Abstract
In the past decades, it has become increasingly clear that women and men significantly differ in the epidemiology, pathophysiology and outcome of cardiovascular diseases that also include certain cardiac electrophysiological aspects leading to different disease phenotypes and disparate outcomes of pharmacological interventions. These dissimilarities stem from numerous differences in ion channel expression, kinetics and regulation. One of the first observations of sex-related differences was the longer QT-interval in women measured on the ECG. Sex hormones can influence the electrophysiological parameters on the genomic level altering gene expression. In this regard, the reduced expression of various ion channels carrying repolarizing currents, including Ito, IK1, IKr, IKs and IK,ATP, have been described in women. Sex hormones can also change ion channel functions by non-genomic effects, including the modulation of specific signalling pathways (such as eNOS). Furthermore, direct effects of sex hormones on ion channels were also described. For example, 17β-oestradiol directly reduced IKr, while testosterone increased IKr and progesterone enhanced IKs. In addition to repolarizing ion currents, sex hormones can influence a large number of transmembrane ion channels and exchangers in various ways, therefore, in this chapter, the sex-related differences regarding an important component of intracellular Ca2+ handling, the Na+/Ca2+ exchanger are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Overview of Cardiac Ca2+ Handling and Its Gender Differences
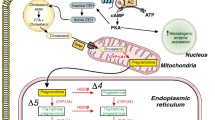
Intracellular Ca2+ has a crucial role in the excitation–contraction coupling. A complex interplay of intracellular Ca2+ fluxes under strict control provides the integrity of the intracellular Ca2+ homeostasis. The actual membrane potential intimately influences Ca2+ handling and vice versa, the intracellular Ca2+ has important roles in the function and kinetics of several ion channels [1].
During depolarization, the opening of the L-type Ca2+ channels provides large Ca2+ influx that triggers Ca2+-induced Ca2+ release by opening the ryanodine receptors (RyR). The RyRs and L-type Ca2+ channels locate in close proximity by the abundant expression in the extensive T-tubule network forming nanodomains, called dyads. The released Ca2+ (Ca2+-transient) interacts with the contractile proteins and initiates several Ca2+-dependent signalling pathways (such as calmodulin-Kinase II signaling) [2]. During relaxation, the intracellular Ca2+ is sequestered to the sarcoplasmic reticulum by the ATP-dependent sarcoplasmic reticulum Ca2+ ATP-ase (SERCA), and extruded to the extracellular space by the Na+/Ca2+ exchanger. In a small extent, the ATP-dependent Ca2+ pump also contributes to the relaxation (Fig. 23.1) [3].
Several studies investigated the possible gender related differences in Ca2+ handling, however, data are often controversial. A study comparing expression levels demonstrated that female ventricular myocytes have markedly higher levels of RyR compared to male animals. Similarly, RyR mRNA was increased in female animals [4]. Ovariectomy caused hyperactivity of the ryanodine receptor, and this increased flux could be reversed by replacement of estrogen and inhibition of protein-kinase A (PKA). This result suggests that estrogen has a role in controlling the Ca2+ flux through the modulation of the ryanodine receptor [5]. Experiments carried out on streptozotocin-induced diabetic rats revealed that expression levels of RyR2 and FKBP12.6 was higher in control females than in control males. In contrast, in diabetes, RyR2 phosphorylation and FKBP12.6 unbinding was lower in females [6]. In 10-week ovariectomized rats the maximum Ca2+ uptake activity of sarcoplasmic reticulum Ca2+ ATPase (SERCA) was reduced together with SERCA protein downregulation and reduction of SERCA mRNA levels. Since supplementation of estrogen and progesterone effectively antagonized the effects of ovariectomy it was concluded that female sex hormones have an important role in SERCA-mediated Ca2+ uptake [7].
It was demonstrated that disruption of the FKBP12.6 gene in mice led to Ca2+ handling mismanagement in both sexes, however, cardiac hypertrophy was observed only in male animals. When female animals were treated with tamoxifen, an estrogen receptor antagonist, similar cardiac hypertrophy could be observed as in the case of male mice. Therefore, it seems possible that estrogen could be protective against hypertrophic response [8].
In contrast, in human atrial tissue it was found that L-type Ca2+ current, RyR, calsequestrin and phospholamban did not show gender differences on the expression level [9].
The Role of the Na+/Ca2+ Exchanger in Ventricular Myocytes
The mammalian Na+/Ca2+ exchanger (NCX) consists of 10 transmembrane segments. In the myocardium, the NCX1 isoform is a critical modulator of cardiomyocyte Ca2+ cycling [10]. A large loop between the 5th and 6th segments has regulatory functions [11,12,13] providing allosteric regulations by cytoplasmic Na+ and Ca2+ ions. It has been found that high intracellular Na+ inactivates NCX [14], however, its physiological significance is questionable since relatively high levels of intracellular Na+ (>20 mM) are required.
The NCX transports three Na+ together with one Ca2+ ion where the Na+ concentration gradient provides the driving force for the exchange. Depending on the intracellular and extracellular Na+ and Ca2+ concentrations, as well as the actual membrane potential, the NCX can work in two operational modes even during the same action potential. When intracellular Na+ is high, the intracellular Ca2+ is low and the membrane potential is depolarized, the reverse mode is favoured where Ca2+ influx takes place. In contrast, the high intracellular Ca2+ and the hyperpolarized membrane potential facilitate forward mode and NCX extrudes the intracellular Ca2+ (Fig. 23.1).
The NCX is abundantly expressed in the sarcolemma, however, it is suggested that the expression level is higher in the t-tubules, having important consequences in Ca2+ handling [15]. 15% of the NCX may be located in close proximity to the ryanodine receptors, therefore, can sense microdomain Ca2+ levels [16]. While the role of the forward mode in the relaxation is clear, the possible role of the reverse mode in the Ca2+ induced Ca2+ release is controversial. There are studies demonstrating that Na+ influx facilitates the reverse mode of NCX and this Ca2+ influx is able to contribute to the Ca2+-induced Ca2+ release mechanism [17,18,19].
Since NCX generates net current, it is feasible that it contributes to the action potential waveform, although the available data are controversial. There are results showing that forward NCX-mediated inward current is a crucial component of the action potential [20]. In contrast, experiments with the novel selective NCX inhibitor ORM-10962 indicate that action potential duration remained unchanged following NCX inhibition [21].
Altered NCX function is described in various pathological conditions. In heart failure, the NCX is upregulated and becomes a better competitor for the SERCA in Ca2+ removal [22]. Therefore, it can contribute to the reduced intracellular Ca2+ content and the generated inward current can be an important source of arrhythmias [22,23,24,25]. In the setting of myocardial ischaemia–reperfusion, the reverse mode of NCX can contribute to the Ca2+-load during ischemia, and the rapid onset of the forward NCX might generate large inward current evoking arrhythmic triggers [26,27,28].
Sex Differences in the NCX-Expression and Genomic Regulation
Chen et al. investigated the sex-related regional expressional differences of NCX in adult rabbits. Furthermore, both the reverse and forward modes of the exchanger were compared [29].
The study found that during the intersex comparison, that in the case of female rabbits, the outward NCX current was larger in the base region, but was smaller in the apex region compared to males. The inward current was identical between genders in both regions. When NCX was compared within the same sex, they observed that in the case of female rabbits, both the outward and inward NCX was larger in the base region. In male animals, the outward NCX was larger in the apex, but the inward NCX had higher amplitude in the base.
Western blot analysis revealed that NCX1 expression was higher in females obtained from the base region of the heart compared to males, as well as was higher compared to apex from both genders (Fig. 23.2). This enhancement of NCX1 could be the consequence of estrogen-induced genomic mechanism.
Distribution of NCX1 protein in male and female rabbits obtained from the apex and base region. Asterisk denotes that NCX1 protein was more abundant in female base compared to male base, and the apex of both sexes (with permission, [29])
The authors also found that NCX and Cav1.2α were upregulated in the base of female hearts that could contribute to the sex-differences in the manifestation of LQT2 syndrome. The higher Ca2+ influx in the base region due to larger ICaL is suggested to be compensated for higher NCX to maintain stable Ca2+ balance [29]. However, the higher ICaL prolongs the action potential and causes sarcoplasmic reticulum Ca2+-overload, and the spontaneous releases elicits early afterdepolarizations via NCX activity. The authors concluded that the apex-base heterogeneity of NCX expression could be an important arrhythmogenic factor in the development of various arrhythmias (Fig. 23.3) [29].
Genomic effect of estrogen on early afterdepolarization (EAD) development. Panel A and B show action potentials from the base (A) and apex (B) regions where the cells were incubated in vehicle and estrogen, respectively. In both cases the IKr inhibitor dofetilide largely prolonged action potentials without eliciting early afterdepolarizations. Panel C: when female base myocytes were incubated in estrogen, the dofetilide induced early afterdepolarizations. Panel D: when female base myocytes were treated with estrogen and its antagonists (ICI), the application of dofetilide prolonged the action potential without evoking early afterdepolarizations (with permission, [29])
Golden et al. have demonstrated that testosterone regulates the expression of the major proteins involved in Ca2+-handling such as NCX. It was found that ventricular myocytes isolated from two-day old rats after 24 h of testosterone treatment had maximal increase in NCX expression. Therefore, the male sex hormone may play a significant role in the gender-related differences of cardiac performance [30].
Furthermore, it has been found that estrogen upregulates the ICaL and NCX in female rabbits, therefore increases the risk of LQT2-type arrhythmias [31]. The same group also investigated whether these results could be confirmed in the human heart. It was found that Cav1.2 and NCX1 protein levels were higher in women than in men or in postmenopausal women in the apex. INCX and ICaL were measured from female and male cardiomyocyte derived human induced pluripotent stem cells, where both ICaL and INCX amplitude were higher in the case of women-derived cells. It was concluded that estrogen upregulated ICaL and INCX in female human ventricular myocytes. These sex-related differences could be attributable, at least in part, to the increased sex-related differences in Ca2+-handling, and arrhythmia propensity [31].
Sex-Related Role of NCX in Ca2+ Handling Balance
In NCX-overexpressed transgenic and wild-type mice, Sugishita et al. investigated the effect of metabolic inhibition on [Ca2+]i and [Na+]i. It was found that metabolic inhibition induced higher [Ca2+]i rise in male transgenic (Tg) animals compered to wild-type, however, in contrast, in female Tg mice, the [Ca2+]i increase was not significant. The increase of [Na+]i was also larger in male animals than in females. The non-selective NCX inhibitor KB-R7943 abolished the effect of NCX overexpression, however, failed to annul all gender differences. In contrast, estrogen significantly decreased the [Ca2+]i and [Na+]i rise in male mice and attenuated gender differences, indicating that estrogen is able to protect cardiac myocytes against [Na+]i and [Ca2+]i elevation during metabolic inhibition [32].
The Ca2+-handling of ovariectomized rats was investigated by Kravtsov et al. [5]. The ovariectomy did not influence the expression level of the NCX, however, increased Ca2+ flux was found via NCX and ryanodine receptor together with enhanced expression of protein-kinase A. These changes suggest that ovariectomy increases contractility, and left ventricular developed pressure [5].
Comparison of the expression levels of different Ca2+-handling proteins in healthy male and female rats revealed that female ventricular myocytes have markedly higher level of CaV1.2, RyR and NCX proteins compared to male animals. Similarly, RyR and NCX mRNA were increased in female animals. Contractile properties were compared by using right ventricular papillary muscles which demonstrated faster maximal rate of force development in female rats [4].
In healthy male and female rats, the key Ca2+-handling proteins were examined. It was found that NCX, RyR and L-type Ca2+ channel mRNA content was higher in female rats [33].
In line with the previous results, it was found that the base of female rabbit hearts exhibited larger ICaL than female apex or males. Estrogen also upregulated ICaL in cultured female myocytes. Mathematical modeling indicated that increased ICaL level increased action potential duration (APD) and promoted arrhythmias. Experimental and modeling data indicates that estrogen upregulates ICaL that promotes APD lengthening and EAD formation [34].
NCX has a crucial role in beat-to-beat Ca2+- handling balance, therefore, it intimately influences the contractile force. The effect of male sex hormones on Ca2+-balance was investigated on orchidectomized male rats, where it was found that the hypogonadal condition caused 50% decrease in the contraction force which could be partially restored by testosterone supplementation. The orchidectomized rats also exerted lower expression levels of NCX with prolonged relaxation of contraction [35].
Sex-Related Differences of NCX in Myocardial Ischemia
Myocardial ischemia often develops following occlusion of a coronary artery establishing serious imbalance between blood supply and demand. The deficit in blood flow initiates several alterations in the kinetics of ion channels, intracellular pH, intracellular Na+ and Ca2+ levels, extracellular K+ level, changes in the secondary messenger system, release of free radicals that altogether largely increase arrhythmia propensity in the heart [36]. Cross et al. investigated the sex-related effects of NCX overexpression during ischemia–reperfusion in transgenic mice. It was found that transgenic male mice exerted lower cardiac performance than male wild type mice, however, there was no difference among female transgenic versus wild type animals. When bilateral ovariectomized and sham-operated female mice were subjected to ischemia, the cardiac performance was lower in the case of ovariectomized mice indicating the role of sex-related hormones [37]. In another study, arrhythmia incidence between left anterior descending artery (LAD) ligation and sham-operated rats from both sexes was also compared. It was found that male gender was a strong predictor of increased arrhythmia vulnerability [38].
Sex-Related Changes of NCX in Heart Failure
Heart failure is a complex clinical syndrome leading to impairment of cardiac performance, pump failure and increased susceptibility to serious cardiac arrhythmias. Various structural (hypertrophy, fibrosis), metabolic as well as electrical alterations (including changes in ion channel protein expression and regulation) can be observed in heart failure, collectively termed ‘remodeling’ [39]. These changes together lead to heterogeneous repolarization and impaired impulse conduction. Repolarizing currents that normally form a strong safety margin by redundant activation (“repolarization reserve”) [40] are seriously compromised and attenuated and the resultant impaired repolarization could serve as a substrate for arrhythmias.
Sex differences also exist in the case of heart failure. Heart failure with reduced ejection fraction is more frequent in males together with ischemic etiology, however in the case of females, heart failure with preserved ejection fraction coupled with hypertension or diastolic dysfunction is more often observed. Transgenic overexpressing TNF1.6 mice exhibit heart failure and increased mortality [41]. It was found that female transgenic mice have slower decay of the Ca2+-transients, however, the transient amplitude, contraction and response to isoproterenol were identical to wild-type mice. In the case of male mice, the transient decline, amplitude, as well as the contraction and isoproterenol response was significantly reduced compared to wild-type animals.
A ventricular tachypacing-induced heart failure swine model was used to investigate the sex differences in NCX function in heart failure. The control (non-failing) ventricular myocytes exerted identical NCX current and beta-adrenergic responsiveness. However, NCX was upregulated in HF and this remodeling was more pronounced in males than in females, however, the beta-adrenergic responsiveness was smaller in male animals (Fig. 23.4) [42].
Gender differences in the NCX current and beta-adrenergic responsiveness in pig heart failure myocytes. Panel A shows NCX currents from male myocytes, panel B from female myocytes. Gray curve illustrates control, black curve illustrates NCX current after isoproterenol treatment. Panel C demonstrates the NCX current density, panel D demonstrates the ratio of outward isoproterenol-induced and basal NCX current. Results indicate that male myocytes have larger NCX current but reduced isoproterenol response in heart failure ([42] with permission)
Conclusion
The expression and function of NCX, a crucial component of cardiac intracellular Ca2+ handling seems to be significantly influenced by gender. It is feasible that in some pathophysiological settings in females, the upregulated NCX function shows heterogeneous distribution via estrogen-mediated genomic mechanisms, and can be an important contributor to increased risk of delayed afterdepolarization development and therefore, increased arrhythmia propensity.
References
Eisner D, Bode E, Venetucci L, Trafford A (2013) Calcium flux balance in the heart. J Mol Cell Cardiol 58:110–117
Bers DM, Grandi E (2009) Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol 54:180–187
Despa S, Bers DM (2013) Na(+) transport in the normal and failing heart - remember the balance. J Mol Cell Cardiol 61:2–10
Chu SH, Sutherland K, Beck J, Kowalski J, Goldspink P, Schwertz D (2005) Sex differences in expression of calcium-handling proteins and beta-adrenergic receptors in rat heart ventricle. Life Sci 76:2735–2749
Kravtsov GM, Kam KW, Liu J, Wu S, Wong TM (2007) Altered Ca(2+) handling by ryanodine receptor and Na(+)-Ca(2+) exchange in the heart from ovariectomized rats: role of protein kinase A. Am J Physiol Cell Physiol 292:C1625–C1635
Yaras N, Tuncay E, Purali N, Sahinoglu B, Vassort G, Turan B (2007) Sex-related effects on diabetes-induced alterations in calcium release in the rat heart. Am J Physiol Heart Circ Physiol 293:H3584–H3592
Bupha-Intr T, Wattanapermpool J (2006) Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 291:H1101–H1108
Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ et al (2002) Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 416:334–338
Lai LP, Su MJ, Lin JL, Lin FY, Tsai CH, Chen YS et al (1999) Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca(2+)-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: an insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol 33:1231–1237
Dong H, Dunn J, Lytton J (2002). Stoichiometry of the Cardiac Na+/Ca2+ exchanger NCX1.1 measured in transfected HEK cells. Biophys J 82(4):1943–1952
Liao J, Li H, Zeng W, Sauer DB, Belmares R, Jiang Y (2012) Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Sci 335:686–690
Ren X, Philipson KD (2013) The topology of the cardiac Na+/Ca2+ exchanger, NCX1. J Mol Cell Cardiol 57:68–71
Philipson KD, Nicoll DA, Ottolia M, Quednau BD, Reuter H, John S et al (2002) The Na+/Ca2+ exchange molecule: an overview. Ann N Y Acad Sci 976:1–10
Hilgemann DW, Matsuoka S, Nagel GA, Collins A (1992) Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gener Physiol 100:905–932
Despa S, Brette F, Orchard CH, Bers DM (2003) Na/Ca exchange and Na/K-ATPase function are equally concentrated in transverse tubules of rat ventricular myocytes. Biophys J 85:3388–3396
Acsai K, Antoons G, Livshitz L, Rudy Y, Sipido KR (2011) Microdomain [Ca(2)(+)] near ryanodine receptors as reported by L-type Ca(2)(+) and Na+/Ca(2)(+) exchange currents. J Physiol 589:2569–2583
Larbig R, Torres N, Bridge JH, Goldhaber JI, Philipson KD (2010) Activation of reverse Na+-Ca2+ exchange by the Na+ current augments the cardiac Ca2+ transient: evidence from NCX knockout mice. J Physiol 588:3267–3276
Neco P, Rose B, Huynh N, Zhang R, Bridge JH, Philipson KD et al (2010) Sodium-calcium exchange is essential for effective triggering of calcium release in mouse heart. Biophys J 99:755–764
Torres NS, Larbig R, Rock A, Goldhaber JI, Bridge JH (2010) Na+ currents are required for efficient excitation-contraction coupling in rabbit ventricular myocytes: a possible contribution of neuronal Na+ channels. J Physiol 588:4249–4260
Armoundas AA, Hobai IA, Tomaselli GF, Winslow RL, O’Rourke B (2003) Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ Res 93:46–53
Kohajda Z, Farkas-Morvay N, Jost N, Nagy N, Geramipour A, Horvath A et al (2016) The effect of a novel highly selective inhibitor of the sodium/calcium exchanger (NCX) on cardiac arrhythmias in in vitro and in vivo experiments. PLoS ONE 11:e0166041
Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM (1999) Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res 85:1009–1019
Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G et al (1994) Gene expression of the cardiac Na(+)-Ca2+ exchanger in end-stage human heart failure. Circ Res 75:443–453
Dipla K, Mattiello JA, Margulies KB, Jeevanandam V, Houser SR (1999) The sarcoplasmic reticulum and the Na+/Ca2+ exchanger both contribute to the Ca2+ transient of failing human ventricular myocytes. Circ Res 84:435–444
Hobai IA, O’Rourke B (2000) Enhanced Ca(2+)-activated Na(+)-Ca(2+) exchange activity in canine pacing-induced heart failure. Circ Res 87:690–698
Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T (1999) Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res 84:1401–1406
Takahashi K, Takahashi T, Suzuki T, Onishi M, Tanaka Y, Hamano-Takahashi A et al (2003) Protective effects of SEA0400, a novel and selective inhibitor of the Na+/Ca2+ exchanger, on myocardial ischemia-reperfusion injuries. Eur J Pharmacol 458:155–162
Kormos A, Nagy N, Acsai K, Váczi K, Ágoston S, Pollesello P et al (2014) Efficacy of selective NCX inhibition by ORM-10103 during simulated ischemia/reperfusion. Eur J Pharmacol 740:539–551
Chen G, Yang X, Alber S, Shusterman V, Salama G (2011) Regional genomic regulation of cardiac sodium-calcium exchanger by oestrogen. J Physiol 589:1061–1080
Golden KL, Marsh JD, Jiang Y (2004) Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolism 36:197–202
Papp R, Bett GCL, Lis A, Rasmusson RL, Baczko I, Varro A et al (2017) Genomic upregulation of cardiac Cav1.2alpha and NCX1 by estrogen in women. Biol Sex differ 8:26
Sugishita K, Su Z, Li F, Philipson KD, Barry WH (2001) Gender influences [Ca(2+)](i) during metabolic inhibition in myocytes overexpressing the Na(+)-Ca(2+) exchanger. Circ 104:2101–2106
Tappia PS, Dent MR, Aroutiounova N, Babick AP, Weiler H (2007) Gender differences in the modulation of cardiac gene expression by dietary conjugated linoleic acid isomers. Can J Physiol Pharmacol 85:465–475
Kalik ZM, Mike JL, Slipski C, Wright M, Jalics JZ, Womble MD (2017) Sex and regional differences in rabbit right ventricular L-type calcium current levels and mathematical modelling of arrhythmia vulnerability. Exp Physiol 102:804–817
Witayavanitkul N, Woranush W, Bupha-Intr T, Wattanapermpool J (2013) Testosterone regulates cardiac contractile activation by modulating SERCA but not NCX activity. Am J Physiol Heart Circ Physiol 304:H465–H472
Carmeliet E (1999) Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 79:917–1017
Cross HR, Lu L, Steenbergen C, Philipson KD, Murphy E (1998) Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ Res 83:1215–1223
Okninska M, Paterek A, Bierla J, Czarnowska E, Maczewski M, Mackiewicz U (2021) Effect of age and sex on the incidence of ventricular arrhythmia in a rat model of acute ischemia. Biomed Pharmacother = Biomedecine & pharmacotherapie 142:111983
Husti Z, Varró A, Baczkó I (2021). Arrhythmogenic remodeling in the failing heart. Cell 10(11):3203
Varro A, Baczko I (2011) Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk. Br J Pharmacol 164:14–36
Janczewski AM, Kadokami T, Lemster B, Frye CS, McTiernan CF, Feldman AM (2003) Morphological and functional changes in cardiac myocytes isolated from mice overexpressing TNF-alpha. Am J Physiol Heart Circ Physiol 284:H960–H969
Wei SK, McCurley JM, Hanlon SU, Haigney MC (2007) Gender differences in Na/Ca exchanger current and beta-adrenergic responsiveness in heart failure in pig myocytes. Ann N Y Acad Sci 1099:183–189
Acknowledgements
This work was supported by grants from the Hungarian National Research, Development and Innovation Office (NKFIH FK-142949, K-128851).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nagy, N., Baczkó, I. (2023). Sex Differences in the Function of Cardiac Sodium-Calcium Exchanger in Physiological and Pathophysiological Settings: Implications for Cardiac Arrhythmias. In: Kirshenbaum, L., Rabinovich-Nikitin, I. (eds) Biology of Women’s Heart Health. Advances in Biochemistry in Health and Disease, vol 26. Springer, Cham. https://doi.org/10.1007/978-3-031-39928-2_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-39928-2_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-39927-5
Online ISBN: 978-3-031-39928-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)