Abstract
Magnetic resonance imaging (MRI) is a noninvasive imaging technology that allows for three-dimesnional imaging of abdomen and pelvis imaging without utilizing any ionizing radiation. It offers unparalleled soft tissue contrast and functional details of anatomy along with high diagnostic sensitivity. For these reasons, MRI is the modality of choice for longitudinal studies, treatment follow-up, and pediatric studies. A typical closed-bore, whole-body MRI system consists of four major components: the primary superconducting magnet that is large enough to encompass the body of an adult subject, the gradient subsystem, the radiofrequency (RF) subsystem for transmitting and receiving the MRI signal, and the computers running the software that controls the entire scanner. To perform high-quality clinical MRI studies of the kidneys, certain MRI hardware specifications are essential to meet the competing demands of image quality, spatial resolution, and imaging speed tailored to the specific needs for accurate diagnosis of renal pathology. In this chapter, we review the basics of MRI, outline the key hardware components of an MRI scanner, and provide an overview of currently employed image acquisition techniques, with the goal of familiarizing the reader with how different hardware configurations impact image quality in state-of-the-art clinical kidney MRI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Magnetic resonance imaging (MRI) is a comprehensive diagnostic imaging modality with high soft tissue contrast capable of generating multiparametric images based on anatomical, metabolic, chemical, or physiological information from inside the body—noninvasively and without the use of ionizing radiation. It is a highly versatile tomographic imaging technique that acquires data as multiple slices or sections in any orientation, including oblique planes. An MRI system is composed of four main hardware components: (1) a primary, high-field strength magnet that is large enough to encompass the body of an adult subject, (2) the gradient subsystem, (3) the radiofrequency (RF) subsystem, and (4) computer systems that interface with and control the various components (Figs. 2.1, 2.2, 2.3, and 2.4). Understanding the interplay of these key components of the MRI system is foundational to acquiring diagnostic-quality MR images.
The most dominant, and usually also the most expensive, component of a closed-bore clinical MRI scanner is the powerful superconducting magnet that generates the strong static, stable, and homogenous magnetic field (denoted by B0) in which the subject is placed for the scan. In general, the higher the field strength, the stronger the resulting signal but also the higher price tag of the system. The second component is a set of three concentric gradient coil systems (oriented orthogonally in X, Y, and Z directions) close to the walls of the bore of the main coil. During the MRI scan, the gradient coils superimpose approximately linear, time-varying magnetic field variations upon the static B0 field used for spatial encoding and localization of the MR signal [1]. These oscillations are also the source of the characteristic knocking sounds that accompany MRI acquisitions. The third component is the RF system. Most notably, it consists of one or more coils near the subject that transmit the megahertz range electromagnetic field oscillations (B1) at the resonant frequency of the imaged nucleus (usually protons) to be excited. In response, the excited nuclei then emit the MRI signal that is detected by a receiver coil. Since transmitting and receiving RF signals are implemented as sequential events, they can be performed by the same coil although many MRI coil arrangements have separate transmit and dedicated receive elements for different anatomy locations to improve MRI image quality and signal to noise. Finally, the fourth component consists of multiple interconnected computer systems that provide the user interface, perform the digital-to-analog conversion for generating the gradient waveforms and the RF-excitation field B1, conduct the analog-to-digital conversion of the collected signals from the patient, and calculate the resulting images that can be displayed and interpreted on the console.
A unique feature of MRI is the intricate dependence of the obtained image information and quality on the temporal arrangement and variation of the applied RF fields and image-encoding gradients in the form of so-called pulse sequences. Over the decades, countless pulse sequences have been developed that are optimized for measuring relevant information about all the main organs and structures in the body. The focus of this book is the detection, staging, and monitoring of kidney diseases (KD) using measurements of renal function and structure that is critical in clinical nephrology as accessible with the help of MRI. In addition to established anatomical imaging techniques, novel pulse sequences such as dynamic contrast-enhanced MRI (DCE-MRI), arterial spin labeling (ASL), blood-oxygen-level-dependent (BOLD) MRI, and diffusion-weighted imaging (DWI) can provide a broad characterization of the kidney, including renal vascular perfusion, oxygenation, and glomerular filtration rate. The high spatial and temporal resolution provided by this modality is essential for functional MR urography techniques, and they can be performed longitudinally even in pediatric population as there is no exposure to ionizing radiation. This chapter is intended to outline the key hardware components and operational features of an MRI scanner and to provide an overview of currently employed renal MR imaging technologies and is written for a target audience of clinicians and MR technicians. The basic concepts and terminologies are explained, and a broad overview of the applications is given. An in-depth explanation of current state-of-the-art kidney imaging methods is provided, followed by novel technologies, approaches, and current advances in the field of kidney MRI in the final sections.
The MRI Signal
The principles of MRI physics can be conceptualized by envisioning that those nuclei of certain atoms produce nuclear magnetic resonance signal. Several different types of nuclei are suitable for MR imaging, but hydrogen nuclei, that is, protons, which are overly abundant in the human body as part of water and fat molecules, are by far the most commonly used. The hydrogen atom, consisting of single proton (nucleon), forms the MRI signal source in routine clinical MR images. Nucleons behave as small current-carrying loop and give rise to a small magnetic field, that is, they behave like tiny little bar magnets. Without an external magnetic field, these tiny magnets are randomly oriented, thereby producing no net magnetization as shown in Fig. 2.3a. However, once a subject is immersed in a magnetic field, these 1H protons align in two energy favorable states either in the direction of the external field or opposite to the direction as shown in Fig. 2.3b. As a consequence, at the field strength of a typical clinical MRI scanner for every million atoms, there is only approximately one atom more aligned than anti-aligned with the field, but due to the huge number of atoms in the human body, this tiny excess provides enough net magnetization to give rise to an easily measurable signal. There are two options available for increasing the number of nuclei that are aligned with the external magnetic field: (1) cooling the sample close to absolute zero, which for obvious reasons would be impractical for human subjects, and (2) increasing the field strength of the external magnetic field B0. Hence the interest lies in imaging at higher field strength to obtain images with higher signal-to-noise ratios.
At equilibrium, the vector of the generated net magnetization Mo is aligned with the direction of the magnetic field vector B0 such that, by definition, only its Mz component, that is, the longitudinal magnetization, is nonzero. Similar to a spinning top that starts to wobble in the Earth’s gravitational field when tipped, this magnetization begins to precess about B0 when tipped by the magnetic vector component B1 of an RF pulse perpendicular to the direction of Mo. Once Mo is tipped out of its complete alignment with B0 its Mx and My components, the so-called transverse magnetization Mxy are no longer zero. As Mo precesses about B0, its transverse magnetization components oscillate, which in turn induces an electric current in an appropriately oriented RF receiver coil as described in more detail below. The frequency of the precession f0 is proportional to the strength of the magnetic field and the gyromagnetic ratio γ, an intrinsic property specific to each type of nucleus. This relationship was reported by Sir Joseph Larmor, summarizing it in what is now called the Larmor equation:
Under the condition that the frequency of an applied RF pulse with a magnetic field B1 perpendicular to B0 matches the precession frequency f0, it is called to be “on resonance,” the origin of the term magnetic “resonance” imaging. This has the effect that Mo is simultaneously precessing about B0 with frequency f0 and B1 with frequency f1, where f1 can be calculated equivalent to Eq. 2.1. For as long as the RF pulse is turned on, Mo continues to be tipped toward the transverse Mxy plane and then becomes inverted before returning to its original alignment with B0 and so on. Therefore, the temporal modulation and amplitude of the applied B1 allow the user to control the tipping angle or flip angle α the magnetization Mo experiences and which fraction of the total available magnetization ends up in the transverse plane, forming the MRI signal amplitude S0 as described by
In the following section, we will describe the hardware that is required to generate, spatially localize, and measure this MR signal.
MRI Hardware
The Primary Magnet
Usually, low magnetic fields are measured using the standard unit Gauss (G). The most familiar and ubiquitous magnetic field we experience, the Earth’s magnetic field, measures about 0.5 G, while most refrigerator magnets fall in the 500–1000 G range. The majority of current clinical MRI scanners are cylindrical closed-bore superconducting magnets, which can produce magnetic fields that are thousands of times more powerful than the Earth’s magnetic field. Because of their very strong magnetic fields, medical magnets are usually measured using a larger unit, much like pounds and tons, called Tesla (T), with 1.0 T equal to 10,000 G. Within the MRI suite, the location of the 5 G line surrounding the periphery of the MRI scanner should have controlled access, that is, the distance from the main magnet where the magnetic field drops to below 5 G is considered to be a safe level of field exposure for the general, unscreened public to move around freely.
MRI scanners can be described by the magnetic field strength of their primary magnet or by the precession frequency of the hydrogen nuclei within the magnet. For example, a 1.5 T scanner can also be referred to as a 63 MHz scanner based on the Larmor frequency of hydrogen at this field strength because \( \frac{\gamma_H}{2\pi }=42.58\;\mathrm{MHz}/\mathrm{T} \) (see Eq. 2.1). Strong magnets are an essential component of all MRI scanners, and in the decades since the introduction of clinical MRI, all three types of magnets, that is, permanent, resistive, and superconducting, have been used. Superconducting magnets have the advantage of producing strong and stable magnetic fields, and once the superconducting windings are energized with electrical power, no additional current is required to maintain the magnetic field produced by superconducting coils. In superconducting and permanent MR scanners, the magnetic field is always on as compared to the resistive electromagnet design where the field can be switched off.
Clinical scanners using superconducting magnets of 3 T and research scanners with even stronger magnetic fields have become commonplace. While there are some theoretical and practical limitations to medical imaging at 3 T, the motivation behind this migration to higher field strengths is to maximize the available MR signal by recruiting a larger proportion of the available hydrogen nuclei in the body for imaging. The linear increase in the signal-to-noise ratio based on field strength that follows can in principle be used to provide some combination of better resolution, faster imaging, or thinner sections. However, there are some disadvantages to utilizing MR scanners with higher field strengths such as 3 T: greater RF power deposition in the body of the patient in the form of heat could pose a safety challenge for patients with implants, decreased T1 contrast, dielectric shading effects due to nonuniform RF penetration especially during body imaging, increased risk of projectile effects from magnetic metal in the room, and larger chemical shift image artifacts. The latter are caused by the spatial misregistration of signals originating from water and fat protons, which have slightly different Larmor frequencies caused by the differences in their chemical environment as will be discussed below in Sect. 2.3.2. A less common, but still potentially problematic, issue is the increased level of signal blowout (loss) artifacts caused by susceptibility differences in air-tissue interfaces or the local magnetic field changes due to the presence of metallic objects inside the body. As shown in Eq. 2.1, such field variations result in changes in resonance frequency which gives rise to signal cancelations in the affected volumes. For some applications, however, like magnetic resonance angiography (MRA), functional MRI, and MR spectroscopy, or for detailed structural imaging, the benefits outweigh these drawbacks. Modern 3 T scanner designs and pulse sequence techniques alleviate many of the negative effects and harness the higher field for advanced imaging applications.
Gradients
The gradient system is a key component of the MRI machine, being responsible for the spatial encoding in image generation and integral to controlling a range of physiological imaging contrasts, most notably diffusion-weighted MRI. The design and performance of the gradient system have substantial influence on the overall quality of the acquired images and have been the focus of intense engineering efforts over the last three decades in the quest for better image quality and ever-faster imaging speed. Gradient performance is parameterized by the maximum gradient amplitude, which is measured in mT/m, and the slew rate, which describes how fast a gradient can attain a desired amplitude within a given amount of time and is measured in T/m/s. Since the inception of MRI, gradient amplitudes and slew rates have increased by orders of magnitude, roughly doubling every 10 years since the 1990s. For localization of the MRI signal, three additional sets of coils called gradients are incorporated in the scanner bore. Gradient coils produce time-varying magnetic fields across the field of view (FOV) to be imaged that linearly increase or decrease the main magnetic field in a spatially dependent manner along the X, Y, and Z directions. Depending on their position along the gradient, the nuclei inside the body are temporarily exposed to magnetic fields of different strength and hence differ in their precessional frequencies. This behavior is exploited during MRI acquisitions in two different ways. For one, as will be explained in more detail in Sect. 2.3.3, the RF pulses used to tip the magnetization vectors are only effective over a certain range of frequencies. Thus, it is possible to excite transverse magnetization and thereby signal, only in specific, well-defined regions of the body, usually in the form of a thin imaging slice. Secondly, once a slice has been selected in this manner, additional spatially encoding with the gradient coils is now required within the slice to obtain a two-dimensional (2D) image.
In MRI, each imaging dimension is encoded separately. In a typical two-dimensional image acquisition, one direction, for example, along the x-axis, is termed the frequency-encoding direction while the other, in this example the y-axis, is called the phase-encoding direction (Fig. 2.5). If no slice selection with an RF pulse was performed, spatial encoding along the z-axis, the second phase-encoding direction, would be necessary and yield a three-dimensional image data set. Encoding the MR signal along the frequency-encoding direction can be easily understood by picturing a piano keyboard. Each of the keys is associated with a specific note: low frequencies on the left and high frequencies on the right. Upon hearing a particular note, a trained listener can exactly identify which key along the length of the keyboard had been pressed by the pianist even without being able to see the keyboard. Similarly, for as long as the frequency-encoding gradient is activated, all the transverse magnetization at a particular location along the x-axis is precessing with a specific frequency. The receiver coil integrates all the signals with their characteristic frequencies, individually weighted by the strength of the signal originating from the associated spatial location and measures the combined oscillations. This would be equivalent to all keys on our imaginary piano being pressed simultaneously but with a force that differs from key to key such that some notes are louder while others much softer. Of course, regardless how well trained, no human observer would now be able to still identify the amplitude of each note in the resulting cacophony. Fortunately, mathematics offers a tool for analyzing a signal consisting of the superposition of many different frequencies by means of the Fourier transform. Applying a one-dimensional (1D) Fourier transform to a frequency-encoded signal reveals the amplitudes of the different signal frequencies as vertical bands in our two-dimensional image matrix. However, to resolve the image information along the y-axis, this information needs to be first encoded during additional steps in the MR data acquisition process.
Unfortunately, simply applying frequency encoding along the y-axis again will not suffice because, after Fourier transform along the y-direction, the image matrix would now only contain horizontal bands, and combining them with the vertical bands of the first frequency encoding will not result in an image. Instead, encoding along the y-direction has to be performed in a multistep phase-encoding procedure (Fig. 2.6). This is achieved by activating the magnetic field gradient along the y-axis just like the frequency-encoding gradient along the x-axis. However, while the frequency-encoding gradient is active for the entire duration of data sampling, the phase-encoding gradient is only turned on for a short amount of time and before data sampling begins. As during frequency encoding, the transverse magnetization precesses with a frequency that is a function of its location but this time as a function of its location along the y-axis. Although no measurements are conducted during phase encoding, spatial information is imprinted on the transverse magnetization in the form of a location-dependent phase shift because the higher the precession frequency at any given y-position, the faster the transverse magnetization at this location is precessing and the more phase is accumulated while the phase-encoding gradient is turned on. Then the signal is measured again while only the frequency-encoding gradient is active. This phase-encoding procedure is repeated once for each line in the measured image but with a higher amplitude of the y-gradient such that each phase-encoding step imparts a steeper linear phase shift along the y-axis. Returning to our piano analogy one last time, phase encoding is the equivalent of adding a position-dependent, linearly increasing delay until any given key is pressed. Once all keys are activated, the sound is recorded in the same manner as before but for each phase-encoding step the rate with which the delay time increases also increases, resulting in a slightly different modulation of the combined notes for each phase-encoding step. Following a two-dimensional Fourier transform, all these modulations in the MRI measurement reveal the signal contributions of each y-position for each of the vertical one-dimensional bands along the x-position, that is, the complete two-dimensional image information. In a three-dimensional measurement, for each phase-encoding step along the z-axis, all phase-encoding steps along the y-axis have to be repeated prior to frequency encoding, lengthening the total acquisition time proportionally.
The final step of image computation, a Fourier transform of the measured raw data, has the interesting and often confusing implication that MRI measurements are actually not performed in image space, such as taking a photograph or acquiring a CT image, but in the spatial frequency space, or k-space, created by the encoding of spatial positions as phase and frequency shifts by the application of magnetic field gradients. This peculiar characteristic has a tremendous impact on how various choices in measurement parameters affect image contrast, signal-to-noise ratios, acquisition speeds, and image artifacts that are highly nonintuitive without this important insight. Further, the concept of phase and frequency encoding provided above, assumes a Cartesian acquisition scheme in which a square (2D) or cube (3D) in k-space is filled with lines of sampled data from left to right, top to bottom, and front to back. However, while the number and location of points in k-space that should be filled with measurement data are dictated by the FOV and spatial resolution of the final image, the order in which they are filled is, in principle, arbitrary. This circumstance is exploited by several advanced acquisition techniques that employ a variety of sampling orders, such as radial or spiral trajectories to name two of the most popular ones, each with their own advantages and disadvantages.
RF Coils
While advances in the MRI hardware such as higher field strengths and improved gradient performance have been substantial, recent progress in RF technology has led to increased spatial and temporal image resolution, improved signal uniformity, and shortened MRI scan durations. MRI RF coils are essential components for every MRI examination, as they are the “antennas” of the MRI system. RF coils have two functions: first, to excite and manipulate transverse magnetization by transmitting RF power (Tx coil), and second, to detect the precessing transverse magnetization of the excited nuclei as an electric current via electromagnetic induction (Rx coil).
RF coils serving both as transmitter and receiver, especially for head and extremity imaging, used to be the norm on older MRI systems. These days, modern scanners mostly utilize the integrated body coil for RF transmission and receive-only RF coils close to the imaged body part for signal reception. These Rx coils are commonly constructed with multiple smaller coil elements, each with its own receive chain, called phased array coils. The combination of multiple surface coils into an array allows for a good signal-to-noise ratio over a large FOV. The signals from each coil in the array are subsequently combined to create a single image. In addition, phased array volume coils can greatly accelerate image acquisition by collecting data from different regions of k-space in parallel.
As mentioned before, the RF transmit coil generates a B1 field vector perpendicular to which the net magnetization vector M is precessing. A typical MRI data acquisition begins with the application of an RF excitation pulse that tips M toward the transverse plane and thereby generates a signal (see Eq. 2.2). However, depending on the size and the design of the transmit coil, the created B1 amplitude can be very location dependent and affects a poorly defined volume of the body. One way to address the problem is the use of field gradients to spatially encode all the excited volume as described in Sect. 2.3.2. The drawback of this approach is that, for a given image resolution, the larger the FOV, the more phase-encoding steps are required and the longer the total acquisition time. In fact, long scan times make the collected image data sets highly susceptible to patient motion, and a full three-dimensional measurement covering a large volume may even be completely unnecessary for the diagnostic objective of the scan. A more practical solution is to limit the effect of the RF pulse to specific, user-selectable thin slices using the same gradient coils as for spatial encoding.
For an RF pulse to tip M, its frequency has to match the precession frequency f0 of M in the transverse plane (see Sect. 2.2). However, an RF pulse never consists of just a single frequency, but rather a band of frequencies around its center frequency and, depending on the amplitude modulation of the pulse, multiple frequency side lobes. Nevertheless, RF excitation pulses optimized for two-dimensional slice selection will have a sharp center frequency band that determines the bandwidth of the pulse and only low-amplitude side lobes. By applying, for instance, a magnetic field gradient along the z-axis, f0 varies linearly in the patient’s head-to-toe direction. Therefore, changing the center frequency of the RF excitation pulse will move the center of the targeted slice up or down the z-axis. The thickness of the selected slice is dictated by the bandwidth of the pulse and the steepness of the field gradient. The smaller the bandwidth and the larger the slope, the thinner the slice and vice versa. Nevertheless, because the slice location and profile are dependent on an appropriate matching of the RF pulse parameters with the spatial precession frequency distribution, any imperfections in the RF pulse amplitude or the linearity of the magnetic field gradients can result in slice distortions and out-of-slice excitations that give rise to image artifacts.
In addition to generating signal in specific locations, RF excitation pulses also have an important function in removing unwanted signal from images. Since in an MR image acquisition, spatial locations are identified by the precession frequencies of the transverse magnetization in the associated volume, the implicit assumption is that, in the absence of frequency-encoding gradients, all magnetization precesses with the same f0. However, the resonance frequency of a nucleus does not truly depend on the applied external field strength B0 but rather on the field strength at the nucleus itself. These two quantities are usually almost identical except for a tiny shielding effect by the electron cloud surrounding the nucleus. It is the size and the shape of this electron cloud as dictated by the type of nucleus and its chemical environment that causes the magnetic field at the nucleus to be slightly lower than B0. For conventional proton MRI of the human body, only the protons in water and fat molecules give rise to strong signals but with water having a 3.5 ppm higher resonance frequency than fat, which translates into a frequency difference of approximately 224 Hz at 1.5 T or 447 Hz at 3 T. Since the MRI acquisition and reconstruction do not “know” about the presence of two resonance frequencies in the signal, the final image will consist of a superposition of a fat and a water image that are shifted along the frequency-encoding direction relative to each other, potentially obscuring important pathological abnormalities.
While there are many techniques available for suppressing the undesirable fat signal in MR images, some of the simplest ones take advantage of the 3.5 ppm difference in resonance frequency of fat and water. By applying a narrow-bandwidth RF pulse with a 90° flip angle centered at the fat resonance frequency, the fat magnetization vector can be tipped into the transverse plane and then be dephased with the help of so-called spoiler gradients (Sect. 2.4.5). Once the fat signal has been thereby saturated, the acquisition of the water signal can begin. Depending on the specifics of the image acquisition, this procedure may need to be performed only once at the start of the measurements or repeatedly for each phase-encoding step.
A Walk Through k-Space
k-space is a raw data matrix which is essentially an array of number representing spatial frequencies in MRI image [2]. The letter “K” represents a wavenumber defined as reciprocal of wavelength of a wave. Wavenumber is a number of waves or cycles per unit distance.
The concept of wavelength was first introduced by Heinrich Kayser, a German physicist when he was working on the emission spectra of elementary substances [3]. Unlike temporal frequency, which is defined as number of cycles per unit time, spatial frequency is defined as number of cycles per unit distance [4].
General Properties of K-Space
-
1.
In general, the K-space grid is usually square and evenly spaced. Regular spacing makes data acquisition and processing easier, faster, and more efficient. The cells of K-space are commonly displayed on rectangular grid with principal axes kx and ky. The kx and ky axes of K-space correspond to the horizontal (x-) and vertical (y-) axes of the image. The K-axes represent spatial frequencies in the x- and y-directions.
-
2.
Each point (kx, ky) in the K-space does not correspond with individual pixel (x,y) in the image. In fact, each point in K-space contains information about spatial frequency and phase about every pixel in the image. Equally, each pixel in the image maps to every point in the K-space.
-
3.
While the data near the center of K-space corresponds to low spatial frequencies and provide information about image signal, brightness, contrast, general shapes, and contours of the object, data from the periphery corresponds to high spatial frequencies and provide information about image resolution (details), edges, and sharp transitions (Fig. 2.7).
-
4.
Center of the K-space has the highest signal intensity because the central row is acquired with no phase-encoding gradient, and therefore no destructive wave interference occurs and no signal loss occurs. Additionally, the central column of K-space coincides with the peak of an echo of a pulse sequence.
-
5.
The K-space have an important property known as conjugate or Hermitian symmetry. It refers to pairs of points that are located diagonally from each other across the origin of K-space. Two data points, which are mirror images across the origin of K-space, possesses identical amplitudes but opposite phases. However, conjugate symmetry may not always be perfect because of the presence of phase errors. The sources of these phase errors include B0 inhomogeneity, susceptibility effects, eddy currents, physiologic motion, and spatial variations in transmit RF uniformity.
-
6.
The distance between adjacent rows and columns is denoted as ΔK. The distance from the center of K-space to the edge is called Kmax.
Location of spatial frequencies in K-space. The center of K-space contains low spatial frequency information which determines overall image signal, general shape, brightness, and contrast. The periphery of K-space contains high spatial frequency information which determines image resolution (general details)
While the reciprocal of ΔK determines field of view (FOV) in the image as ΔK = 1/FOV, the Kmax determines the pixel size (ΔW) as ΔW = 1/KFOV where KFOV = (+kmax) − (−kmax) = 2kmax.
Partial Fourier Techniques and K-Space
Phase-Conjugate Symmetry
Phase-conjugate symmetry is also known as half Fourier or half scan. In this case, top half of the data is actually acquired and lower half of the data is synthesized using the property of conjugate (Hermitian) symmetry. Phase-conjugate symmetry allows acquisition of data using only half the normal number of phase-encoding steps, therefore potentially reducing scanning time by 50%. However, in the actual practice, more than half the lines of K-space (approximately 60%) are acquired (Fig. 2.8). These extra lines are then used to generate phase correction maps of K-space. Using phase-conjugate symmetry, spatial resolution is preserved. However, scanning time is reduced at expense of lower signal-to-noise ratio (SNR).
Read-Conjugate Symmetry
Read-conjugate symmetry is also known as partial-echo or half-echo imaging. In this case, right half of the K-space is acquired and left half of the K-space is synthesized (Fig. 2.9). Unlike phase-conjugate symmetry, the full number of phase-encoding steps are acquired in read-conjugate symmetry. Hence, there is no direct scan time saving. However, the advantage in read-conjugate symmetry is that only a part of echo is sampled, thus reducing the echo time (TE). Both read-conjugate and phase-conjugate symmetry techniques are useful but these two techniques cannot be used together.
K-Space Trajectories and Image Reconstruction
There are different sampling methods of filling K-space by digitized MR signals, and these methods include Cartesian (row by row, also known as rectilinear), radial, spiral, and zigzag. Cartesian filling of K-space can be sequential filling of K-space lines from one end to another end of K-space or centric ordering of K-space lines starting from the center to periphery or starting from periphery to center of K-space.
Cartesian data acquisition method is very popular. The main advantage of using Cartesian sampling is that the data elements are regularly spread out and can be placed directly into the standard array processors which are used for efficient fast Fourier transform (FFT).
However, radial or spiral sampling of K-space data is fast gaining momentum. Unlike Cartesian methods, radial sampling does not have unique frequency and phase-encoding directions. Noise arising from moving anatomic structures does not propagate as discrete ghosts along a particular phase-encoding direction, but the noise is simply distributed more diffusely across the entire image. Therefore, radial imaging is relatively less sensitive to motion artifacts. Moreover, the center of K-space is oversampled, and this redundancy is usually exploited to detect and correction for any motion artifacts, if any. The reduction in motion and aliasing artifacts are some of the advantages of using radial sampling. The disadvantage includes nonuniform collection of data points, thus requiring an iterative process known as gridding in which data points are morphed into a Cartesian arrangement prior to performing FFT. In addition to the Fourier transform and iterative reconstruction techniques, newer reconstruction algorithms are developed to improve speed of acquisition or generate higher spatial resolution from limited samples ok K-space data collected. Compressed sensing (CS) reconstruction is a type of MRI reconstruction that uses mathematical algorithms to reduce the amount of data required to produce an image, thus reducing the scan time. For compressed sensing to work, underdamping of the data must be performed in an incoherent (semi-random) manner. CS requires that the acquired image is compressible. In other words, it needs to have a sparse representation in a well-known transform domain. If this condition is met, and K-space is undersampled such that the undersampling artifacts have a noise-like appearance in this transform domain, the image can be reconstructed using a nonlinear iterative optimization. Current CS reconstruction approaches employ variable density random Poisson disk and golden-angle radial semi-random acquisition of data with preferential sampling near the center of K-space. The level of scan acceleration is governed by the compressibility of the image rather than the factors that affect standard parallel imaging and may highly depend on the application and the basic SNR conditions at hand. While CS is typically suited to three-dimensional applications and excellent results are obtained in two-dimensional functional studies, the sparsity requirements are often not met with static two-dimensional sequences. The most recent innovation and potentially the most exciting is the use of artificial intelligence (AI) image reconstruction techniques to denoise and sharpen our data in our quest to reduce scan times. We know from the literature and every MR lecture we attend that the final MR image is a compromise between three tightly associated factors: SNR, resolution, and acquisition time. Minor modifications of one factor significantly affect the others—and AI or deep learning (DL) reconstruction methods have the potential to minimize these effects. DL-based reconstruction is a type of MRI reconstruction that uses artificial neural networks trained on lots of prior data to reconstruct the images. Deep learning reconstructions have been successfully employed by vendors to perform image denoising and artifact removal. By using deep learning, we can increase the resolution, introduce higher parallel acceleration factors, or reduce the number of averages. We can intentionally create an image with more noise present in the data and use DL in the reconstruction phase to produce images of diagnostic quality. These techniques have enabled us to reduce the scan times of our two-dimensional acquisitions that were previously not suitable for iterative reconstruction techniques. These DL techniques have gained popularity in recent years due to their ability to produce higher SNR images with reduced scan time or generate higher spatial resolution images at clinically acceptable scan times.
Signal Relaxation Mechanisms and Image Contrasts
At thermal equilibrium, M and B0 are aligned without any signal-generating transverse magnetization components. If this equilibrium is disturbed by an RF pulse, any created signal will eventually decay away, and M will return to its original alignment again. This process is caused by several types of relaxation mechanisms that have environment- and tissue-specific time constants with the two most important being: T1 (longitudinal or spin lattice) and T2 (transverse or spin/spin) [1]. It is the large range of these constants in biological tissues that gives MR its excellent soft tissue contrast.
T1 Relaxation
T1 relaxation describes the recovery of the longitudinal magnetization Mz back to its thermal equilibrium value M0 following a perturbation by an RF pulse (Fig. 2.10) with an exponential recovery time constant T1:
where t is the time after an ideal 90° RF excitation pulse. Thus, T1 is the time at which Mz(t) has returned from 0 to 1/e (~63%) of M0. In the process, the excited nuclei transfer the absorbed RF energy to surrounding water and macromolecules, the “lattice,” realigning M with B0. Hence, T1 relaxation is also called longitudinal, thermal, or spin-lattice relaxation [1].
The rate at which the spin magnetization (Mz) at time t recovers to the original magnetization (M0) at time t = 0 is called T1 relaxation time, where B0 is the external magnetic field. T1 relaxation time measured is the time for the longitudinal magnetization to re-grow to about 63% of its final value (the flipped nuclei realign with the main magnetic field). (© [Suraj D. Serai, 2023. All rights reserved])
T1 relaxation is stimulated by the presence of local magnetic fields oscillating at the resonance frequency f0 of the nuclei. The main source for these fields is the dipole moment of nearby protons attached to the same or other molecules. As the molecules tumble around due to thermal motion, their dipole moments create rapidly fluctuating magnetic fields in their vicinity. Because of their small size, free water molecules at body temperature rotate, on average, at a much higher rate than f0 even for the strongest clinical MRI systems. As a result, only a relatively small number of water molecules happen to generate magnetic field fluctuations at f0 at any given point in time, and T1 in pure water is quite long (~4 s). The motion of large protein molecules or lipid chains, on the other hand, is much slower than that of water molecules, and their mean rotational frequency is much closer to f0, giving rise to a much shorter T1 relaxation. It is, therefore, the ratio of free water to protons bound to macromolecules that determines the T1 of a given tissue type. As a rule of thumb, the more “water-like” the tissue, the longer the T1 and the more protein dense or fatty the shorter the T1. For instance, the T1 of cerebrospinal fluid is close to that of water (4 s) while the T1 of body fat is only about 250 ms with all other body tissues falling somewhere in between.
How to Measure T1 Relaxation Time for Body Imaging
According to Eq. 2.3, T1 maps could be generated by saturating Mz with a 90° excitation pulse and then, after a certain delay time, perform an image acquisition. If the transverse magnetization has been sufficiently spoiled (see Sect. 2.4.5), then the resulting images for very short delay times would have very low signal intensities but would rapidly brighten as the delay increases until reaching their normal intensities for delay times that are much longer than T1 (>~3 * T1). Tissues with short T1 would thereby appear brighter sooner than those with long T1, and by fitting Eq. 2.3 to the signal intensity in each pixel as a function of the delay time, T1 maps could be calculated. Although this approach would be most accurate, it is also so time consuming that it is impractical for diagnostic studies.
The preferred way of measuring T1 relaxation in body MR imaging is the look-locker method. For a more accurate measurement, it applies a 180° inversion pulse such that Mz recovers according to
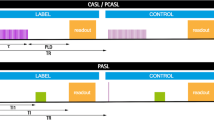
Instead of waiting after each inversion pulse until a specific delay time is reached before beginning with the image acquisition, the recovery of Mz is sampled by a series of RF excitations with small flip angles. In other words, not just one but multiple delay times are measured as a train of signals after each saturation. Although the additional RF excitations interfere with the T1 relaxation process toward thermal equilibrium by tipping part of the regrowing longitudinal magnetization back into the transverse plane, for low flip angles, the effect is sufficiently small to still permit fitting with Eq. 2.4. In clinical settings, liver T1 is measured using a modified look-locker inversion recovery (MOLLI) pulse sequence that allows measurement of T1 times in a single breath-hold (Fig. 2.11). This has become the most popular T1-mapping method for abdomen and cardiac imaging.
Acquisition strategy for the modified look-locker (MOLLI) sequence. In this example, after a 180° inversion pulse, images are acquired in diastole over five heartbeats, followed by a rest period of three heartbeats. Then, after another inversion, another three images are acquired with slightly offset inversion times (TI) to sample more points along the inversion recovery curves. Based on the number of heartbeats for acquiring images after each inversion pulse, and on a rest period of three heartbeats between the two cycles, this MOLLI acquisition scheme is termed 5(3)3
T2 Relaxation
Equivalently to T1 relaxation, T2 relaxation is the exponential return of the transverse magnetization to its thermal equilibrium value in the absence of static magnetic field inhomogeneities following a 90° RF excitation pulse (Fig. 2.12):
T2 relaxation. Following turning off of the radiofrequency pulse, differences in magnetic fields experienced by the spins cause them to precess at slightly different frequencies and fan out about the transverse plane. T2 relaxation time is the time for the transverse magnetization to decay to about 37% of its initial value (the flipped nuclei that started spinning together become incoherent). S(TE) is the signal intensity measured at each individual echo time (TE) and S0 is the initial signal intensity at time t = 0. Mxy is the magnetization in the transverse plane. (© [Suraj D. Serai, 2023. All rights reserved])
Under equilibrium conditions, Mxy = 0, which is clearly fulfilled when Mz = M0, and therefore T2 is always shorter than T1 but this is not the only possibility. It is important to keep in mind that the magnetization measured macroscopically by the MRI scanner is generated by a myriad of nuclei such that the net magnetization vector for even the smallest pixel in a high-resolution scan still consists of the superposition of a huge number of microscopic magnetization vectors. Following an ideal 90° excitation pulse, all of these microscopic vectors are in phase, that is, having the same orientation in the transverse plane, and add up to the largest possible transverse net magnetization vector. However, as phase coherence disappears and the microscopic magnetization vectors begin to point in different directions, the net magnetization decreases until, at the point of complete phase randomization, the magnetization vectors cancel each other out and the macroscopic transverse magnetization reaches zero despite Mz < M0. Still, it is noteworthy that the microscopic transverse magnetization is not zero, though, and that it can be further manipulated by the scanner, which is both a feature of MRI and the source of various kinds of image artifacts that are beyond the scope of this book.
There are multiple mechanisms that give rise to phase randomization and, thus, relaxation of transverse magnetization beyond that caused by T1 relaxation. One of them, static field inhomogeneities cause a recoverable phase coherence loss that is not attributed to T2 relaxation and will be discussed in more detail in Sects. 2.5.4–2.5.6. Two irrecoverable T2 relaxation mechanisms are dipole-dipole interactions and nanoscale field inhomogeneities. In the case of the former, also referred to as spin-spin relaxation, two nuclei in very close proximity, one of them with its magnetic moment aligned with B0 and the other one anti-aligned, switch their alignments. While the net magnetic moment remains unaffected, the phase is randomized during the switching. The latter case, nanoscale field inhomogeneity, is the dominant factor of T2 relaxation. As already mentioned in Sect. 2.5.1, thermal motion causes nuclei in solution to tumble around at a wide range of frequencies. The magnetic moments of those that move the slowest generate sufficiently stationary magnetic field inhomogeneities to slightly change the value of B0 in their immediate vicinity and thereby alter the precession frequencies of other nuclei as they enter the affected volume. This results in a small, random phase change for those other nuclei that, over time, dephases the net transverse magnetization. As for T1 relaxation, T2 is longest in pure water because the number of slowly moving molecules is much smaller than in solutions with a high concentration of macromolecules.
How to Measure T2 Relaxation Time for Body Imaging
Since T2 mapping measures the decay of the transverse magnetization without the contributions from static magnetic field inhomogeneities, the signal dephasing by the latter needs to be undone before the signal is measured. Static magnetic fields alter the precession frequencies of nuclei at a specific location, which means that the associated transverse magnetization in this area accumulates phase at a different rate than in other locations, resulting in a net signal cancellation over time. However, the phase accumulation can be reversed again with the help of 180° RF refocusing pulses. While the 180° RF inversion pulses described in Sect. 2.4.2 act on the longitudinal magnetization, refocusing pulses are designed to flip the entire transverse plane by 180° about an axis within the plane. This in effect turns the direction of phase accumulation around such that the different magnetization vectors are in phase again and form a spin echo for t = TE, where TE is the so-called echo time, with the refocusing pulses being activated at t = TE/2. The mechanism can be pictured as runners sprinting down a track at different but constant speeds. If, once the first runner reaches the halfway point of the track, all runners turn around, sprinting back the way they came with the same speed as before, all runners will return to the starting line at the same time.
Although the transverse magnetization will peak at the echo time, its peak amplitude decreases as TE is increased because of irreversible T2 relaxation processes. Therefore, T2 can be quantified by applying a 90° RF excitation pulse at t = 0 s, followed by a refocusing pulse at t = TE/2, measuring the signal at t = TE, and then fitting the pixel intensity S as a function of TE to the mono-exponential decay curve
with S0 = S(0). In practice, the T2 decay is measured by applying a train of refocusing pulses spaced apart by Δt = TE, in the form of multi-echo spin-echo (MSME) and fast spin-echo (FSE) MRI pulse sequences. T2 relaxation times are routinely used in the clinic for liver and musculoskeletal applications [5,6,7,8].
T2* Relaxation
T2* relaxation is the decay of the transverse magnetization following an RF excitation pulse including all contributions from static magnetic field inhomogeneities [1]. Similar to Eq. 2.5, it is described by a mono-exponential decay function:
The relationship between T2 and T2* can be expressed as
where T2’ is the relaxation time constant exclusively tied to the static field inhomogeneities. Therefore, the three main relaxation time constants can be ordered as T2* < T2 < T1 (Fig. 2.13).
T2* measurement. While T2 relaxation time is the “true” T2 caused by spin-spin molecular interactions, T2* relaxation time is the “observed” T2, reflecting true T2 as well as the effect of magnetic and gradient inhomogeneity. T2 or T2* as measured is the time required for the transverse magnetization to fall to approximately 37% of its initial value
There are numerous causes for magnetic field inhomogeneities in the human body that can give rise to image distortions (see Sect. 2.4.2) and rapid signal decay due to the associated short T2*. The most common one is a difference in magnetic susceptibility between neighboring tissue types. This effect is particularly pronounced at air-tissue interfaces such as around the ear canal, the sinuses, and the oral cavity and in the lungs. Other field inhomogeneities are created by implants or metallic objects in or near the body (dental fillings, jewelry, zippers, belt buckles, etc.), which is one of the reasons that patients need to be screened and should remove such objects prior to an MRI scan whenever possible. As discussed in Sect. 2.5.4, the impact of magnetic field inhomogeneities can be partially or completely alleviated by the application of RF refocusing pulses in the form of spin-echo MRI measurements. However, these types of acquisitions have their own shortcomings in the form of increased acquisition times, decreased T1 weighting, and much higher RF power deposition in the body due to the large number of high flip angle pulses required.
While a short T2* can cause considerable problems for MRI acquisitions, a reduction of T2*, in a controlled manner, can also be very beneficial under certain circumstances. For instance, ferromagnetic nanoparticles can cause signal voids in the image even at extremely low concentrations, making them an excellent marker for molecular imaging applications. However, the most mundane use of T2* shortening is the application of spoiling gradients during MR imaging [6]. A single MRI acquisition can contain hundreds or even thousands of RF pulses, each creating additional transverse magnetization. If RF excitation pulses could only be applied after the transverse magnetization from the pulse before had decayed away due to T2 relaxation to prevent interference between the magnetization components from different excitations, such scans might take hours. Fortunately, this problem can be mitigated by applying a spoiling gradient using the built-in gradient coils once the data from any given excitation pulse has been spatially encoded and sampled. If the amplitude of the spoiling gradient is chosen such that the phase of the transverse magnetization increases by a multiple of 360° over the width of a pixel, then the net transverse magnetization within each pixel, under ideal conditions, is zero. Of course, care needs to be taken that subsequent RF pulses do not refocus the spoiled transverse magnetization until it has been irrecoverably dephased due to T2 relaxation.
How to Measure T2* Relaxation Time for Body Imaging
MRI acquisitions that do not contain RF refocusing pulses, so-called gradient-recalled echo (GRE) pulse sequences, are inherently T2* weighted. Like the quantification of T1 and T2, T2* can be measured by fitting the signal intensity S of each pixel from multiple data sets to the associated exponential decay function:
with each data set acquired at a different TE and S0 = S(TE = 0). In a GRE pulse sequence, TE is defined as the time from the center of the RF excitation pulse to the point in time when the gradient moment, that is, the gradient amplitude integrated over time, along the frequency-encoding axis has been refocused to zero. T2*-based methods have been validated and are frequently used to measure iron in body organs such as the liver and heart [6, 9,10,11,12,13].
T1ρ Relaxation
T1ρ, or spinlock T1 relaxation time, is the time constant for magnetic relaxation in the presence of continuous RF irradiation [1, 14]. When a long, on-resonance RF pulse, a so-called spinlock pulse, is applied, it generates a magnetic field vector B1 in the transverse plane. Throughout the activation of this spinlock pulse, its B1 field is in all respects equivalent to the main B0 field, except that its amplitude is only on the order of a few microtesla. As described in Sect. 2.4.1, magnetic field fluctuations at the resonance frequency associated with B0 in the tens of Megahertz range give rise to T1 relaxation along B0. An equivalent T1 relaxation effect, that is, T1ρ, can be observed along B1 in the transverse plane, but it is induced by field fluctuations in the Kilohertz range, the resonance frequency associated with B1. However, the increase of transverse magnetization due to T1ρ relaxation is counteracted the simultaneous process of T2 relaxation. Hence, T1ρ relaxation is detectable as a lengthening of T2. T1ρ is believed to be sensitive to the slow interactions between free water and proton nuclei in large macromolecules, such as collagen and proteoglycans. For instance, because fibrosis involves the accumulation of extracellular macromolecules, including collagen and proteoglycans, it is hypothesized that T1ρ imaging ρ is a direct measure of fibrosis.
How to Measure T1ρ Relaxation in Body Imaging
T1ρ mapping is the most common form of T1ρ imaging and is frequently used for various applications [1, 14, 15]. It requires measurements for at least two but usually several different spinlock times (TSL), the time during which the spinlock RF pulse is activated, to obtain a series of images with different levels of T1ρ-weighted contrast. An efficient way of acquiring such images is the application of a 90° excitation pulse followed by the spinlock pulse for a duration of TSL. The transverse magnetization is then flipped back to the longitudinal axis with a −90° pulse. The now T1ρ-weighted longitudinal magnetization is subsequently imaged with a rapid acquisition using RF excitation pulses with low flip angles. The image voxel intensities at different TSLs, S(TSL), are then fitted to a mono-exponential decay function to calculate the T1ρ values, yielding a T1ρ map ρ, using
where and S0 = S(TSL = 0).
Diffusion-Weighted Imaging
MRI permits turning the molecular diffusion of the nuclei into an image contrast. Section 2.4.2 describes the use of frequency-encoding gradients to spatially localize the excited transverse magnetization. While the gradient is activated, the precession frequency of the transverse magnetization is modified according to the amplitude of the frequency-encoding gradient at that position, changing the phase of the magnetization as a function of the gradient duration. If, after this gradient is switched off, a second gradient identical to the first except that its amplitude is reversed is turned on the entire effect of the first gradient is undone, the phase of the transverse magnetization returns to its original value. Hence, the MRI signal with and without these gradients is the same. However, the rephasing of the magnetization is contingent upon the nuclei being completely stationary while such a gradient pair is applied. If, on the other hand, the nuclei are diffusing around, they will move into regions with different gradient amplitudes, changing their precession frequency and, therefore, their rate of phase accumulation. As a consequence of the random nature of the diffusion process, the phase change of the transverse magnetization after the first gradient will no longer be completely reversed by the second gradient, and part of the transverse magnetization within a pixel will cancel out. The faster the diffusion of the nuclei, the larger the phase discrepancy and the lower the resulting signal compared to a measurement without diffusion-weighting gradients.
The sensitivity of the diffusion weighting, the so-called b-value (measured in units of s/mm2), depends on the amplitude of the applied gradients, their duration, and the delay time between the two bipolar gradients comprising the diffusion-weighting gradient pair. The higher the b-value, the stronger the diffusion weighting (Fig. 2.14). Strong gradients and high slew rates are important hardware requirements for diffusion-weighted imaging (DWI) because they reduce the diffusion encoding time, which improves the signal-to-noise ratio (SNR) by shortening the TE. High slew rates also enable faster image encoding, which can further decrease TE and help mitigate distortions by shortening the echo spacing. On clinical scanners, DWI is typically performed with single-shot K-space trajectories, most commonly using the echoplanar imaging (EPI) technique [1]. For additional imaging acceleration, diffusion images are often under-sampled to minimize the effective TE and length of the echo train. Parallel imaging techniques are employed to restore the unaliased images by using spatial information inherent in the coil sensitivity profiles of a multichannel receiver coil.
In diffusion-weighted imaging, a progressive decrease in image signal intensity is evident and can be measured with increasing b-values, as in these three representative axial images obtained at b = 0 s/mm2, b = 400 s/mm2, and b = 800 s/mm2 in a 14-year-old boy. The signal intensity can be measured and fitted to a mono-exponentially decaying curve to generate an apparent diffusion coefficient (ADC) map (top-right). (© [Suraj D. Serai, 2023. All rights reserved])
How to Measure Apparent Diffusion Coefficients for Body Imaging
The application of diffusion-weighting gradients prior to data sampling will generate a qualitative diffusion-weighted image contrast in which the signals from regions with more rapid diffusion will appear more attenuated than those from regions with slower diffusion relative to the signal from an equivalent acquisition without diffusion gradients [1]. Nevertheless, the self-diffusion D constant of the nuclei, typically that of water, can principally be quantified based on the relative signal difference in just two images measured with different b-values because in the case of unrestricted diffusion, the signal decreases exponentially with increasing b-value:
where S1 and S2 are the pixel intensities in the measurements with diffusion weightings of b1 and b2, respectively. Although this relation holds for any b-value combination, b1 is usually chosen to be 0 s/mm2, that is, no diffusion gradients are applied. However, in biological specimen, pure water diffusing without restrictions is rarely encountered. Rather, water exists as a solvent containing numerous macromolecules that slow down the diffusion of water molecules. Further, various structural obstacles such as cell membranes block the diffusion pathways. As a result, MR diffusion measurements yield an apparent diffusion coefficient (ADC) that tends to be considerably smaller than D and that depends on the selected acquisition parameters.
In clinical practice, measurements with 3–5 different b-values are typically used. The number of signal averages is usually increased for higher b-values to ensure a sufficient SNR while maintaining clinically acceptable scan times. The ADC is calculated by fitting the measured signal intensities S(b) to a mono-exponential decay curve:
where S0 = S(b = 0).
Pulse Sequence and Imaging Parameters
-
1.
Echo time (TE): It is the time interval between application of radiofrequency pulse and measurement of an echo.
-
2.
Field of view (FOV): It is the image area that contains the object of interest to be measured. The smaller is the FOV, the higher is the image resolution and smaller is the voxel size and hence lower signal-to-noise ratio.
FOV is expressed as
$$ \mathrm{FOV}=\mathrm{BW}/\gamma G $$where BW, receiver bandwidth (Hz); γ, gyromagnetic ratio of a nucleus (MHz/T); and G, amplitude or strength of a gradient (mT/m).
-
3.
Flip angle: It is the extent of rotation experienced by the net magnetization during the application of a RF pulse. The flip angle over an image depends on B0 inhomogeneity, type of RF pulse, pulse profile, slice select gradient, and off-resonance effects.
For a strong and rectangular RF pulse of constant amplitude (B1) and duration (τ), the flip angle (α) is given by
-
4.
Image contrast: It is the relative difference in signal intensities between two adjacent regions of an image. Image contrast depends upon tissue properties such as T1, T2, proton density, susceptibility, chemical shift, diffusion, perfusion, and flow. It also depends upon type of pulse sequences, several sequence parameters, and factors such as temperature and pH of the tissues.
-
5.
Image resolution: Resolution is an ability of the human eyes to distinguish one structure from another; in other words, the resolution is the level of detail of an image. The resolution is defined by number of pixels in a given FOV. The higher the number of pixels, the greater the image resolution. The pixel size is measured by dividing the FOV by matrix size. There are two types of image resolution used in MRI.
-
(a)
Base resolution is defined as the number of pixels in the frequency encoding-direction. Base resolution is directly proportional to scan time and inversely proportional to pixel size and hence the SNR. Increasing the base resolution more than an acceptable range results in grainy image due to low SNR, and decreasing the base resolution more than an acceptable range produces a blurry image due to increased SNR.
-
(b)
Phase resolution is defined as the number of pixels in the phase-encoding direction. Phase resolution is generally expressed a percentage of base resolution. Decreasing phase resolution will increase the pixel size in one direction resulting in rectangular pixel shape. Phase resolution is directly proportional to scan time and inversely proportional to pixel size and hence SNR (Fig. 2.15).
-
(a)
-
6.
Inversion time (TI): It is the time interval between applications of 180° and 90° RF pulses.
-
7.
Number of excitations (NEX): It is defined as the number of times each line of K-space is acquired. Doubling the NEX increases the SNR by a factor of √2 because random noise is also sampled. However, it increases the scan time.
-
8.
Receiver bandwidth: It is also known as digitization rate of MR signal. It is reciprocal of dwell time (time interval between digitized samples). The dwell time is defined as the number of complex sample points multiplied by sampling time. Different MRI vendors express the receiver bandwidth in different ways. While Siemens and Canon scanners use bandwidth per pixel, GE scanners use the total bandwidth across an entire image. The receiver bandwidth is also defined as range of frequencies from −fmax to +fmax.
BW is also defined as 1/ΔTs or Nx/Ts
where ΔTs is a dwell time (time interval between complex data points), Nx is the number of frequency-encoding steps, and Ts is the sampling time (Nx * ΔTs).
-
9.
Repetition time (TR): It is the time interval between two successive applied radiofrequency pulses.
-
10.
Scan time for conventional spin-echo sequence: TR * NEX * number of phase-encoding steps
-
11.
Scan time for fast spin-echo sequence: TR * NEX * number of phase-encoding steps/echo train length
-
12.
Scan time for gradient recalled echo sequence: TR * NEX * number of phase-encoding steps * number of slices
-
13.
Scan for three-dimensional imaging: TR * NEX * number of phase-encoding steps * number of phase-encoding steps (partitions) in z-direction
-
14.
Signal-to-noise ratio (SNR)
A digital MR image consists of signal and noise. The parameter signal-to-noise ratio (SNR) is used to describe the image quality. A number of factors affect the SNR of an image, and these factors are the following:
-
(a)
SNR depends upon the composition and characteristics of a biological tissue being imaged. Tissues with higher number of proton spins produce higher signal intensities and hence possess higher SNR.
-
(b)
SNR varies with primary magnetic field as SNR α B07/4. High magnetic field strength tends to align a greater number of spins along the direction of magnetic field. Henceforth, high field strength increases the net longitudinal magnetization and SNR.
-
(c)
To achieve high SNR, the RF receiver coil should be placed as close as possible to the anatomical structure being imaged. Therefore, a coil with smaller radius will produce higher SNR. Additionally, the greater the number of receiver channels, the greater the SNR.
-
(d)
SNR increases with increase in TR as a high TR will allow longitudinal magnetization to recover back to high values and will produce high signal.
-
(e)
SNR decreases with increase in TE as a high TE will allow transverse magnetization to decay to low values and will result in signal loss
-
(f)
SNR decreases with increasing matrix size as voxel size is inversely proportional to matrix size.
-
(g)
SNR increases with increasing FOV as voxel size is proportional to FOV
-
(h)
SNR is directly proportional to square root of number of phase-encoding steps. Therefore, doubling the NEX causes 1.44 times increase in SNR.
-
(i)
SNR increases with increasing interslice gap which is the distance between two adjacent slices and is usually described as percentage of slice thickness. Slice gap reduces or eliminates slice overlapping and cross-talk that may be caused by imperfect slice profiles. This crosstalk causes a saturation effect (signal loss) in the area of slice overlap, resulting in lower SNR.
-
(j)
SNR increases with increasing voxel size. A larger voxel contains a greater number of proton spins per unit volume.
-
(k)
SNR increases with increasing slice thickness.
-
(l)
SNR is directly proportional to square root of number of excitations (NEX). Therefore, doubling the NEX causes 1.44 times increase in SNR.
-
(m)
SNR is inversely proportional to square root of receiver bandwidth, and hence, SNR decreases as receiver bandwidth increases.
-
(n)
The use of lower receiver bandwidth increases SNR and allows selection of a smaller FOV for scanning small tissues of interest. However, lower receiver bandwidth causes more chemical shit artefacts, susceptibility artifacts, and more spatial distortion of an image.
-
(o)
Application of any saturation pulses decreases the overall SNR in the image. For example, nullifying the fat proton signals reduces the overall signal intensity from anatomical structure being imaged.
-
(p)
Partial filling of K-space lines in the phase-encoding direction decreases SNR.
-
(a)
-
15.
Transmitter bandwidth: Range of frequencies (measured in Hz) is involved in transmitted radiofrequency pulse. It is expressed as
where ΔF is the transmitter bandwidth (Hz), γ the gyromagnetic ratio of a nucleus (MHz/T), Gss the amplitude/strength of slice select gradient (mT/m), and Δz the slice thickness (mm).
-
16.
Voxel: It is a volume element representing a value in the three-dimensional space, corresponding to a pixel (pictorial element) for a given slice thickness.
Kidney MRI
Image Acquisition
MR image acquisition requires four basic steps: (1) place the patient in a uniform magnetic field generated by the combination of the MRI components, (2) displace the equilibrium magnetization vector with an RF pulse, (3) collect the signal as the magnetization vector returns to equilibrium, and (4) convert the collected signals into images using the computer’s signal processing algorithms.
With MR, smaller picture elements mean that there are fewer hydrogen nuclei per “voxel.” This three-dimensional projection of the pixel (voxel) is defined by the face of the pixel and the slice thickness. Decreasing either the slice thickness or the pixel size results in fewer protons per voxel (Fig. 2.16). With fewer hydrogen protons in each voxel, and assuming the noise stays the same, the signal-to-noise ratio decreases, which may decrease image contrast. The scan time for a simple spin-echo acquisition is based on the facts that a 90° pulse is necessary for each gradient step and that one gradient step is necessary for each line of information in the phase-encoding direction. As a result, a 512 × 512 matrix requires twice as much time to acquire as a 256 × 256 scan, but the pixels at 512 × 512 will be one-fourth the size. While it might seem that this would decrease the signal by one-fourth, since each phase-encoding step contributes to the signal of the entire image, the signal loss decreases by only one-half since there are twice as many phase-encoding steps in a 512 versus a 256 square matrix. The decrease in the signal-to-noise ratio can be corrected in part by repeating the entire pulse sequence more than once and averaging the results. This variable is sometimes abbreviated as NEX, meaning “number of excitations.” Each excitation adds time, of course, so using a NEX of 2 doubles the scan time (Fig. 2.17). You might think that one would double the signal by doubling the scan time. Unfortunately, you still will not come out even because when you double the scan time, the signal-to-noise ratio increases only by a factor of 1.4 (square root of 2), not 2. To double the signal, you would need to use a NEX of 4 or a scan time four times as long (square root of 4 = 2) to break even. For this reason, it is prudent to avoid an extremely fine matrix unless your scanner provides signal to burn (remember the drive to high field strength) or the indications warrant this added time. One compromise commonly used is an asymmetric matrix such as 128 × 256. In MRI image acquisition, the scan time, image resolution, and signal-to-noise ratio (SNR) are interdependent on each other (Fig. 2.18). Increasing the matrix size in the frequency direction requires applying a stronger gradient that does not add time. In addition, using phase encoding for the short side of this rectangular matrix will take the same time as a scan with a 128 × 128 matrix while still providing a finer detail.
Physiological Monitoring and Motion Consideration
One of the main challenges that continues to drive innovation in pediatric imaging is motion. Much time and effort are devoted to reducing the numerous sources of motion and their resultant artifacts (Fig. 2.19). These sources of motion include gross body (i.e., voluntary) motion, respiratory motion, cardiac contractions, vascular pulsations, and bowel peristalsis. Obtaining diagnostic-quality images requires the patient to “lie still” during image acquisition; however, many patients and especially children are unable to comply with this request for various reasons. Physiology-synchronized acquisition techniques (e.g., triggering, gating, and tracking) may mitigate respiratory and cardiac motion at the expense of additional scan time.
There are clinically established and emerging MRI techniques for the abdominal imaging. These techniques fall under two general categories: undersampling the K-space for accelerated imaging and motion compensation and correction techniques. Some of the newer methods combine the two as self-navigating accelerated imaging techniques. Most of the techniques can be employed with physiological motion compensation strategies such as breath-holding and respiratory synchronized acquisition.
Respiratory Synchronization
Respiratory motion can be monitored and tracked in real time using pneumatic respiratory bellows placed on the abdomen or two-dimensional radiofrequency (RF) excitation pulses, known as RF pulse navigators, positioned at the lung/diaphragm interface in foot to head direction. Data acquisition can be synchronized to the respiratory motion in three different modes: (1) triggering, where typically data is acquired at certain delay after the detection of expiration; (2) gating, where data is acquired during a prescribed acceptable diaphragmatic displacement; and (3) tracking, where upon detection of expiration, the slice offset is adjusted in real time prior to data acquisition.
The bellows track the body wall motion as a surrogate to abdominal organ motion. The RF navigator tracks the diaphragmatic movement directly and allows slice offset correction in real time. Respiratory gating restricts data acquisition to a designated phase of the respiratory cycle, typically expiration (Fig. 2.20). While physiologically more accurate, RF navigator may not be effective in patients with extremely shallow breathing due to the inability to pick up synchronous signal and in patients with iron overload due to low signal to noise ratio. Similarly, the respiratory gating may not work well in older children with heavy chest breathing and larger diaphragmatic excursions, which cause larger abdominal organ motion (Fig. 2.21). Generally, respiratory gating also results in an approximately threefold increase in total scan time. To reduce scan time to a reasonable duration, a single-shot acquisition with a triggered approach is employed.
Signal Averaging
When breath-hold and respiratory synchronization is not feasible, averaging the signal over multiple number of excitations (NEX) may help reduce motion artifacts. This technique cohesively adds up the signal intensity in the static anatomy and reduces the signal intensity of ghosts caused by the anatomy moving out of synchronization with the acquisition. Increasing NEX also increases the SNR by a factor of the square root of the NEX value. To achieve adequate artifact reduction, the NEX is often set to 3 or greater, resulting in a threefold or proportional increase in total scan time. Careful attention should be paid to increase in total RF deposition with higher NEX values.
Swapping Encoding Direction and Saturation Bands
Motion artifacts are predominant in the phase-encoding direction. Typically, because the anterior to posterior (AP) dimension is the smallest and most favorable for coil topology, phase encoding is prescribed in AP direction. In certain cases, by swapping the phase-encoding direction, motion artifacts can be constrained to outside the anatomy of diagnostic interest. Subcutaneous abdominal fat generates a higher signal in almost all types of contrasts (T1W, T2W, and PDW) and, being closer to coil elements, dominates the signal intensity spectrum. Therefore, respiratory artifacts from the moving anterior abdominal wall are generated distinctly over the rest of the abdomen. The signal from moving tissue, especially abdominal fat, can be suppressed with fat saturation techniques or by placing a regional saturation band over the anterior abdominal wall.
Practical Points to Consider When Performing a Kidney MRI Study
Scheduling a Renal MRI Scan
Renal MRI studies are typically scheduled into timeslots of between 30 min and 1 h depending on the complexity of the study and capabilities of the MRI scanner and/or if contrast will need to be injected. Examinations that need sedation or anesthesia may require more time for patient preparation and recovery. Either at the time of the appointment or when confirming the appointment time with the patient, it is prudent for the staff to briefly review the patient’s reason for the scan and perform a prescreening of metallic implants, pacemakers, or other electronic implants and inquire about claustrophobia, allergies, or any other special needs the patient may have. This is a good opportunity for staff to also give some information to the patient about the length of the visit, how a patient should dress (e.g., no jewelry, hair clips, makeup, etc.), or if they will need to change into a hospital gown for the study. It is quite costly to an MRI facility to have an unexpected open time slot if the patient does not arrive for an appointment or arrives late. A late arrival will cause stress to the delays for the rest of the day. After the study is scheduled, the radiologist reviews the indication for the requested study and decides on the scanning protocol. The team then determines if the examination is scheduled appropriately based on the time of the day, available staff, resources, and the length of the exam. Most scheduled exams will need few alterations when handled by experienced personnel. Renal MRI studies are becoming more complex, and certain examinations require detailed clinical information for the MRI team to plan properly the scanning protocol. Continuous flow of communication is essential among all team members. An adequately prepared team reduces the chance of unanticipated problems, allowing for smooth execution of the examination.
Patient Positioning
In order to obtain images, an MRI technologist must first select the appropriate RF coil and position the patient. The patient should be made as comfortable as possible to achieve cooperation. For most renal MRI scans, the patient lies supine. Prone positioning may help reduce claustrophobia since the patient can look outside the bore, but it is less comfortable than supine position. Phased array receive coils with posterior elements must be positioned on the table before the patient lies down. Most new MRI scanners now have the posterior elements built into the table, which simplifies the positioning workflow. The anterior portion of the phased array coil is then positioned and stabilized using Velcro straps. Adequate cushioning must be placed between the patient and the coil to ensure that the patient will be comfortable for the duration of the study.
Physiological Monitoring
Depending on the MR system, and as described in Sect. 2.5.3, available respiratory gating methods include using mechanical bellows and navigator gating. While these methods are highly effective in many patients, the results vary according to the regularity and depth of the patient’s breathing. Extra time is required to position the bellows optimally and to choose the thresholds for accepting the data or, in case of navigator echoes, to position the navigator appropriately and obtain baseline data about respiratory patterns. With respiratory gating methods, some data gets rejected, so the scan times are increased, typically by a factor of 2.
Coil Selection and Image Acquisition
The first step for image acquisition is to choose the appropriate receiver coil. Recollect that our receiver coil acts like an antenna for the radio signal tuned to the specific frequency, and choosing the correct coil for the anatomy of interest can make a big difference in SNR. A series of surface coils are combined to form a phased array coil; individual coils are magnetically decoupled, thus providing sensitivity for imaging over a large volume that is often required for renal imaging. Depending on the scanner capabilities, phased array coils nowadays may have as few as 4 to as many as 32 or more elements. The development of phased array technology is partly driven by the development of parallel imaging methods. In general, higher acceleration factors work most effectively with coils that have larger number of elements. It is important to know the number of receive channels available on the scanner hardware and the number of elements on the coil being used. With certain phased array coil combinations, there may be more coil elements, than the number of receive channels. The scanning MR technologist should prefer to activate only those coil elements that are needed to image the specific anatomy of interest. If too many elements are activated, multiple elements are multiplexed into a single receiver channel. Moreover, some elements might be located relatively far away from the magnet isocenter. The result is a worsening SNR along with the potential to introduce artifacts into the image. Moreover, image reconstruction time increases with the number of elements as does the amount of space needed to store raw data. Long reconstruction times can limit the practical application of some advanced imaging techniques such as compressed sensing, particularly when large amount of data is rapidly acquired in conjunction with the use of phased array coils with numerous elements. For body imaging, coil centering and imaging on isocenter are equally important for better image quality and uniformity. The magnetic field is most uniform at the magnet isocenter. The magnet uniformity and linearity of the gradients decline as we move away from the isocenter. Therefore, the coil should be centered, and imaging should be performed at isocenter. In general, image quality will not suffer greatly if the body part is a few centimeters away from the isocenter. However, with large displacements, SNR may degrade, and shim may worsen resulting in poor fat suppression that is often done for renal imaging. For a clinical renal MRI scan, and especially for quantitative imaging, a good SNR is essential for image quality. A higher SNR means image will appear to be less grainy and fine details will be more apparent. Consequently, a low SNR results in a grainy image in which fine details are obscured. Although a high SNR is desirable, increasing the SNR often requires more scan time, and a balance needs to be played between acceptable image quality and image acquisition time. Figure 2.22 provides a summary of factors that affect image quality and acquisition time.
Finally, there are several practical ways to optimize and adjust the imaging parameters during a renal MRI scan to maximize SNR or spatial resolution in an attempt to produce high-quality MR images. The overview of the physics and the details of the currently employed image acquisition techniques are included in this chapter, and in subsequent chapters in this section, we intend to familiarize the reader on how different various configurations impact image quality in state-of-the-art clinical kidney MRI. Ultimately, with this background information, the user can experiment with different imaging parameters and decide what suits the needs of the particular MRI study. The power of MRI lies in its flexibility, and its clinical potential will continue to grow with developments in the underlying technology.
Fat Suppression Methods
Due to short relaxation times, fat has a high signal on MRI. This high signal due to fat may be responsible for artifacts such as ghosting and chemical shift. Moreover, a contrast-enhancing tumor may be hidden by the surrounding fat. These problems have led to the development of fat suppression techniques in MRI [16, 17]. Fat suppression depends on different magnetic properties of water and fat protons differ in two ways:
-
1.
The T1 values for fat protons are much shorter than those of water protons.
-
2.
The water protons resonate slightly faster than those of fat protons. This difference in resonance frequency is known as water-fat chemical shift.
These differences in relaxation and chemical shift-dependent magnetic properties can be used to selectively suppress the signals of fat-bound protons.
Inversion Recovery (STIR, Short TI Inversion Recovery)
This technique is based on the different relaxation behavior of water and fat tissues. Because fat has a much shorter T1 relaxation time than other tissues, its signal can be selectively nulled using a magnitude-reconstructed inversion recovery (IR) sequence. Prior to the excitation pulse of the sequence, an inversion pulse (α = 180°) is applied which inverts the spins of all the tissues, which subsequently undergo T1 relaxation. By choosing intervention time (TI) such that the longitudinal magnetization of fat at that time is zero, fat spins will not contribute to the MR signal. STIR images have an inverted T1 contrast: tissue with long T1 appears brighter than tissue with short T1.
Advantages
It is relatively insensitive to B0 inhomogeneities and can be used near metallic implants and over large fields of view.
Disadvantages
Additional inversion pulse increases minimal TR and hence total scan time. It can also increase the specific absorption rate (SAR) due to additional 180° pulse.
The signal suppression is not specific to fat tissues. In fact, any material with short T1 value such as melanin, methemoglobin, and mucus will be nulled. Signal-to-noise ratio can decrease as tissues of interest may have lower net magnetization recovery at null point.
Spectral Fat Saturation
This is the most widely used fat suppression technique. It is based on the difference in resonance frequencies (~3.5 ppm) between fat and water protons. In this technique, a frequency-selective RF pulse tuned to the resonance frequency of fat protons is applied in association with a crusher gradient to excite and then dephase the fat protons (Fig. 2.23). Thus, no fat magnetization is left for imaging and only water protons are present to produce an MR signal.
Advantages
The technique is simple and versatile and can be added to any pulse sequence.
Tissue contrast is not affected.
Disadvantages
It is sensitive to B0 and B1 inhomogeneities.
It works better on higher field strengths because of larger chemical shift dispersion between water and fat protons at higher field strength.
It requires homogeneous B0 field and does not work properly around the metallic implants or around the air-tissue interfaces.
Dixon Technique
The Dixon technique is based on the difference in resonance frequencies between fat and water-bound protons. In this technique, signals from water and fat protons in the same voxel can be made to interfere constructively or destructively by changing TE [18, 19]. This method is routinely used in body applications for fat quantification [19,20,21,22].
Advantages
It is insensitive to B0 and B1 inhomogeneities.
Four contrast images (in-phase, opposed-phase, water, and fat images) are obtained in a single measurement.
Fat quantification is also possible.
Disadvantages
It increases minimal TR because in and opposed phase data must be acquired.
Typical Body Imaging Artifacts and Tips to Mitigate Them
An imaging artifact is a feature appearing in an image that is not usually present in the original object. Many different artifacts can occur during MRI affecting the diagnostic quality and mimicking with a pathological condition [23].
Motion Artifacts
Motion artifact is one of the most common artifacts observed on MR images that is generally present along the phase-encoding direction. The reason for mainly affecting data sampling in the phase-encoding direction is the significant difference in the time of acquisition in the frequency and phase-encoding directions [23]. Frequency-encoding sampling in all the rows of the matrix takes place during a single echo (milliseconds). On the other hand, phase-encoded sampling takes several seconds or even minutes because of the collection of all the K-space data. The most of physiological movements are of the order of milliseconds to seconds duration and thus too slow to affect the frequency-encoded sampling, but they have a pronounced effect in the phase-encoding direction. Periodic movements such as cardiac movement and blood vessel or CSF pulsation produce ghost images, while nonperiodic movements result in diffused image noise.
Several methods can be used to reduce motion artifacts including patient immobilization (by using foam pads, taping, bite bars, etc.), employing cardiac and respiratory gating, increasing the number of excitations/averaging, and using ultrafast sequences, radial/spiral sampling of K-space, flow compensation, and fat, spatial, and flow saturation pulses or techniques. The swapping of frequency and phase-encoding directions will displace the artifact out of the tissue of interest but will not eliminate it.
Chemical Shift Artifact
The chemical shift refers to change in resonance frequencies of two biological tissues owing to different in chemical environment of nuclei. The chemical shift artifact occurs at the water and fat interface especially along the frequency-encoding direction (Figs. 2.24 and 2.25). These artifacts arise due to the difference in resonance frequency of water and fat protons, which causes spatial mismatching of fat and water pixels. These artifacts are reflected as alternative bright and dark bands along the frequency-encoding direction. The chemical shift distance is defined as
Chemical shift distance (mm) = Frequency shift (Hz) × FOV (mm)/receiver bandwidth (Hz).
The best strategy to reduce chemical shift artifact is to use fat suppression technique for reducing the signal from fat and thereby minimize this artifact. The other method is to increase total receiver bandwidth by reducing the field of view or increasing the strength of the frequency-encoding gradient. Swapping the frequency and phase-encoding directions prior to imaging will not eliminate the chemical shift artifact but will shift it to a different anatomical region.
Magnetic Susceptibility and Magnetic Susceptibility Artifact
The degree to which a substance gets magnetized when placed in an externally applied magnetic field is called magnetic susceptibility (χ). While diamagnetic substances are feebly repelled by magnetic field, paramagnetic substances are feebly attracted by magnetic field. Almost all biological tissues are diamagnetic in nature. The presence of metallic ions (Mn, Gd) in tissues produces weak paramagnetism. The globular nonheme iron protein complexes such as ferritin and hemosiderin create strong positive paramagnetic effect known as super-paramagnetism. The most powerful magnetic field susceptibility occurs in solid materials containing Fe, Co, or Ni and is called ferromagnetism (Fig. 2.26).
Magnetic properties of materials. Diamagnetic materials (almost all biological tissues, oxyhemoglobin, and calcium) are feebly repelled by magnetic fields. These materials do not have permanent magnetic dipole moments (MDMs). Paramagnetic materials (deoxyhemoglobin, methemoglobin) and superparamagnetic materials (ferritin, hemosiderin) are attracted by magnetic field. In the absence of externally applied magnetic field, permanent MDMs of paramagnetic/superparamagnetic materials are randomly oriented. However, these MDMs become oriented in the presence of magnetic field along its direction. Ferromagnetic materials (iron, cobalt, and nickel) are strongly attracted by magnetic field. The MDMs in ferromagnetic materials exert strong forces on their neighbors and appear to be groped magnetically into domains. In the absence of magnetic field, all MDMs within a domain are aligned, but the direction of alignment varies from domain to domain. In the presence of magnetic field, all these domains are aligned along the direction of magnetic field
The magnetic susceptibility effects create magnetic field distortions resulting in variations in precessional frequency within a voxel. This frequency shift, in turn, produces signal loss from T2*-dephasing and spatial mismapping of the MR signal. The susceptibility artifacts are usually present at the air-tissue interfaces and around the metallic objects and implants.
The intensity of susceptibility artifact depends on difference in magnetic susceptibilities between tissues, magnetic field strength (B0), echo time (TE), and receiver bandwidth. This relationship is expressed as
Size of susceptibility artefact ∝ [(Δsuceptibility ) ∗ B0 ∗ TE]/receiver bandwith.
The swapping of the frequency and phase-encoding does not eliminate the susceptibility artifacts but changes the shape of artifact. The susceptibility artifacts can be reduced by using shorter TE values, increasing receiver bandwidth, increasing gradient strength for a given FOV, and using smaller voxel size, thinner slices, and parallel imaging. The susceptibility artifacts are most prominent on echo planar sequences and decrease in the order of using gradient-echo, conventional spin-echo, and fast spin-echo sequences.
Dielectric (Standing Wave) Artifacts
These artifacts arise because of interaction between electric field components of electromagnetic radiation with matter. RF wavelengths in a biological tissue vary as a function of field strength as shown in the following equation:
where λ = wavelength, B0 = magnetic field strength, and ε = dielectric constant of the medium.
In fact, RF wavelength is known to decrease as field strength increases and becomes similar or smaller than the anatomical dimension. When this happens, a standing wave pattern of electric current occurs, creating constructive and destructive interference and producing alternate bright and dark bands separated by one-fourth wavelength across an anatomical region. The dielectric artifacts can be reduced by using dielectric pads (which are filled with conducting solutions) between a patient and a receiver RF coil. The usage of multi (dual) transmitter RF coil also known as parallel transmission, especially at 7 T field strength, has been shown to reduce dielectric artifacts.
Gibbs/Ringing or Truncation Artifact
This artifact occurs near the sharp boundaries, where high contrast transitions occur between the tissues (brain/CSF, meniscus/fluid in the knee). It appears as multiple, regularly spaced parallel bands of alternating bright and dark signal intensities. An MR signal is defined as an infinite summation of sine waves of different amplitudes, phases, and frequencies. However, due to time constrains, only a finite number of frequencies are sampled. In fact, Gibbs artifact is caused by under sampling of high spatial frequencies at sharp boundaries in the image. Lack of appropriate high-frequency components leads to an oscillation at a sharp transition known as a ringing artifact. Since fewer samples are typically taken in the phase-encoding direction, this artifact is mostly noticeable along the phase-encoding direction. Gibbs artifact can be minimized by increasing the number of phase-encoding steps or by reducing the FOV.
Aliasing or Wraparound Artifact
Aliasing or wraparound occurs when the dimension of an objects exceeds the defined FOV and FOV = BW/γGfwhere FOV = field of view, BW = receiver bandwidth, γ = gyromagnetic ratio, and Gf = amplitude of frequency-encoding gradient.
Also, receiver bandwidth is defined as BW = 2fmax = ±fmax
where fmax is the Nyquist frequency or the highest frequency that a computer system can recognize. As a result, a computer system cannot recognize the portion of object present outside the defined BW or FOV. This portion of the object is wrapped around to the opposite side of the image, presenting as low-frequency signals. The perceived frequency is given by
Although aliasing or wraparound artifact may occur along the frequency-encoding direction, it generally occurs along the phase-encoding direction. These artifacts may also occur between end slices in three-dimensional imaging (Fig. 2.27). Phase wraparound artifacts can be reduced by increasing FOV along the phase-encoding direction so that the entire anatomic dimension of the object can be encompassed in that direction. If the FOV is increased, spatial resolution will suffer. To maintain the spatial resolution, the number of phase-encoding steps is increased, however at the cost of increased scanning time. Using surface coil or applying saturation bands outside the FOV may also reduce the wraparound artifacts.
Partial Volume Artifacts
This artifact occurs when portions of several tissue components are averaged together in a voxel or slice of interest. Imperfect RF pulse profiles may cause partial volume effects by exciting tissues outside the desired slice. Partial volume artifacts are reduced by selecting higher imaging matrix sizes, smaller FOVs, and thinner slices. The use of longer duration RF pulses improves slice profiles resulting in precisely defined voxels with less excitation of adjacent tissues, thereby reducing the partial volume artifacts.
Slice Overlapping
When multiple overlapping oblique slices are used to cover a tissue of interest, dark bands appear. It is because protons in the overlapped slices receive multiple RF irradiation and their steady-state magnetization is reduced due to magnetic saturation, resulting in signal loss or appearance of dark bands [23]. The possible solution is to obtain multiple oblique slices in concatenated rather than in sequential acquisition mode.
Summary
In this chapter, we reviewed the basics of MRI, outlined the key hardware components of an MRI scanner, and provided an overview of currently employed image acquisition techniques, with the goal of familiarizing the reader on how different hardware configurations impact image quality in state-of-the-art clinical kidney MRI. We also included some clinical examples of image artifacts, troubleshooting methods, and tips to mitigate them.
References
Serai SD. Basics of magnetic resonance imaging and quantitative parameters T1, T2, T2*, T1rho and diffusion-weighted imaging. Pediatr Radiol. 2022;52(2):217–27.
Moratal D, Vallés-Luch A, Martí-Bonmatí L, Brummer M. k-Space tutorial: an MRI educational tool for a better understanding of k-space. Biomed Imaging Interv J. 2008;4(1):e15.
Jacobs MA, Ibrahim TS, Ouwerkerk R. AAPM/RSNA physics tutorials for residents: MR imaging: brief overview and emerging applications. Radiographics. 2007;27(4):1213–29.
Plewes DB. The AAPM/RSNA physics tutorial for residents. Contrast mechanisms in spin-echo MR imaging. Radiographics. 1994;14(6):1389–404; quiz 1405.
Calle-Toro JS, Barrera CA, Khrichenko D, Otero HJ, Serai SD. R2 relaxometry based MR imaging for estimation of liver iron content: a comparison between two methods. Abdom Radiol (NY). 2019;44(9):3058–68.
Serai SD, Fleck RJ, Quinn CT, Zhang B, Podberesky DJ. Retrospective comparison of gradient recalled echo R2* and spin-echo R2 magnetic resonance analysis methods for estimating liver iron content in children and adolescents. Pediatr Radiol. 2015;45(11):1629–34.
Towbin AJ, Serai SD, Podberesky DJ. Magnetic resonance imaging of the pediatric liver: imaging of steatosis, iron deposition, and fibrosis. Magn Reson Imaging Clin N Am. 2013;21(4):669–80.
Xia W, Zhang Y, Liu C, Guo Y, He Y, Shao J, et al. Quantitative T2 mapping magnetic resonance imaging of articular cartilage in patients with juvenile idiopathic arthritis. Eur J Radiol. 2023;160:110690.
Reeder SB, Yokoo T, França M, Hernando D, Alberich-Bayarri Á, Alústiza JM, et al. Quantification of liver iron overload with MRI: review and guidelines from the ESGAR and SAR. Radiology. 2023;307:221856.
Venkatakrishna SSB, Otero HJ, Ghosh A, Khrichenko D, Serai SD. Rate of change of liver iron content by MR imaging methods: a comparison study. Tomography. 2022;8(5):2508–21.
Barrera CA, Khrichenko D, Serai SD, Hartung HD, Biko DM, Otero HJ. Biexponential R2* relaxometry for estimation of liver iron concentration in children: a better fit for high liver iron states. J Magn Reson Imaging. 2019;50(4):1191–8.
Barrera CA, Otero HJ, Hartung HD, Biko DM, Serai SD. Protocol optimization for cardiac and liver iron content assessment using MRI: what sequence should I use? Clin Imaging. 2019;56:52–7.
Serai SD, Trout AT, Fleck RJ, Quinn CT, Dillman JR. Measuring liver T2* and cardiac T2* in a single acquisition. Abdom Radiol (NY). 2018;43(9):2303–8.
Hectors SJ, Bane O, Kennedy P, El Salem F, Menon M, Segall M, et al. T1ρ mapping for assessment of renal allograft fibrosis. J Magn Reson Imaging. 2019;50(4):1085–91.
Serai SD, Trout AT, Miethke A, Diaz E, Xanthakos SA, Dillman JR. Putting it all together: established and emerging MRI techniques for detecting and measuring liver fibrosis. Pediatr Radiol. 2018;48(9):1256–72.
de Kerviler E, Leroy-Willig A, Clément O, Frija J. Fat suppression techniques in MRI: an update. Biomed Pharmacother. 1998;52(2):69–75.
Kalovidouri A, Firmenich N, Delattre BMA, Picarra M, Becker CD, Montet X, et al. Fat suppression techniques for breast MRI: dixon versus spectral fat saturation for 3D T1-weighted at 3 T. Radiol Med. 2017;122:731.
Yokoo T, Serai SD, Pirasteh A, Bashir MR, Hamilton G, Hernando D, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology. 2018;286(2):486–98.
Serai SD, Dillman JR, Trout AT. Proton density fat fraction measurements at 1.5- and 3-T hepatic MR imaging: same-day agreement among readers and across two imager manufacturers. Radiology. 2017;284(1):244–54.
Navaratna R, Zhao R, Colgan TJ, Hu HH, Bydder M, Yokoo T, et al. Temperature-corrected proton density fat fraction estimation using chemical shift-encoded MRI in phantoms. Magn Reson Med. 2021;86(1):69–81.
Hu HH, Yokoo T, Bashir MR, Sirlin CB, Hernando D, Malyarenko D, et al. Linearity and bias of proton density fat fraction as a quantitative imaging biomarker: a multicenter, multiplatform, multivendor phantom study. Radiology. 2021;298(3):640–51.
Joshi M, Dillman JR, Singh K, Serai SD, Towbin AJ, Xanthakos S, et al. Quantitative MRI of fatty liver disease in a large pediatric cohort: correlation between liver fat fraction, stiffness, volume, and patient-specific factors. Abdom Radiol (NY). 2018;43(5):1168–79.
Zhuo J, Gullapalli RP. AAPM/RSNA physics tutorial for residents: MR artifacts, safety, and quality control. Radiographics. 2006;26(1):275–97.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Serai, S.D., Ruppert, K., Chawla, S., Jambawalikar, S. (2023). MR Physics, Hardware Considerations, and Practical Steps for Clinical MRI of the Kidney. In: Serai, S.D., Darge, K. (eds) Advanced Clinical MRI of the Kidney. Springer, Cham. https://doi.org/10.1007/978-3-031-40169-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-40169-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40168-8
Online ISBN: 978-3-031-40169-5
eBook Packages: MedicineMedicine (R0)