernandez-Ruiz 2018, 2020, 2021; Huang et al. 2019; Mohamed et al. 2020; Siddiqui et al. 2020; Sun et al. 2021). However, the number of studies on the roles of phytomelatonin on reproductive development is considerably fewer but equally exciting. The present section gives a brief account of the role of melatonin during plant reproductive development.
2.1 Flowering Time
An environmentally coordinated circadian clock is important for the growth and development of plants. The well-synchronized flowering rhythm ensures adequate pollination and normal seed/fruit development. Studies have shown that the transition from the vegetative phase to flowering in plants is under the control of environmental (photoperiod and temperature), physiological (phytohormones and nutritional status), and genetic factors (gene regulation and developmental stage) (Cao et al. 2021). The role of melatonin in controlling circadian rhythms in animals is well known. Taking cues from that, initial studies indicating a possible role of melatonin as a chrono-regulator of circadian rhythms in plants were done on short-day plant Chenopodium rubrum (Kolar et al. 1997; Wolf et al. 2001). These studies showed that during the light period, melatonin concentration remained low or undetectable. As the dark period ensues the melatonin concentration starts to increase reaching a maximum at 4–6 hours of the dark period before decreasing rapidly. Similar fluctuating levels of melatonin in a 24-hr cycle have been seen in various other plant species, such as Eichhornia crassipes, Vitis vinifera, Prunus avium, and Hordeum vulgare (Tan et al. 2007; Boccalandro et al. 2011; Zhao et al. 2013; Arnao and Hernandez-Ruiz 2015).
The effect of melatonin on flowering rhythm was also studied for the first time in C. rubrum and it was shown that melatonin interferes in the photoperiod induction of flowering (Machackova and Krekule 2002; Kolar et al. 2003; Kolář and Macháčková 2005). In C. rubrum, flower induction was shown to be inhibited by an average of 40–50% when high concentrations of melatonin is applied 2 h before and after the beginning of the inductive dark period. However, melatonin treatment had no effect per se on the duration of flowering which suggests that it controls some process related to floral transition (Kolář and Macháčková 2005). Also, in transgenic rice plants which were rich in melatonin flowering was seen to be delayed by 1 week indicating some role of melatonin in regulating flowering time. Delayed flowering resulted in a reduction of grain yields by an average of 33% in the melatonin-rich transgenic lines (Byeon and Back 2014).
More direct evidence for the restrictive role of melatonin in flowering was provided by Shi et al. (2016). The authors showed that exogenous application of melatonin retards flowering in Arabidopsis. In this study, Arabidopsis plants which were treated with 500 μM melatonin exhibited delayed flowering by 5 days and plants had more rosette leaves as compared to the untreated plants. The study also demonstrated the novel involvement of DELLAs and flowering Locus C (FLC) in melatonin-mediated flowering in Arabidopsis. However, recently Lee et al. (2019) have presented contradictory results in snat2 knockout mutants of Arabidopsis. Serotonin N-acetyltransferase (SNAT) catalyzes the formation of N-acetylserotonin (NAS) from serotonin and is known to play important roles both in melatonin biosynthesis and function (Zheng and Cole 2002). snat2 mutants produce less melatonin than the wild type. Interestingly, it was discovered that the snat2 seedlings showed delayed flowering despite having a lower concentration of melatonin (Lee et al. 2019). According to the authors, exogenously applied melatonin cannot translate the effects of endogenous melatonin on flowering and hence their results were in contradiction with the results of Shi et al. (2016) Also, high-dose melatonin (500 μM, as used by Shi et al. 2016) probably retarded the growth of Arabidopsis seedlings. This contradictory report warrants more attention as most of the studies to date have pointed to delayed flowering in the presence of high concentrations of melatonin. Another interesting study was done in Arabidopsis mutants for strigolactone (SL) synthesis or signaling, a carotenoid-derived compound involved in regulating flowering in plants (Zhang et al. 2019). It was shown that the flowering time of Arabidopsis is delayed if the tissue content of melatonin is higher than ~8 ng/g F.W, or accelerated if it falls below ~0.9 ng/g. Authors proposed that melatonin acts downstream of SL, and if its concentration is not within a certain range it can cause a delay in flowering.
Another recent study that has shown a suppressive effect of melatonin on flowering if present in high concentrations was done in apples by Zhang et al. (2018). The authors monitored apple trees for two consecutive years and reported a significant reduction in endogenous melatonin content in apple trees before flowering. Apple trees were also subjected to different concentrations (0, 20, 200, and 1000 μM) of exogenous melatonin through spraying. It was found that in comparison to the control plants, 20- and 200-μM melatonin treatments delayed apple bloom by 2 days, and 1000-μM melatonin treatment delayed flowering by 3 days (Zhang et al. 2018). Hence, the application of melatonin in a dose-dependent manner before flowering could delay the flowering in apple trees (Zhang et al. 2018).
Thus, based on the current evidence, melatonin can be assumed to be a chrono-regulator of flowering time and the concentration of melatonin likely decreases just before flowering. However, this notion should be thoroughly investigated in other plant species for conclusive evidence.
2.2 Floral Meristem Formation
The development of a flower is a highly coordinated multistep procedure that involves floral induction, floral meristem formation, and floral organ development. All these steps are under the strict control of a network of interacting genes and their protein products known as the Gene Regulatory Network (GRN) (Kinoshita and Richter 2020). After reaching the right developmental stage and perceiving the right environmental cues, flowering-time genes are triggered resulting in the conversion of the vegetative shoot apical meristem (SAM) into an inflorescence meristem (IM) (Liu et al. 2009).
In the model plant, A. thaliana, several regulators which are involved in the flowering induction are recognized. These regulators facilitate the transition of vegetative meristem to the reproductive meristem by integrating the gene interactions and resultant signal transduction pathways (Liu et al. 2015b). The main flowering genes recognized in A. thaliana include FLOWERING LOCUS C (FLC), FLOWERING LOCUS T (FT), SUPPRESSSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), APETALA1 (AP1), CONSTANS (CO), LEAFY (LFY), and TARGET OF EAT1 (TOE1). Out of these, FLC is a MADS-box transcription factor which is a negative regulator of floral transition as it represses the transcription of some floral genes like SOC1 and FT by binding to their promoter regions (Li et al. 2016). Phytohormone Gibberellic Acid (GA) also plays an important role in the formation of floral meristem in A. thaliana (Sun and Gubler 2004). GA promotes the ubiquitin-mediated degradation of DELLA proteins which mediate different genetic pathways that repress plant flowering (Wigge et al. 2005; Searle et al. 2006). DELLAs also affect the transcriptional activity of FLC leading to late flowering (Li et al. 2016).
The role of melatonin in the transition from the vegetative to the reproductive phase was first described in Arabidopsis by Shi et al. (2016). According to this study, melatonin mediates the stabilization of DELLA proteins which activates FLC and represses the transcription of FT resulting in delayed flowering (Fig. 7.1). Authors treated plants with exogenous melatonin and it was proposed that stabilization of DELLAs by melatonin is without regulation of transcription of DELLAs and endogenous GA level. Notably, floral transition in della mutants was not influenced by exogenous melatonin as there was a decrease in melatonin-induced FLC transcripts in della mutants. Thus, results suggested that melatonin mediated flowering in Arabidopsis through DELLAs-activated FLC. According to Mukherjee (2019), melatonin induces endogenous nitric oxide (NO) levels and it has been speculated that NO may play some role in melatonin-mediated DELLA stabilization and consequently delayed flowering (Shi et al. 2016).
Proposed role of melatonin in floral transition. Melatonin upregulates the expression of DELLAs and FLC, consequently delaying the transition of SAM to IM (Shi et al. 2016). During this process, SL acts upstream of melatonin to delay flowering (Zhang et al. 2019). SL strigolactone, FLC flowering locus C, FT flowering locus T, SOC1 suppressor of overexpression of CONSTANS1, API1 Apetala-1, SAM shoot apical meristem, IM inflorescence meristem
A study by Zhang et al. (2019) revealed the interaction between melatonin and other signaling molecules in regulating floral transition. As mentioned in the previous 3.1, this study was done in Arabidopsis mutants for SL synthesis or signaling. SL is a carotenoid-derived compound involved in regulating various developmental pathways in plants including flowering. The authors suggested that floral transition in Arabidopsis is mediated by a combination of melatonin and SL. The study proposed that whenever the melatonin content exceeds a certain threshold, SL acts upstream of melatonin to delay flowering due to the activation of FLC.
The above-mentioned studies unravel the role of melatonin in a key developmental event of the transition of vegetative meristem to reproductive meristem (Fig. 7.1). However, exactly how SL regulates melatonin and melatonin regulates the transcription of FLC needs more investigation. Table 7.1 summarizes the effect of melatonin on the expression of various genes involved in flowering.
2.3 Flower Development
Flowers harbor the reproductive organs of a plant which in turn store the male and female gametophytes i.e., the pollen grains and the embryo sac respectively. The gametophytes are the most vulnerable and vital tissues produced in the life cycle of a plant having a direct role in plant reproduction. It is well known that the development of reproductive tissues is highly sensitive to potential environmental damage which may induce the generation of oxidants like ROS and RNS. These oxidants need to be in a redox balance, or else, they may cause oxidative damage to reproductive tissues and hamper the reproductive success of plants.
In the initial years of research, phytomelatonin was reported from a wide variety of tissues like roots, stems, leaves, fruits, and seeds except flowers (Murch et al. 1997; Chen et al. 2003; Cao et al. 2006; Arnao and Hernandez-Ruiz 2006). In the subsequent years, one of the roles attributed to melatonin in plants was protecting from oxidative damage through direct radical scavenging (Hardeland 2005; Tan et al. 2007; Schaefer and Hardeland 2009). The hypothesis that melatonin may serve as an antioxidant served as the basis for the detection of melatonin in flowers. The first study to determine the presence of melatonin during flower development was done in Hypericum perforatum (Murch and Saxena 2002). It was reported that during the uninucleate stage of microsporogenesis, concentrations of the indole were highest and at the elevated concentration of melatonin the regenerative potential of isolated anthers was also maximum. Thus, the authors proposed that melatonin may play a significant role in the regulation of the reproductive physiology and flower development of H. perforatum. Authors also speculated a similar pattern of melatonin accumulation in flowers of other species. High levels of melatonin were also reported in the floral tissues of Datura metel (Murch et al. 2009). It was found that melatonin levels were high in the developing flower buds and ovules of D. metel and which progressively declined in fruits. Authors proposed that melatonin in D. metel acts as an antioxidant in protecting the early stages of reproductive tissues. The hypothesis was further supported by the observation of elevated levels of melatonin in flower buds exposed to cold stress.
Accumulation of melatonin during flower development was also shown in rice (Park et al. 2013). In the study, melatonin content was estimated during reproductive stages (pre-flowering, flowering, and post-flowering) and it was demonstrated that the melatonin contents were sixfold higher in the flowering stage than the pre-flowering stage. Authors also reported induction of melatonin biosynthesis was marked by the induction of required proteins such as tryptophan decarboxylase, tryptamine 5-hydroxylase, and N-acetylserotonin methyltransferase. In Prunus avium, melatonin levels were reported to increase later in the season by Zhao et al. (2013), which authors attributed to defense against high light stress and increased ROS load in the tissues.
In the herbaceous ornamental plant Paeonia lactiflora, the melatonin content has been studied in different color series and developmental stages of flowers viz. flower-bud stage (Stage 1, S1), initiating bloom stage (Stage 2, S2), bloom stage (Stage 3, S3) and wither stage (Stage 4, S4) (Zhao et al. 2018). It has been reported that peony flowers are rich in melatonin, however, the color series vary in melatonin content. The highest amount of melatonin was found in the white series, followed by the ink series, the red series, and then the pink series. Also, during flower development, the melatonin content first increases in the S1 stage and then decreases in S2 before peaking in the bloom stage (S3). The melatonin content again decreases in the S4 stage but was still higher than the content in S2. Zhao et al. (2018) also studied the effect of different parts of the light spectrum on the melatonin content during flowering. They demonstrated that sun exposure and blue light induce melatonin production whereas shade conditions, and white and green lights lower melatonin production. Also, “dual peaks” of melatonin were reported at 2 p.m. and 2 a.m. in a 24-h light/dark cycle. Authors linked this fluctuation in the melatonin content during different stages, at different times, and in different light conditions to a matching expression pattern of the tryptophan decarboxylase gene (TDC).
Melatonin has been reported from flowers of many other plants like Malus domestica, Tanacetum parthenium, Tripleurospermum disciforme, Viola odorata, Oryza sativa, Solanum lycopersicum, and Capsicum annum (Okazaki and Ezura 2009; Ansari et al. 2010; Park et al. 2013; Lei et al. 2013; Korkmazab 2014). All these studies show that the induction of melatonin occurs during flower development and that melatonin may have a protective role during flower development. However, how, where and at which stage of flower development exactly melatonin functions are still a matter of investigation. Also, more direct evidence of its function during flower development will be more revealing of its role.
2.4 Floral Volatiles
The majority of flowering plants rely on biotic pollination for reproductive success (Ollerton et al. 2011). To cause this effect plants develop a variety of contrivances to attract potential pollinators. Floral volatiles are one of the key floral attractants for pollinators which other than that also defend the plants from floral antagonists (Schiestl et al. 2014; Junker and Parachnowitsch 2015). Other than its role in flower reproduction, floral volatiles also have immense economical value in perfumes, cosmetics, flavorings, and therapeutic industries. Chemically floral volatiles can range from terpenoids to benzenoids, fatty acid derivatives, nitrogen-containing compounds, amino acid derivatives, and sulfur-containing compounds (Farré-Armengol et al. 2020).
Floral volatiles is synthesized through complex biochemical pathways which are regulated by various internal and external stimuli (Dudareva and Pichersky 2008; Abbas et al. 2017). A recent study by Abbas et al. (2021) in Hedychium coronarium has proposed a putative regulatory role of melatonin floral scent production. Flowers of H. coronarium are known to release abundant amounts of volatiles during the blooming period. The major volatiles found in the scent are terpenoids (monoterpenes and sesquiterpenes) and benzenoids/phenylpropanoids. Through integrated metabolomic and transcriptomic approaches, authors analyzed the changes triggered by melatonin exposure during the half bloom (HS), full bloom (FB), and fade stage (FS) of flower development in H. coronarium. The study revealed that volatile organic compound emission was significantly enhanced at all the stages of flowering after exposure to melatonin. The metabolomic analysis led to the identification of 15 volatile compounds whose concentration was enhanced by the melatonin treatments. According to the transcriptomic analysis, around seventy-six genes and some transcription factors, such as MYB/bHLH, were found to be significantly upregulated and were speculated to be directly involved in the biosynthesis of floral aromatic compounds. Thus, the authors suggested that melatonin mediates the expression of certain genes involved in the biosynthesis of volatile compounds and enhances the production of aroma in H. coronarium flowers (Abbas et al. 2021).
2.5 Parthenocarpy
Parthenocarpy is the production of fruits without the fertilization of ovules such that fruits are seedless. It is of common occurrence in the horticultural varieties of banana, pineapple, cucumber, tomatoes, figs, oranges, grapes, kiwi, blackberry, pepper, etc. One of the main advantages of parthenocarpy is that the fruit set is not dependent on pollination and fertilization. Therefore, it ensures reproduction even in environmental conditions that are not conducive to pollination. Moreover, the absence of seeds increases the palatability of fruits and in turn their commercial viability.
Parthenocarpy is a genetically inherited trait and the potential to form parthenocarpic fruit is dependent on the genetic makeup of the cultivar. Parthenocarpy can also be induced artificially by exogenous applications of plant hormones. For instance, indole-3-acetic acid (IAA) is used to induce parthenocarpy in many horticultural plants, such as tomatoes, cucumbers, and zucchini (Martinelli et al. 2009; Pomares-Viciana et al. 2017). Also, the treatment of certain cultivars of oranges, tomatoes, blueberries, garden peas, and Arabidopsis by GA3 (or GA1) causes parthenocarpic fruit development (Cano-Medrano and Darnell 1997).
The similarity between the functions of melatonin and IAA in plants, the fact they share a common precursor, tryptophan, and the already-known role of melatonin in the GA pathway of flowering led Liu et al. (2018) to explore the involvement of melatonin in inducing parthenocarpy. Authors used ‘Starkrimson’ pear for their study and found that the exogenous application of melatonin promoted the development of ovaries in the absence of pollination same as pollinated ovaries. Melatonin-treated ovaries led to the development of fruits without seeds. Investigation into the changes of related hormones in the ovaries led to the revelation of a significant increase in the contents of the gibberellins (GAs) GA3 and GA4. The authors also studied the relationship between melatonin and GA using paclobutrazol (PAC), a GA-biosynthesis inhibitor. It was seen if a prior treatment of PAC was given, neither GA content increased nor parthenocarpic fruit development happened even after spraying with melatonin. Also, transcriptome analysis has shown that melatonin can cause significant upregulation of PbGA20ox (GA 20-oxidase) and downregulation of PbGA2ox (GA 2-oxidase), enzymes involved in the biosynthesis of GA. Thus, it has been suggested that melatonin induces parthenocarpy in pears by promoting the biosynthesis of GA biosynthesis.
3 Role of Melatonin During Stress Tolerance in Reproductive Tissues
Over the past decade, huge amounts of evidence have amassed which suggests that melatonin protects plants against biotic stress (Arnao and Hernandez-Ruiz 2014, 2015, 2018; Chen and Li 2017) and abiotic stress (Zhang et al. 2014; Arnao and Hernandez-Ruiz 2015; Zhang et al. 2015; Li et al. 2015, 2021; Nawaz et al. 2015; Cao et al. 2018; Ahammed et al. 2019; Huang et al. 2019; Siddiqui et al. 2020). Currently, several review articles discuss the protective effects of melatonin in improving plant tolerance and the role of melatonin in regulating epigenetic and transcriptional changes in plants under stress. Most of these studies have focussed on the role of melatonin in response to abiotic stresses in vegetative tissues. Although melatonin is known to accumulate in high quantities during flower development, very limited literature is available on the role of melatonin in stress tolerance in reproductive organs. Nevertheless, the few studies conducted on the aspect have put forward some very interesting findings and helped in understanding the functions of melatonin in plant reproductive development.
The study by Qi et al. (2018) through exhaustive data reported that melatonin protects pollen activity in Solanum lycopersicum under high-temperature stress. Authors reported that irrigation treatment with 20 μM of melatonin can alleviate high temperature-induced pollen abortion. Under high temperature, both pollen viability and the mean germination ratio of pollen grains was found to be significantly higher in plants treated with melatonin as compared to untreated plants. It was also shown that melatonin alleviates high temperature-induced ROS accumulation in tomato anthers as there was a decrease in H2O2 content by 35.3% after 3 h of high-temperature stress in the plants treated with melatonin. The gene expression analysis has shed light on the genes which might be upregulated after melatonin pre-treatment. Transcript levels of antioxidant-related genes like CAT1, APX1, DAHR, and Fe-SOD are known to be accentuated by melatonin under oxidative stress. All these findings suggest that melatonin helps in the protection of anthers from oxidative stress as triggered by high temperature by either directly scavenging the ROS or indirectly stimulating the expression of antioxidative enzymes (Qi et al. 2018). The study also revealed the ultrastructural changes specifically, premature degeneration of the tapetum cells which lead to pollen abortion in response to high temperature can be assuaged by melatonin. Pre-treatment with melatonin also enhances the expression of heat shock protein genes HSP21 and HSP70 which help in refolding the unfolded proteins. Autophagy is a degradation system employed by cells to destroy dysfunctional proteins and organelles by delivering them to lysosomes. It is involved in numerous biological processes of plants including responses to biotic and abiotic stress (Qi et al. 2021). Investigating if melatonin can enhance the occurrence of autophagy in heat-stressed anthers of tomato revealed that the expression of the autophagy-related (ATG) genes was greater in melatonin-pre-treated anthers. This results in the manifestation of autophagy upon high-temperature stress.
Drought stress during flowering can significantly reduce the yield of plants by damaging the reproductive organs (Fang et al. 2010). Numerous studies have reported drought-induced yield loss due to low male sterility (Fang et al. 2010; Fu et al. 2011). The role of melatonin in overcoming the drought-induced suppression of seed germination and root elongation is known in many plants (Zhang et al. 2014; Li et al. 2015; Liu et al. 2015a; Wei et al. 2015). However, the first study to explore the role of melatonin in drought tolerance in male reproductive organs was attempted by Hu et al. (2020). The authors studied the effects of exogenous melatonin (100, 200, and 1000 μM) on male fertility and related carbohydrate metabolism in drought-stressed anthers of cotton cultivar Yuzaomian 9110. Results showed that exogenous melatonin can enhance the concentration of endogenous melatonin in drought-stressed anthers and also improve the water status by 1.4–14.2 folds. Also, melatonin application significantly improves the translocation of carbon assimilates to drought-stressed anthers which otherwise is inhibited by drought. Drought lowers male fertility in plants by modifying carbohydrate metabolism. Under the conditions of drought, pollen viability and germination are restricted due to a decline either in the deposition of starch or the hydrolysis of sucrose into hexoses, or the generation of adenosine triphosphate (ATP) in anthers. Hu et al. (2020) reported that exogenous melatonin can improve male fertility under drought conditions by regulating the carbohydrate metabolism. In their study, the application of exogenous melatonin in drought-stressed anthers led to enhancement in the activities of ADP-glucose pyrophosphorylase and soluble starch synthases which in turn increases the starch accumulation. Exogenous melatonin was also reported to generate more ATP for reproductive activities and also accelerate the hydrolysis of sucrose by increasing the activities of sucrose synthase and acid and alkaline invertases.
Cut flowers suffer from a short life span post-harvest. To meet the demands for high-quality freshly cut flowers, preservation of cut flowers is essential. Low-temperature storage is one of the most important post-harvest handling procedures for cut flowers. However, flowers develop chilling injuries during this time which decreases their quality and negatively affects consumer preferences. Many compounds are used as protective and preservative factors in the cut flowers industry like γ-aminobutyric acid (GABA), putrescine, spermidine, etc. The role of melatonin in abating chilling injury in cut flowers was studied for the first time in cut anthurium flowers (Aghdam et al. 2019). It was shown that exogenous melatonin at 1, 10, 100, and 1000 μM can ameliorate chilling injury in cut anthurium flowers during storage at 4 °C for 21 d by 11, 29, 51 and 31%, respectively, compared with that of untreated flowers, (Aghdam et al. 2019). Flowers treated with 100 μM melatonin show lower electrolyte leakage and malondialdehyde concentration during cold storage and authors speculated that high NADPH oxidase activity may be responsible for signaling H2O2 concentration in treated flowers. Authors also reported higher alternative oxidase gene expression which was accompanied by higher activities of catalase, superoxide dismutase, ascorbate peroxidase, and glutathione reductase, and higher concentrations of ascorbate and glutathione. It was linked to protection from the damaging effects of H2O2 at 4 °C. A recent study on a similar aspect in carnations also shows the efficacy of melatonin in prolonging the vase life of a cut flower (Lezoul et al. 2022). The authors evaluated the effect of different concentrations of melatonin (0.01, 0.1, and 1 mM) on the vase life of cut carnations flowers cv. Baltico. It was observed that melatonin at 0.1 mM concentration increases the vase life of cut carnations by up to 10 days.
The results obtained from the above studies underline the potential role of melatonin in improving reproductive performance, thereby yield of crop plants under unfavorable environmental conditions, and also as a tool for post-harvest management of horticultural crops.
4 Conclusions and Future Perspectives
Melatonin acts at various levels of flowering and flower development. High amounts of melatonin before flowering leads to a delay in flowering. Molecular mechanisms show that melatonin delays flowering by upregulating the transcription of FLC and consequently inhibiting the meristem transition. Melatonin also increases the stabilization of DELLA proteins which induce a late-flowering effect. It is speculated that melatonin-mediated stabilization of DELLAs and consequently delayed flowering may involve NO. Another aspect of melatonin-mediated control of flowering is the suppression of melatonin signaling and/or biosynthesis by SL which induces earlier flowering. Although downregulation of melatonin is required before flowering, it is the opposite during flower development. Melatonin concentrations are highest in the initial stages of flower development and decrease progressively till fruit development. Melatonin is required for the development and protection of male gametophytes and is probably involved in the scavenging of ROS in general. Melatonin also has a putative regulatory role in floral aroma enhancement and inducing parthenocarpy. It is involved in enhancing stress tolerance during male gametophyte development through ROS scavenging and carbohydrate metabolism under heat stress and drought stress respectively.
Accumulation of enormous data on the functions of melatonin clearly shows that it is a regulator of multiple aspects of plant growth and development. So much so, that the possibility of melatonin as a phytohormone has also been raised after the identification of the putative melatonin receptor CAND2/PMTR1 in plants. However, there are several aspects especially ones related to flowering that need to be deciphered and could be the aim of future studies. In-depth investigations are required to understand the exact role of melatonin in delaying flowering. Future studies should explore how melatonin signaling is switched off or downgraded before flowering. Also, the precise cross-talk between SL and melatonin in flowering is an area of further investigation. The data generated can be of immense value to horticultural species. The involvement of NO in many of the responses mediated by melatonin has been the subject of many studies. Investigation into the genetic regulation of NO and melatonin can increase our knowledge of the effects of melatonin on flowering.
Understanding of the functions of melatonin during flower development is largely limited. Investigations in the area will help clarify how melatonin is involved in so many cellular and physiological activities during flower development. The effect of melatonin on female gametophyte development and stress tolerance is completely untouched. Likewise, investigation of melatonin during the progamic phase in plants will be interesting as it is a very important phase for successful reproduction. Concerted interactions occur between pollen and pistil during the progamic phase. Carbohydrates in the pistil are essential for normal pollen tube growth. However, heat stress results in substantial changes in the carbohydrate balance of pollen and pistil. Future studies can explore applications of exogenous melatonin to rescue plants from the ill-effects of heat stress during the progamic phase. Given the diverse roles of melatonin in plants, it will be beneficial to convert these findings into commercial outputs.
References
Abbas F, Ke Y, Yu R, Yu Y, Amanullah S, Jahangir MM et al (2017) Volatile terpenoids: multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 246:803–816
Abbas F, Zhou Y, He J, Ke Y, Qin W, Yu R, Fan Y (2021) Metabolite and transcriptome profiling analysis revealed that melatonin positively regulates floral scent production in Hedychium coronarium. Front Plant Sci 12:808899
Aghdam MS, Jannatizadeh A, Nojadeh MS, Ebrahimzadeh A (2019) Exogenous melatonin ameliorates chilling injury in cut anthurium flowers during low temperature storage. Postharvest Biol Technol 148:184–191
Ahammed GJ, Xu W, Liu A, Chen S (2019) Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ Exp Bot 161:303–311
Ahn H-R, Kim Y-J, Lim Y-J, Duan S, Eom S-H, Jung K-H (2021) Key genes in the melatonin biosynthesis pathway with circadian rhythm are associated with various abiotic stresses. Plan Theory 10:129
Ansari M, Rafiee K, Yasa N, Vardasbi S, Naimi SM, Nowrouzi A (2010) Measurement of melatonin in alcoholic and hot water extracts of Tanacetum parthenium, Tripleurospermum disciforme and Viola odorata. Daru 18:173–178
Arnao MB, Hernandez-Ruiz J (2006) The physiological function of melatonin in plants. Plant Signal Behav 1:89–95
Arnao MB, Hernandez-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19:789–797
Arnao MB, Hernandez-Ruiz J (2015) Functions of melatonin in plants: a review. J Pineal Res 59:133–150
Arnao MB, Hernandez-Ruiz J (2018) Melatonin and its relationship to plant hormones. Ann Bot 121:195–207
Arnao MB, Hernandez-Ruiz J (2020) Melatonin in flowering, fruit set and fruit ripening. Plant Reprod 33:77–87
Arnao MB, Hernandez-Ruiz J (2021) Melatonin as a plant biostimulant in crops and during post-harvest: a new approach is needed. J Sci Food Agric 101:5297–5304
Back K (2021) Melatonin metabolism, signaling and possible roles in plants. Plant J 105:376–391
Boccalandro HE, González CV, Wunderlin DA, Silva MF (2011) Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: evidence of its antioxidant role in fruits. J Pineal Res 51:226–232
Byeon Y, Back K (2014) An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J Pineal Res 56:408–414
Cao J, Murch SJ, O’brien R, Saxena PK (2006) Rapid method for accurate analysis of melatonin, serotonin and auxin in plant samples using liquid chromatography-tandem mass spectrometry. J Chromatogr A 1134:333–337
Cao S, Shao J, Shi L, Xu L, Shen Z, Chen W, Yang Z (2018) Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci Rep 8(1):806
Cao SH, Luo XM, Xu DG, Tian XL, Song J, Xia XC, Chu CC, He ZH (2021) Genetic architecture underlying light and temperature mediated flowering in Arabidopsis, rice and temperate cereals. New Phytol 230:1731–1745
Chen S, Li H (2017) Heat stress regulates the expression of genes at transcriptional and post-transcriptional levels, revealed by RNA-seq in Brachypodium distachyon. Front Plant Sci 7:2067
Chen GF, Huo YS, Tan DX, Liang Z, Zhang WB, Zhang YK (2003) Melatonin in Chinese medicinal herbs. Life Sci 73:19–26
Cano-Medrano RA, Darnell RL (1997) Cell number and cell size in parthenocarpic vs. Pollinated blueberry (Vaccinium ashei) fruits. Ann Bot 80:419–425
Dubbels R, Reitter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 18:28–31
Dudareva N, Pichersky E (2008) Metabolic engineering of plant volatiles. Curr Opin Biotechnol 19:181–189
Fang X, Turner NC, Yan G, Li F, Siddique KHM (2010) Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J Exp Bot 61:335–345
Farré-Armengol G, Fernández-Martínez M, Filella I, Junker RR, Peñuelas J (2020) Deciphering the biotic and climatic factors that influence floral scents: a systematic review of floral volatile emissions. Front Plant Sci 11:1154
Fu G, Jian S, Xiong J, Li Y, Chen H, Le M, Tao L (2011) Changes of oxidative stress and soluble sugar in anthers involve in rice pollen abortion under drought stress. Agric Sci China 10:1016–1025
Hardeland R (2005) Antioxidative protection by melatonin. Endocrine 27:119–130
Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int 35:627–634
Hu W, Cao Y, Loka DA, Harris-Shultz KR, Reiter RJ, Ali S, Liu Y, Zhou Z (2020) Exogenous melatonin improves cotton (Gossypium hirsutum L.) pollen fertility under drought by regulating carbohydrate metabolism in male tissues. Plant Physiol Biochem 151:579–588
Huang B, Chen YE, Zhao YQ, Ding CB, Liao JQ, Hu C, Zhou LJ, Zhang ZW, Yuan S, Yuan M (2019) Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front Plant Sci 10:677
Junker RR, Parachnowitsch AL (2015) Working towards a holistic view on flower traits-how floral scents mediate plant-animal interactions in concert with other floral characters. J Indian Inst Sci 95:43–67
Kinoshita A, Richter R (2020) Genetic and molecular basis of floral induction in Arabidopsis thaliana. J Exp Bot 71:2490–2504
Kolář J, Macháčková I (2005) Melatonin in higher plants: occurrence and possible functions. J Pineal Res 39:333–341
Kolar J, Machackova I, Eder J, Prinsen E, van Dongen W, van Onckelen H, Illnerova H (1997) Melatonin: occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry 44:1407–1413
Kolar J, Johnson C, Machackova I (2003) Exogenously applied melatonin affects flowering of the short-day plant Chenopodium rubrum. Physiol Plant 118:605–612
Korkmazab A, Değera Ö, Cucic Y (2014) Profiling the melatonin content in organs of the pepper plant during different growth stages. Sci Hortic 172:242–247
Lee YH, Lee K, Back K (2019) Knockout of Arabidopsis Serotonin N-acetyltransferase-2 reduces melatonin levels and delays flowering. Biomol Ther 9:712
Lei Q, Wang L, Tan D-X, Zhao Y, Zheng X-D, Chen H, Li Q-T, Zuo B-X, Kong J (2013) Identification of genes for melatonin synthetic enzymes in “Red Fuji” apple (Malus domestica Borkh. cv. Red) and their expression and melatonin production during fruit development. J Pineal Res 55:443–451
Lerner AB, Case JD, Takahashi Y, Lee TH, Wataru M (1958) Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc 80:2587
Lezoul NE, Serrano M, Ruiz-Aracil MC, Belkadi M, Castillo S, Valero D, Guillén F (2022) Melatonin as a new postharvest treatment for increasing cut carnation (Dianthus caryophyllus L.) vase life. Postharvest Biol Technol 184:111759
Li C, Tan DX, Liang D, Chang C, Jia D, Ma F (2015) Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J Exp Bot 66:669–680
Li M, An F, Li W, Ma M, Feng Y, Zhang X, Guo H (2016) DELLA proteins interact with FLC to repress flowering transition. J Integr Plant Biol 58:642–655
Li X, Ahammed GJ, Zhang X-N, Zhang L, Yan P, Zhang LP, Fu JY, Han WY (2021) Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J Hazard Mater 403:123922
Liu C, Xi W, Shen L, Tan C, Yu H (2009) Regulation of floral patterning by flowering time genes. Dev Cell 16:711–722
Liu N, Jin Z, Wang S, Gong B, Wen D, Wang X, Wei M, Shi Q (2015a) Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato. Sci Hortic 181:18–25
Liu YP, Yang J, Yang MF (2015b) Pathways of flowering regulation in plants. EMBO Rep 31:1553–1566
Liu J, Zhai R, Liu F, Zhao Y, Wang H, Liu L, Yang C, Wang Z, Ma F, Xu L (2018) Melatonin induces Parthenocarpy by regulating genes in gibberellin pathways of ‘Starkrimson’ pear (Pyrus communis L.). Front Plant Sci 9:946
Machackova I, Krekule J (2002) Sixty-five years of searching for the signals that trigger flowering. Russ J Plant Physiol 49:451–459
Martinelli F, Uratsu SL, Reagan RL, Chen Y, Tricoli D, Fiehn O, Rocke DM, Gasser CS, Dandekar AM (2009) Gene regulation in parthenocarpic tomato fruit. J Exp Bot 60:3873–3890
Mohamed MF, Abdulwareth A, Ahmed M, Elkelish A, Arnao MB, Linfeng L, Shaoying A (2020) Melatonin and its protective role against biotic stress impacts on plants. Biomol Ther 10:54–54
Mukherjee S (2019) Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide 82:25–34
Murch SJ, Saxena PK (2002) Mammalian neurohormones: potential significance in reproductive physiology of St John’s wort (Hypericum perforatum L.)? Naturwissenschaften 89:555–560
Murch SJ, Simmons CB, Saxena PK (1997) Melatonin in feverfew and other medicinal plants. Lancet 350:1598–1599
Murch SJ, Alan AR, Cao J, Saxena PK (2009) Melatonin and serotonin in flowers and fruits of Datura metel L. J Pineal Res 47:277–283
Nawaz MA, Huang Y, Bie Z, Ahmed W, Reiter RJ, Niu M, Hameed S (2015) Melatonin: current status and future perspectives in plant science. Front Plant Sci 6:1230
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Okazaki M, Ezura H (2009) Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivar Micro-Tom. J Pineal Res 46:338–343
Park S, Le TNN, Byeon Y, Kim YS, Back K (2013) Transient induction of melatonin biosynthesis in rice (Oryza sativa L.) during the reproductive stage. J Pineal Res 55:40–45
Poeggeler B, Balzer I, Hardeland R, Lerchl A (1991) Pineal hormone melatonin oscillates also in the dinoflagellate Gonyaulax polyedra. Naturwissenschaften 78:268–269
Pomares-Viciana T, Die J, Del Rio-Celestino M, Roman B, Gomez P (2017) Auxin signalling regulation during induced and parthenocarpic fruit set in zucchini. Mol Breed 37:56
Qi ZY, Wang KX, Yan MY, Kanwar MK, Li DY, Wijaya L, Alyemeni MN, Ahmad P, Zhou J (2018) Melatonin alleviates high temperature-induced pollen abortion in Solanum lycopersicum. Molecules 23:386
Qi H, Xia F, Xiao S (2021) Autophagy in plants: physiological roles and post-translational regulation. J Integr Plant Biol 63:161–179
Schaefer M, Hardeland R (2009) The melatonin metabolite N-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J Pineal Res 46:49–52
Schiestl FP, Kirk H, Bigler L, Cozzolino S, Desurmont GA (2014) Herbivory and floral signaling: phenotypic plasticity and tradeoffs between reproduction and indirect defense. New Phytol 203:257–266
Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G (2006) The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 20:898–912
Shi H, Wei Y, Wang Q, Reiter RJ, He C (2016) Melatonin mediates the stabilization of DELLA proteins to repress the floral transition in Arabidopsis. J Pineal Res 60:373–379
Siddiqui MH, Alamri S, Khan MN, Corpas FJ, Al-Amri AA, Alsubaie QD, Ali HM, Kalaji HM, Ahmad P (2020) Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J Hazard Mater 398:122882
Song S, Qi T, Huang H, Xie D (2013) Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol Plant 6:1065–1073
Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55:197–223
Sun C, Liu L, Wang L, Li B, Jin C, Lin X (2021) Melatonin: a master regulator of plant development and stress responses. J Integr Plant Biol 63:126–145
Tan DX, Manchester LC, Mascio PD, Martinez GR, Prado FM, Reiter RJ (2007) Novel rhythms of N-1-acetyl-N-2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. FASEB J 21:1724–1729
Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. Exp Bot 66:695–707
Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309:1056–1059
Wolf K, Kolar J, Witters E, van Dongen W, van Onckelen H, Machackova I (2001) Daily profile of melatonin levels in Chenopodium rubrum L. depends on photoperiod. J Plant Physiol 158:1491–1493
Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQ, Li DB, Cao YY, Weeda S, Zhao B, Ren S (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57:269–279
Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo YD (2015) Roles of melatonin in abiotic stress resistance in plants. J Exp Bot 66:647–656
Zhang H, Wang L, Shi K, Shan D, Zhu Y, Wang C, Bai Y, Yan T, Zheng X, Kong J (2018) Apple tree flowering is mediated by low level of melatonin under the regulation of seasonal light signal. J Pineal Res 66:e12551
Zhang Z, Hu Q, Liu Y, Cheng P, Cheng H, Liu W, Xing X, Guan Z, Fang W, Chen S, Jiang J, Chen F (2019) Strigolactone represses the synthesis of melatonin, thereby inducing floral transition in Arabidopsis thaliana in an FLC-dependent manner. J Pineal Res 67:e12582
Zhao Y, Tan DX, Lei Q, Chen H, Wang L, Li QT, Gao Y, Kong J (2013) Melatonin and its potential biological functions in the fruits of sweet cherry. J Pineal Res 55:79–88
Zhao D, Rong W, Ding L, Yanqing W, Jing S, Jun T (2018) Melatonin and expression of tryptophan decarboxylase gene (TDC) in herbaceous peony (Paeonia lactiflora pall.) flowers. Molecules 23:1164
Zheng W, Cole PA (2002) Serotonin N-acetyltransferase: mechanism and inhibition. Curr Med Chem 9:1187–1199
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khanduri, P., Roy, S.K. (2023). Functions and Prospects of Melatonin During Pre-fertilization Reproductive Stages in Plants. In: Mukherjee, S., Corpas, F.J. (eds) Melatonin: Role in Plant Signaling, Growth and Stress Tolerance. Plant in Challenging Environments, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-031-40173-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-40173-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40172-5
Online ISBN: 978-3-031-40173-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)