Abstract
Before menopause women are protected against cardiovascular disease morbidity and mortality, relative to age-matched men. After menopause cardiovascular disease mortality rises sharply in women to match or exceed levels in men. A higher rate of cardiovascular mortality is seen in women who experience menopause at an early age which supports the idea that cardiovascular risk is primarily driven by ovarian failure and not age. While the dramatic shift in cardiovascular disease risk is associated with the loss of estrogens in menopause, there are multiple biological and sociological phenomenon driving the threat. Shifts in adipose patterning, vasomotor symptoms and the damage to endothelial function, along with increased levels of inflammation, are all postmenopausal changes that pose threats to cardiovascular health. The inequitable treatment of women with cardiovascular disease compounds biological hazards, as does a lack of education and training for women’s cardiovascular health. Estrogen replacement therapy offers some potential benefits for postmenopausal women but the knowledge gaps in our understanding of how estrogens impact cardiovascular health specifically, and postmenopausal well-being generally, limits its use. Research into the cardiovascular impact of menopause, the mechanisms of estrogen replacement therapy, and improved training for healthcare professionals could improve the health of post-menopausal women.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Prior to menopause, women are relatively protected against cardiovascular disease, as evidenced by a lower incidence of cardiovascular disease morbidity and mortality when compared to men of the same age [1]. However, protection against cardiovascular disease is starkly diminished in women after menopause, as seen by the sharp rise of cardiovascular mortality later in life that matches or exceeds levels reported in the male population [2]. Despite the relative protection enjoyed by premenopausal women against cardiovascular disease mortality, cardiovascular disease in general—and ischemic heart disease specifically—is the leading cause of death of women globally [3,4,5].
The reasons for increased cardiovascular disease mortality in women after menopause are poorly understood. There are, however, a number of biological and sociological risk factors that change or emerge after menopause that contribute to this phenomenon. The rise in cardiovascular mortality reported in postmenopausal women is not explained by any single risk factor and is likely the result of interactions between several of these elements.
This chapter will outline the physiological changes that occur during menopause, provide mechanisms to explain the biological basis for the postmenopausal rise in cardiovascular mortality, and discuss sociological factors that compound biological risk.
Setting the Stage: Defining Key Elements of Menopause
-
(a)
Menopause
Menopause is a physiological process that typically occurs over several years and results in a decline in ovarian function and the end of menstrual cycles. Some women experience iatrogenic menopause following the surgical removal of the ovaries or ovarian failure in association with chemotherapy, radiation therapy, or other medical treatments. The removal of ovaries (oophorectomy) in association with hysterectomy was once a relatively common occurrence, but current guidelines recommend retaining the ovaries unless there are medical concerns, such as a genetic risk of ovarian cancer [6, 7]. The onset of menopause is defined as the time 12 months after the final menstrual period as a result of ovarian follicle depletion, or at the time of bilateral oophorectomy [8].
Natural menopause can take up to 10 years to occur [9], with a mean age of onset of 51 years of age. However, age of menopause is impacted by a wide range of factors including genetics [10, 11]; diet and exercise; socio-economic status; and ethnicity, giving rise to variations across the world [12]. In much of the world the expanded life expectancy now means that women spend more than one-third of their lives in a postmenopausal state.
Menopause is more than a transition into a post-reproductive phase: it impacts health and quality of life. Estrogens—a key group of hormones produced by the ovaries—affect systems in the body beyond the reproductive organs, including bone, brain, adipose, muscle, blood vessels, and heart. The ovarian estrogens also regulate estrogen production by extragonadal tissues like adipose. The decline in circulating estrogens creates an imbalance with other hormones and regulatory factors in the body including testosterone and glucocorticoids, whose actions are normally countered by estrogens. Overall, the loss of estrogens and hormonal homeostasis creates a physiological environment that is associated with an increased risk for a number of health conditions including dementia, obesity, osteoarthritis, cardiovascular disease, and sarcopenia [13].
-
(b)
Perimenopause
A standardized set of criteria developed by ‘The Stages of Reproductive Aging Workshop + 10’ (STRAW + 10) has divided the menopausal transition into 4 periods: late reproductive phase, early menopausal transition, late menopausal transition, and early postmenopause [8]. The postmenopausal stage has historically garnered the most attention in terms of research and medical interventions because of the rise in health conditions associated with this phase. Although the postmenopausal period is when most health conditions associated with menopause overtly manifest, the groundwork for risks is likely set much earlier, during the early and late menopausal transitions, more commonly referred to as “perimenopause”.
Perimenopause is broadly defined as a period of irregular menstrual cycles coupled with amenorrhea that describes the time between the reproductive stage and menopause [8]. Early menopausal transition starts when women experience persistent differences in their menstrual cycle duration of 7 or more days. Cycle variability increases and there are periods of amenorrhea which last 60 days or more in the late menopausal transition. Perimenopause is completed at the end of a 12-month period of amenorrhea. In total, perimenopause can have a normal duration of 2–8 years [14].
Perimenopause is not simply a smooth and gradual decline in ovarian hormones. It is marked by highly variable and increasing levels of follicle stimulating hormone (FSH), as well as low levels of antimüllerian hormone (AMH) [9]. Perhaps the most well-known hormonal change in perimenopause are decreasing but punctuated levels of 17β-estradiol [8]. FSH levels rise up to 6 years before menopause and the decline in estrogens does not start until 2 years before the final menstrual period [15, 16]. Circulating levels of testosterone do not change significantly during the perimenopausal phase, but the declining levels of estrogens produces an imbalance between estrogens and androgens that creates a state of relative androgenic excess [17].
-
(c)
Early/Premature Menopause
Menopause typically does not occur until middle age, an age that also corresponds with an increased cardiovascular disease risk in men. Because ageing is a risk factor for cardiovascular disease, it is difficult to quantify how much of the increased risk seen in postmenopausal women is a natural process of ageing and how much is the consequence of ovarian failure.
The case for ovarian involvement is evident in women who experience early menopause or premature ovarian insufficiency. Early menopause is defined as an onset that occurs before age 45, and premature ovarian insufficiency results in menopause before 40 years of age. These conditions affect 12 and 4% of women, respectively [18]. Meta-analyses of observational studies found premature ovarian insufficiency is associated with an increased rate of cardiovascular disease generally, and ischaemic heart disease specifically [19,20,21]. A review of 32 observational cohort, case–control, and cross-sectional studies including 310,329 women shows early menopause creates a higher risk of coronary heart disease and cardiovascular disease mortality, but not stroke [22]. Zhu and colleagues reported a similarly tight relationship between early and premature menopause with coronary heart disease, but they also report an elevated risk of stroke in women who enter menopause earlier than expected [20]. Overall, the data clearly show an elevated risk for cardiovascular disease development and mortality in women who experience either early menopause or premature ovarian insufficiency, compared to women of a similar age.
Hormone replacement therapy studies show cardiovascular disease development is reduced when women with premature ovarian insufficiency are treated until the typical age of menopause [23, 24], supporting the argument that the loss of ovarian function is a critical risk factor for cardiovascular disease. A review of 15 observational studies across 5 countries reported women with premature or early menopause who used hormone therapy for more than 10 years had a reduced risk of cardiovascular disease compared to women who did not [20]. Recommendations published in the Journal of Obstetrics and Gynaecology Canada recommend “women with premature or early-onset menopause appear to be at an increased risk of adverse cardiovascular outcomes, and this risk may be prevented by the use of menopausal hormone therapy until the average age of menopause” [25].
Together, these studies uncouple the risk of cardiovascular disease development in postmenopausal women from ageing, and establish a link between the decline in ovarian estrogen production and risk factors for cardiovascular disease. These data, coupled with studies demonstrating an ability of estrogen replacement to mitigate some cardiovascular disease hazard, make a strong case for a key role of ovarian failure in the increased rate of cardiovascular disease seen in postmenopausal women.
Emergence of Risk
The physiological changes that accompany menopause create a favourable environment for the development of cardiovascular disease and co-morbidities that worsen or promote cardiovascular illness. Diabetes [26, 27], hypertension [28, 29], and dyslipidemia [30] are among the co-morbidities observed at higher rates in postmenopausal women. Metabolic syndrome, which is characterized by increased abdominal obesity, insulin resistance, elevated blood pressure, and increased inflammation, is more prevalent in women after menopause [31, 32]. These comorbidities exacerbate injury associated with cardiovascular events like myocardial infarction and complicate the clinical management of patients, giving rise to higher adverse events and mortality rates. How they arise is a reflection of the diverse impact estrogens have on the body and the systematic effects of menopause.
-
(a)
Adipose
Regional adiposity is a risk factor in cardiovascular disease. The loss of estrogens coupled with a relative increase in androgens after menopause creates a hormonal milieu in which body fat distribution is altered in women [33, 34]. Premenopausal adipose distribution is primarily subcutaneous in the gluteofemoral region and is associated with a lower risk of metabolic diseases and other pre-cursors to cardiovascular disease [35,36,37]. After menopause, there is a shift towards an increase in visceral storage of adipose [38, 39]. A key role for the location of adipose deposits in determining risk of disease was demonstrated in a study of normal weight postmenopausal women that showed visceral obesity was associated with increased mortality, but increased subcutaneous levels were not [40]. The adjustment in adipose distribution towards higher levels in abdominal and intraperitoneal regions more closely resembles the male pattern of adipose distribution and creates an elevated risk for cardiovascular risk factors like diabetes [41], and cardiovascular conditions such as hypertension [41] and coronary artery disease [42].
Sex differences in adipose patterning has long been known, but the mechanisms underlying shifts in adipose deposition with menopause remains poorly understood. There is emerging evidence for interactions between sex steroids and the adipose microenvironment to drive the alterations in adipose distribution that follow menopause. Abildgaard and colleagues reported menopause is associated with metabolic dysfunction in visceral and subcutaneous adipose [43]. Both visceral and subcutaneous adipose showed adipocyte hypertrophy, increased inflammation, hypoxia, and fibrosis, while visceral adipocytes exhibited a decrease in insulin sensitivity. Subcutaneous adipose tissue dysfunction causes an inability to store fats in this location and creates a spill-over that is taken up by visceral adipose. Price et al. previously speculated that a decline in circulating progesterone coupled with increased production of estrone in the gluteofemoral region decreases lipoprotein lipase activity regionally and explains the decreased ability to store fats in this adipose site [44]. After menopause, the visceral adipose tissue is the primary source of estrogens and increased local levels may create a cellular milieu that promotes visceral expansion [45]. Despite studies offering data to support a local mechanism of adipose regulation after menopause, the details of local adipose storage both before and after menopause remain largely unknown.
Increased postmenopausal obesity may also be centrally mediated. In mouse models, central nervous system estrogen receptor-α (ERα) influences abdominal obesity and physical activity, two areas that are commonly affected in postmenopausal women. Deletion of ERα in hypothalamic steroidogenic factor-1 neurons decreased energy expenditure in the form of physical activity and increased deposition of fat in the abdomen [46]. Deletion of the same estrogen signaling target in pro-opiomelanocortin neurons of the hypothalamus stimulated appetite. Interestingly, ERα reductions in ventromedial hypothalamic nucleus steroidogenic factor-1 neurons produces a similar hypoactive state, possibly through decreased SNS activity, which would also increase lipid storage [47]. The postmenopausal reduction in circulating estrogens produces less stimulant for central nervous system ERα, providing a feasible mechanism to explain some body composition alterations.
-
(b)
Vasomotor Symptoms
Estrogens help maintain a healthy, functioning endothelium, which is diminished with menopause. The onset of endothelial dysfunction begins early in menopause [48,49,50] and may set the stage for later coronary artery lesions. The process of atherosclerosis and vascular dysfunction is enhanced with other menopausal changes, including increased activation of the renin–angiotensin–aldosterone [51, 52] and sympathetic nervous systems [53,54,55].
Vasomotor symptoms (VMS) are the cardinal symptom of the menopausal transition. Over 70% of women report VMS [56] which include hot flushes and night sweats. The intensity and frequency of VMS vary widely, and are impacted by ethnic background, genetics, diet, and level of physical activity [57]. In addition to impacting quality of life by disrupting sleep and mood, VMS appear to be a harbinger of future cardiovascular risk.
Women who experience more severe symptoms, or for a prolonged period of time, are at increased risk of developing cardiovascular disease [58, 59]. A study of 11,725 women who had frequent VMS before age 50 found an increased risk of coronary artery disease [60]. The SWAN [61, 62], WISE [63], and WHI-OS [59] studies all found VMS were associated with preclinical signs of cardiovascular disease, although KEEPS [64] found no association in women at low risk for cardiovascular disease.
Early onset [65] and prolonged VMS duration [66] are individually linked to subclinical changes in vascular structure and function that are risk factors for cardiovascular disease. How menopausal VMS increase the risk of cardiovascular disease is not definitively known, although there are several plausible mechanisms. VMS are a consequence of a more narrow thermoeneutral zone that arises during perimenopause. The constricted tolerance for temperature variations triggers a physiological response to dissipate the perceived increase in heat, largely through the autonomic nervous system. Increased SNS and/or decreased PNS activity work to mediate this effect, but the impact is systematic, including the cardiovascular system. Chronic SNS activation in association with VMS could lead to increased blood pressure and the risk for cardiovascular disease. Increased SNS activity is also linked to dyslipidemia [67] which may explain unhealthy lipid profiles that can develop in women with VMS, independent of BMI and other known CVD risk factors [68, 69]. Some [70], but not all [71] studies have demonstrated a positive correlation between VMS and increased levels of pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α [72, 73], which promote cardiovascular disease including endothelial dysfunction.
Sleep disturbances and mood imbalances that are linked to VSM may also provide connections between the menopausal effects of VMS and the increased risk for CVD. Studies have shown that reduced or disrupted sleep is associated with subclinical CVD [74, 75]. VSM also negatively impacts mood, which creates CVD risk [76, 77].
Whether VSM increase cardiovascular risk directly or are a coincidental occurrence in women who develop chronic conditions that have been linked to this menopausal phenomenon has been difficult to determine, given the number of other postmenopausal changes that occur simultaneously. For example, women with obesity [78, 79]—a known cardiovascular risk factor—are more likely to experience severe VMS.
-
(c)
Inflammation
Both preclinical animal models of menopause and human studies have reported increased levels of circulating proinflammatory cytokines supporting a heightened level of inflammation [80,81,82]. Fernandes and colleagues were the first to report changes in myocardial levels of cytokines in a mouse model of menopause [83]. The links between inflammation and cardiovascular disease are well established [84–86, 90,91,92,93].
Estrogens—in particular 17β-estradiol—have direct impact on inflammatory cytokine levels by supressing expression of pro-inflammatory IL-1 [88], IL-6 [88,89,90], and TNF-α [89, 90], and stimulating release of anti-inflammatory IL-10 [91, 92]. These effects are mediated through ER which are expressed in a number of cell types including macrophages, B cells, T-cells, neutrophils, and monocytes [93]. The anti-inflammatory actions of estrogens occur when hormonal levels are high, but estrogens can be pro-inflammatory at low concentrations [94]. Progesterone, which is also lost during menopause, promotes a predominantly anti-inflammatory cytokine profile [95].
Estrogens can also impact immune function and inflammation through indirect pathways. Metabolically stressed cells secrete inflammatory cytokines and chemotactic molecules to recruit immune cells such as macrophages, lymphocytes, and eosinophils to the dysfunctional tissue. The loss of estrogens and an ability to protect against metabolic stress with menopause increases the likelihood of energetic disturbances which create a pro-inflammatory environment. The disruption in metabolism that occurs with menopause and promotes a pro-inflammatory state is referred to as “metainflammation” [96].
-
(d)
The Inequity of Medicine
Menopause is associated with numerous biological changes that individually and in combination increase the risk of cardiovascular disease. But beyond the biological changes, social and medical management factors that influence care also contribute to the elevated risk of morbidity and mortality that accompanies menopause.
Myocardial infarctions with obstructive coronary artery disease (type 1) are 3 times more common in men than women, while myocardial infarctions with no obstructive coronary arteries (MINOCAs, type 2) are more prevalent in women [2]. The lack of coronary obstructions associated with MINOCA leads to a more optimistic prognosis and less aggressive treatment. This benign prediction stands in contrast to clinical trials showing that clinical outcomes with MINOCA patients are similar to those with obstructive coronary artery disease [97]. The disproportionate representation of postmenopausal women who experience MINOCA as compared to similarly aged men may contribute to the higher rates of acute myocardial infarction mortality, but the relatively small number of MINOCA cases as a proportion of all acute myocardial infarctions limits the ability to use this issue to explain a significant amount of the sex-dependent discrepancies in mortality.
Beyond the risks specifically associated with MINOCA, studies have consistently shown that women are less likely to be given timely treatment following acute myocardial infarctions. A study of 192,204 adults over the age of 60 diagnosed with STEMI found that women are less likely to receive Percutaneous Coronary Intervention (PCI) or coronary artery bypass grafts, and that the gap between the sexes for revascularization increased between 2005 and 2014 [98]. While the presence of co-morbidities that increase the risk of bleeding may explain some of the differences in PCI between the sexes, women who did receive revascularization therapy underwent procedures after a more significant delay than men. These data suggest that even when revascularization therapy is not contraindicated because of co-morbidities, there is still a difference in interventional therapies between the sexes. The combination of fewer interventions and delayed treatment was associated with a higher in-hospital mortality in older women as compared to men.
Medical management of patients at risk of cardiovascular disease or who have previously had a cardiovascular event is critical to limit the risk of recurrence or a first-time event. Guideline therapies for individuals at risk or recovering from cardiovascular disease generally do not differ between the sexes. The lack of sex-specific guidelines are the result of a paucity of clinical and preclinical research involving female patients or animals, as opposed to a need to treat similar pathophysiological mechanisms between the sexes [99, 100]. Despite similarities in treatment plans, women are generally less likely than men to receive pharmaceutical treatments according to guidelines to manage cardiovascular risk in both primary and secondary care setting [1, 101, 102].
Hormone Replacement Therapy (HRT): Restoring the “Protection”?
Throughout its history HRT has undergone extensive investigation with paradoxical findings: some studies report beneficial effects on women’s health, while others find no benefits or even increased risk for a variety of health conditions.
-
(a)
A Brief History of HRT
In the 1960s, American gynecologist Robert Wilson published the book “Feminine Forever” in which he advocated for the use of estrogen supplements to avoid menopausal “symptoms” that led to a “horror of…living decay” [103]. Wilson’s work generated significant philosophical debate about the view of menopause as a medical condition which persisted decades after publication [104].
In 1975 two reports linking HRT to an increased risk of endometrial cancer presented the first significant obstacle to the clinical use of exogenous estrogens to mitigate the symptoms and risks of menopause [105, 106]. Concerns about a link to cancer were addressed by lowering the dose of estrogens and adding progesterone [107].
Over the next several decades preclinical research and observational studies produced a remarkably consistent body of evidence demonstrating clear cardiovascular benefits of HRT for postmenopausal women. However, in 1998 the Heart and Estrogen/progestin Replacement Study (HERS) yielded data questioning the benefits of HRT and offered evidence of some increased risk [108]. These concerns were hotly debated until 2002 when the Women’s Health Initiative (WHI) released the results of a trial where data showed a 29% increase in heart attacks alongside a 26% increased risk of breast cancer [109]. The clear risks of HRT coupled with no obvious cardiovascular benefits shown in the WHI study seemed to mark the end of HRT.
-
(b)
Solving the Paradox
Despite the clearly negative results of WHI and other clinical trials investigating the health benefits of HRT, a closer examination of the findings raised a number of issues that may see the re-emergence of estrogen replacement therapy as a tool to reduce risk and improve the health of postmenopausal women. Among the variables that differ across studies and may explain some of the variations in outcomes are the timing and duration of estrogen replacement therapy; dose and route of estrogen administration; as well as the formulation of estrogens used.
In general, studies have found that initiating hormone therapy within 10 years of the onset of menopause (< 60 years of age) reduces rates of myocardial infarctions, cardiac deaths, and all-cause mortality [110, 111]. Initiation of therapy within 6 years is beneficial in slowing the progression of atherosclerosis, but not when started after 10 years postmenopause [112]. A Cochrane review of placebo-controlled RCTs found HRT reduced coronary artery deaths and all-cause mortality when HRT was started within 10 years of menopause, with a neutral impact beyond 10 years [113]. Even in studies that found negative results, women who initiated treatment at earlier ages demonstrated benefits with respect to cardiovascular disease risk and mortality [113]. Together, these studies suggest that early intervention with HRT appears to have cardiovascular benefits, but initiating or continuing treatment beyond a decade after menopause is not widely supported.
Vaginally administered estrogens have beneficial cardiovascular outcomes, while transdermal estrogens may increase the risk of cardiovascular death [113]. Interestingly, vaginal estrogens are weaker than those administered using dermal patches, suggesting that low dose estrogens may have more benefits than higher doses, and that route of delivery impacts cardiovascular outcomes.
Estrogen is not a singular compound, but rather a group of hormones. Hormone replacement therapies are often comprised primarily of 17β-estradiol along with other estrogens [114]. The variable formulations of HRT are likely to contain agonists that differentially activate the various estrogen receptors in the body, creating diverse and even paradoxical effects.
Although the data are not clear in terms of fully understanding the risks and benefits of estrogen replacement therapy, on the whole, most medical societies support the use of hormone replacement therapy to relive menopausal symptoms for women who experience early menopause or premature ovarian insufficiency; postmenopausal women under 60 years of age; and those who are at low-to-moderate CVD risk [115,116,117]. While there are suggestions that HRT may be protective, there are contraindications which preclude its universal application. Women with a history of venous thromboembolism, or histories of cardiovascular disease or breast cancer should be excluded.
The effectiveness of hormonal replacement therapy has been a hotly debated topic with evidence supporting a cardiovascular advantage for use, as well as no benefit against cardiovascular disease or even an increased risk of negative outcomes. These discrepant findings and the inability to adequately explain their existence underscores the knowledge gap that exists concerning our understanding of how estrogens work in the body; how ageing impacts their effects; and how these sex hormones respond to stressors.
-
(c)
Mechanisms of HRT
The three known estrogen receptors—α (ERα), β (ERβ), and G protein-coupled estrogen receptor 1 (GPER)—are expressed in numerous cell types. Depending on their distribution and level of expression they can induce and initiate different cascades and actions. The beneficial effects of estrogens seen on the vascular system are mainly due to an ERα mediated response [118, 119]. The activation of vascular ERα releases arterial vasodilatation actors and modulates the inflammatory response through genomic mechanisms [120]. In fact, with the hormonal changes occurring with menopause, it is believed that there is a shift toward a higher expression of ERβ which is associated with higher inflammation and endothelial dysfunction, possibly explaining the negative results of clinical trials when HRT was applied well after menopause.
Several studies have shown the beneficial effects of estrogens in downregulating vascular inflammation through the decrease of cytokines, intracellular adhesion molecules (VCAM-1 and ICAM-1), and the accumulation of leucocytes in the endothelium [94, 121,122,123]. In addition, the estrogen-dependent decrease in TNF-α and IL-1β in different cell types has been proposed as a driving force behind the anti-inflammatory and vasculo-protective actions [94, 124]. Some studies have, on the contrary, shown an increase in inflammatory cytokines as a result of estrogen excess [125]. However, it seems that this connection is only consolidated in the context of auto-immune diseases such rheumatoid arthritis and atherosclerosis [126].
Other effects of estrogen deficiency have highlighted their role as antioxidants. Estrogen loss leads to increased production of reactive oxygen species which promotes LDL oxidation [127]. LDL oxidation is a key step in the process of atherosclerosis by inducing the formation of foam cells on blood vessel walls. This disease is driven by an immune-mediated inflammation and increased production of proinflammatory cytokines, both of which damage vascular endothelium [128]. The endothelial damage arising from inflammation and oxidative stress leads to a decrease in endothelial-derived nitric oxide production, which impairs vascular function. The introduction of exogenous estrogens can prevent the oxidation of LDL and reduce endothelial damage [127, 129].
The balance between ERα and ERβ signaling is a crucial factor in maintaining the regulation of inflammation, oxidative stress, and other potentially damaging processes which contribute to cardiovascular disease. Most of the beneficial effects of estrogens on the cardiovascular system are mediated through ERα which promotes vasodilation and reduces platelet aggregation. By contrast, ERβ activation is highly expressed in conditions such as oxidative stress, hypoxia, and inflammation, and restrains the beneficial effects of estrogen through ERα activation [130]. Of some note is the recent finding that in a mouse model of menopause the responsiveness of cardiac estrogen receptors is significantly altered during the perimenopausal transition [83]. These findings support the concept that estrogen replacement therapy has time-dependent benefits and risks, and that the remodelling of organ systems with menopause creates a fundamentally different physiology whose regulation by estrogens may be altered. The idea that a simple replacement of a complex group of hormones with a relatively static delivery system in an altered physiological environment ignores the complexity and diversity of estrogen regulation and the profound changes associated with menopause.
Women’s Health Education
Following prescribed treatment plans and implementing lifestyle modifications to reduce risk is highly correlated with understanding cardiovascular disease [131]. A number of cardiovascular organizations and societies have undertaken public education campaigns to increase awareness of cardiovascular disease in women. These initiatives have been tremendously successful in making both women and men aware of the cardiovascular disease risks faced by women; the unique symptoms that may occur in women with cardiovascular disease; and empowering women to advocate for appropriate care [132].
Despite the success of public education campaigns, there remain significant knowledge gaps in both the general public and amongst healthcare practitioners concerning cardiovascular disease and women. In 2013, the Canadian Women’s Heart Health Centre (CWHHC) surveyed Canadian women to determine their knowledge and understanding of heart health [133]. The CWHHC found 75% of women had low to medium level knowledge of cardiovascular disease and risk factors. A greater understanding of cardiovascular disease was associated with a university education and higher household income. Of some concern were the findings that those whose knowledge of cardiovascular disease was lowest, tended to have the greatest overestimation of their understanding, and that 60% of women deemed to be at high risk for cardiovascular disease described their risk as low or moderate. On the positive side, women who were over 55 and most likely to be postmenopausal were the most likely to discuss heart disease risks and prevention with their healthcare providers. More recent studies and surveys conducted in other countries have reported similar results concerning public understanding of cardiovascular disease in women [132, 134]. Thus, while the efforts of research societies and medical organizations have dramatically improved awareness of cardiovascular disease in women, there remains a significant deficiency in public education.
A similarly concerning knowledge deficiency has been identified among healthcare professionals. A 2004 survey found 70% of medical school trainees received no formal education or training on sex or gender-based medicine [135]. A more recent review of medical school curriculum in Canada shows that the problem persists, as evidenced by a general lack of material on women’s health or sex differences [136]. The lack of education and training on women’s health has resulted in a lack of confidence among healthcare professionals when it comes to treating women [137]. Only 39% of primary healthcare providers recognized cardiovascular disease as a top health concern for women, and less than half of primary care physicians and cardiologists felt well prepared to manage cardiovascular disease in women [132].
Path Forward
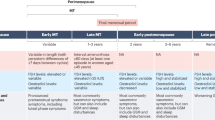
Menopause presents a complex and intricate series of biological stressors on a women’s cardiovascular system (Fig. 10.1). The addition of sociological issues which complicate treatment creates an environment ripe for cardiovascular risk. Despite the bleak picture, there are several opportunities to advance and improve women’s cardiovascular health, specifically by targeting the challenges posed by menopause.
Cardiovascular risk in postmenopausal women. a The decline in ovarian function throughout the perimenopausal phase presents a stress to the cardiovascular system that is driven largely by the punctuated decline in estrogens. b Systemic challenges including changes in adipose patterning, vascular dysfunction, inflammation, and oxidative stress cause damage and dysfunction across the cardiovascular system. Social inequities including unequal access to timely and guideline-driven medical care exacerbates biological risk. c The application of treatments according to recommended guidelines improves outcomes for postmenopausal women with cardiovascular disease. Public and professional education programs whose content is based on preclinical and clinical research dedicated to understanding women’s cardiovascular physiology including the changes produced by menopause are critical for improved care and prevention strategies. Research into the mechanisms of HRT may reshape its delivery or advance alterative therapies that mitigate cardiovascular disease risk after menopause. Figure created with BioRender.com
Discovery and preclinical research have advanced our understanding of the role estrogens play in the body and how their loss impacts health and disease. The emergence of a new animal model of menopause to create and study the transitional perimenopausal phase offers an important opportunity to recapitulate the hormonal changes that characterizes menopause [138]. The more physiologically accurate model will allow for an investigation of the Timing Theory—the idea that menopause creates distinct windows of risk and that HRT may be most effective when applied within distinct phases. Studies using this model have already shown that cardiovascular changes occur during the perimenopausal period, and have identified potential targets to mitigate risk [83, 139].
The initial public education campaigns by professional societies and advocacy groups should be lauded for their success in improving knowledge about the risk of cardiovascular disease faced by women. However, there remain knowledge gaps in both the public and healthcare professional groups that require a new approach. In an effort to improve healthcare training and correct the inequity of treatment that negatively impacts women, the Canadian Women’s Heart Health Alliance developed an accredited physician education program that specifically deals with women and cardiovascular disease [140]. The women’s heart health curriculum targets physician specialists in internal medicine and cardiology; nurses; medical trainees; cardiac rehabilitation specialists; and women at risk for cardiovascular disease. A survey found most healthcare professionals are willing to pursue additional training to address their knowledge gaps concerning sex and cardiovascular disease, which bodes well for these educational initiatives.
The concerning results of the WHI and other studies which found no cardiovascular benefits of HRT were a stark reminder of how little is known about estrogens, their role in cardiovascular physiology, and their potential as therapeutic tools. Preclinical research investigating the physiological changes that occur during menopause coupled with studies designed to understand the fundamental role of estrogens in regulating cardiovascular function could be used to re-design HRT. The use of specific estrogen receptor agonists/antagonists for more precise prophylactic treatment would limit unwanted side effects and increase effectiveness. Identifying the lowest effective dose and the best route of treatment would also limit side effects and provide the most impactful interventions.
Women make up slightly more than half the global population and on average spend nearly one-third of their lives in menopause. The cardiovascular risk posed by this phase of life is significant and profoundly impacts quality of life as well as life expectancy. Addressing the unique challenges of women’s cardiovascular health demands a focused and significant investment of time and resources. Without a dedicated undertaking women will continue to receive substandard care. These costs are both economic and social, and reach beyond the women directly affected by cardiovascular disease to profoundly impact the societies in which they live.
References
Irani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW (2020) American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circ 141(9):e139–e596
Maas AHEM et al (2021) Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J 42:967–984
Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Leal J, Luengo-Fernandez R, Burns R, Rayner M, Townsend N (2017) European cardiovascular disease statistics, 2017 edn. European Heart Network, pp 1–192
Kaptoge S et al (2019) World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Heal 7:e1332–e1345
Abbafati C et al (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396:1204–1222
Novetsky AP, Boyd LR, Curtin JP (2011) Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstet Gynecol 118:1280–1286
Mikhail E et al (2015) National trends of adnexal surgeries at the time of hysterectomy for benign indication, United States, 1998–2011. Am J Obstet Gynecol 213(713):e1–713.e13
Harlow SD et al (2012) Executive summary of the stages of reproductive aging workshop 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 97:1159–1168
Randolph JF et al (2003) Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 88:1516–1522
Ruth KS et al (2021) Genetic insights into biological mechanisms governing human ovarian ageing. Nat 596:393–397
Bae H et al (2019) Genetic associations with age of menopause in familial longevity. Menopause 26:1204–1212
Gold EB (2011) The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 38:425–440
Van Dijk GM, Kavousi M, Troup J, Franco OH (2015) Health issues for menopausal women: the top 11 conditions have common solutions. Maturitas 80:24–30
Brambilla DJ, Mckinlay SM, Johannes CB (1994) Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol 140:1091–1095
Randolph JF et al (2011) Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 96:746–754
Burger HG et al (1999) Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84:4025–4030
Hall JE (2015) Endocrinology of the menopause. Endocrinol Metab Clin North Am 44:485–496
Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z (2019) The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric 22(4):403–411
Roeters van Lennep JE, Heida KY, Bots ML, Hoek A (2016) Collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol 23(2):178–186
Zhu D et al (2019) Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 4:553–564
Tao XY, Zuo AZ, Wang JQ, Tao FB (2016) Effect of primary ovarian insufficiency and early natural menopause on mortality: a meta-analysis. Climacteric 19(1):27–36
Muka T, Oliver-Williams C, Kunutsor S, Laven JSE, Fauser BCJM, Chowdhury R, Kavousi M, Franco O (2016) Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality. JAMA Cardiol 1:767–776
Webber L et al (2016) ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 31:926–937
Webber L, Anderson RA, Davies M, Janse F, Vermeulen N (2017) HRT for women with premature ovarian insufficiency: a comprehensive review. Hum Reprod Open (2):hox007
Abramson BL, Black DR, Christakis MK, Fortier M, Wolfman W (2021) Guideline No. 422e: menopause and cardiovascular disease. J Obstet Gynaecol Canada 43:1438–1443
Ren Y et al (2019) Association of menopause and type 2 diabetes mellitus. Menopause 26:325–330
Slopien R et al (2018) Menopause and diabetes: EMAS clinical guide. Maturitas 117:6–10
Pollow DP, Uhlorn J, Husband N, Brooks HL (2019) Regulation of postmenopausal hypertension. Sex Differ Cardiovasc Physiol Pathophysiol 105–118
Lima R, Wofford M, Reckelhoff JF (2012) Hypertension in postmenopausal women. Curr Hypertens Rep 14:254
Wietlisbach V, Marques-Vidal P, Kuulasmaa K, Karvanen J, Paccaud F (2013) The relation of body mass index and abdominal adiposity with dyslipidemia in 27 general populations of the WHO MONICA Project. Nutr Metab Cardiovasc Dis 23:432–442
Carr MC (2003) The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88:2404–2411
Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K (2008) Menopause and the metabolic syndrome: the study of women’s health across the nation. Arch Intern Med 168:1568–1575
Rebuffe-Scrive M, Cullberg G, Lundberg PA, Lindstedt G, Bjorntorp P (1989) Anthropometric variables and metabolism in polycystic ovarian disease. Horm Metab Res 21:391–397
Killinger DW, Perel E, Daniilescu D, Kharlip L, Lindsay WRN (1990) Influence of adipose tissue distribution on the biological activity of androgens. Ann NY Acad Sci 595:199–211
Hill JH, Solt C, Foster MT (2018) Obesity associated disease risk: the role of inherent differences and location of adipose depots. Horm Mol Biol Clin Investig 33(2):/j/hmbci.2018.33.issue-2/hmbci-2018-0012/hmbci-2018-0012.xml.
Piché ME, Vasan SK, Hodson L, Karpe F (2018) Relevance of human fat distribution on lipid and lipoprotein metabolism and cardiovascular disease risk. Curr Opin Lipidol 29:285–292
Diaz-Canestro C, Xu A (2021) Impact of different adipose depots on cardiovascular disease. J Cardiovasc Pharmacol 78:S30–S39
Svendsen OL, Hassager C, Christiansen C (1995) Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism 44:369–373
Lovejoy JC, Champagne CM, De Jonge L, Xie H, Smith SR (2008) Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32:949–958
Sun Y, Liu B, Snetselaar LG, Wallace RB, Caan BJ, Rohan TE, Neuhouser ML, Shadyab AH, Chlebowski RT, Manson JE, Bao W (2019) Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw Open 2(7):e197337
Kissebah AH, Peiris AN (1989) Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes Metab Rev 5:83–109
Lapidus L et al (1984) Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 289:1257–1261
Abildgaard J, Ploug T, Al-Saoudi E, Wagner T, Thomsen C, Ewertsen C, Bzorek M, Pedersen BK, Pedersen AT, Lindegaard B (2021) Changes in abdominal subcutaneous adipose tissue phenotype following menopause is associated with increased visceral fat mass. Sci Rep 11:14750
Price TM et al (1998) Estrogen regulation of adipose tissue lipoprotein lipase—possible mechanism of body fat distribution. Am J Obstet Gynecol 178:101–107
Bracht JR et al (2020) The role of estrogens in the adipose tissue milieu. Ann NY Acad Sci 1461:127–143
Xu Y et al (2011) Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14:453–465
Musatov S et al (2007) Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104:2501–2506
Celermajer DS et al (1994) Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24:471–476
Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM (2012) Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97:4692–4700
Moreau KL, Hildreth KL, Klawitter J, Blatchford P, Kohrt WM (2020) Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. GeroScience 42:1699–1714
Gersh FL, O’Keefe JH, Lavie CJ, Henry BM (2021) The renin-angiotensin-aldosterone system in postmenopausal women: the promise of hormone therapy. Mayo Clin Proc 96:3130–3141
Yanes LL et al (2010) Postmenopausal hypertension: role of the Renin-Angiotensin system. Hypertens. (Dallas, Tex. 1979) 56:359–363
Mercuro G et al (2000) Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol 85:787–789
Barnes JN et al (2014) Aging enhances autonomic support of blood pressure in women. Hypertens (Dallas, Tex. 1979) 63:303–308
Vongpatanasin W (2009) Autonomic regulation of blood pressure in menopause. Semin Reprod Med 27:338–345
Thurston RC (2018) Vasomotor symptoms: natural history, physiology, and links with cardiovascular health. Climacteric 21:96–100
Talaulikar V (2022) Menopause transition: physiology and symptoms. Best Pract Res Clin Obstet Gynaecol 81:3–7
El Khoudary SR, Thurston RC (2018) Cardiovascular implications of the menopause transition: endogenous sex hormones and vasomotor symptoms. Obstet Gynecol Clin North Am 45:641–661
Szmuilowicz ED et al (2011) Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause 18:603–610
Herber-Gast GCM, Brown WJ, Mishra GD (2015) Hot flushes and night sweats are associated with coronary heart disease risk in midlife: a longitudinal study. BJOG 122:1560–1567
Thurston RC et al (2021) Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in SWAN. J Am Heart Assoc 10:1–17
Thurston RC et al (2011) Hot flashes and carotid intima media thickness among midlife women. Menopause 18:352–358
Thurston RC et al (2017) Menopausal symptoms and cardiovascular disease mortality in the Women’s Ischemia Syndrome Evaluation (WISE). Menopause 24:126–132
Wolff EF et al (2013) Self-reported menopausal symptoms, coronary artery calcification, and carotid intima-media thickness in recently menopausal women screened for the Kronos early estrogen prevention study (KEEPS). Fertil Steril 99:1385–1391
Thurston RC et al (2016) Trajectories of vasomotor symptoms and carotid intima media thickness in the study of women’s health across the nation. Stroke 47:12–17
Thurston RC, Kuller LH, Edmundowicz D, Matthews KA (2010) History of hot flashes and aortic calcification among postmenopausal women. Menopause 17:256–261
Valensi P (2021) Autonomic nervous system activity changes in patients with hypertension and overweight: role and therapeutic implications. Cardiovasc Diabetol 20
Sassarini J, Fox H, Ferrell W, Sattar N, Lumsden MA (2011) Vascular function and cardiovascular risk factors in women with severe flushing. Clin Endocrinol (Oxf) 74:97–103
Thurston RC et al (2012) Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol 119:753–761
Bechlioulis A et al (2012) Increased vascular inflammation in early menopausal women is associated with hot flush severity. J Clin Endocrinol Metab 97:E760–E764
Chedraui P et al (2011) Pro-inflammatory cytokine levels in postmenopausal women with the metabolic syndrome. Gynecol Endocrinol 27:685–691
Huang WY et al (2017) Circulating interleukin-8 and tumor necrosis factor-α are associated with hot flashes in healthy postmenopausal women. PLoS One 12
Gordon JL et al (2016) Cardiovascular, hemodynamic, neuroendocrine, and inflammatory markers in women with and without vasomotor symptoms. Menopause 23:1189–1198
Liu X, Yan G, Bullock L, Barksdale DJ, Logan JG (2021) Sleep moderates the association between arterial stiffness and 24-hour blood pressure variability. Sleep Med 83:222–229
Kundel V et al (2021) Sleep duration and vascular inflammation using hybrid positron emission tomography/magnetic resonance imaging: results from the Multi-Ethnic Study of Atherosclerosis. J Clin Sleep Med 17:2009–2018
Mulvagh SL et al (2021) The Canadian women’s heart health alliance atlas on the epidemiology, diagnosis, and management of cardiovascular disease in women—Chapter 4: sex- and gender-unique disparities: CVD across the lifespan of a woman. CJC Open 4:115–132
Kahl KG, Stapel B, Correll CU (2022) Psychological and psychopharmacological interventions in psychocardiology. Front Psychiatry 13:831359
Saccomani S et al (2017) Does obesity increase the risk of hot flashes among midlife women?: a population-based study. Menopause 24:1065–1070
Anderson DJ et al (2020) Obesity, smoking, and risk of vasomotor menopausal symptoms: a pooled analysis of eight cohort studies. Am J Obstet Gynecol 222(478):e1–478.e17
Pfeilschifter J, Koditz R, Pfohl M, Schatz H (2002) Changes in proinflammatory cytokine activity after menopause. Endocr Rev 23:90–119
Cioffi M et al (2002) Cytokine pattern in postmenopause. Mat 41:187–192
Deswal A et al (2001) Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone Trial (VEST). Circ 103:2055–2059
Fernandes RD et al (2019) Cardiac changes during the peri-menopausal period in a VCD-induced murine model of ovarian failure. Acta Physiol 227
Lu X, Crowley SD (2022) The immune system in hypertension: a lost shaker of salt 2021 Lewis K. Dahl Memorial Lecture. Hypertens (Dallas, Tex. 1979) 79:1339–1347
Anzai A, Ko S, Fukuda K (2022) Immune and inflammatory networks in myocardial infarction: current research and its potential implications for the clinic. Int J Mol Sci 23:5214
Evans BR, Yerly A, van der Vorst EPC, Baumgartner I, Bernhard SM, Schindewolf M, Döring Y (2022) Inflammatory mediators in atherosclerotic vascular remodeling. Front Cardiovasc Med 9:868934
Mikkelsen RR et al (2022) Immunomodulatory and immunosuppressive therapies in cardiovascular disease and type 2 diabetes mellitus: a bedside-to-bench approach. Eur J Pharmacol 925:174998
Miller AP et al (2004) Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulat 110:1664–1669
Frink M, Thobe BM, Hsieh YC, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH (2007) 17beta-Estradiol inhibits keratinocyte-derived chemokine production following trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol 292(2):L585–591
Kan WH, Hsu JT, Ba ZF, Schwacha MG, Chen J, Choudhry MA, Bland KI, Chaudry IH (2008) MAPK-dependent eNOS upregulation is critical for 17beta-estradiol-mediated cardioprotection following trauma-hemorrhage. Am J Physiol Heart Circ Physiol 294(6):H2627-2636
Klein PW, Easterbrook JD, Lalime EN, Klein SL (2008) Estrogen and progesterone affect responses to malaria infection in female C57BL/6 mice. Gend Med 5:423–433
Brunsing RL, Prossnitz ER (2011) Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunol 134:93–106
Rosenzweig R, Gupta S, Kumar V, Gumina RJ, Bansal SS (2021) Estrogenic bias in T-Lymphocyte biology: Implications for cardiovascular disease. Pharmacol Res 170:105606
Straub RH (2007) The complex role of estrogens in inflammation. Endocr Rev 28:521–574
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nat 444:860–867
Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM, D'Onofrio G (2018) Presentation, clinical profile, and prognosis of young patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc 7(13):e009174
Ye G et al (2022) Sex differences and temporal trends in revascularization and outcomes of ST-elevation myocardial infarction in older adults in the United States. Arch Med Res 53:441–450
Pacheco C et al (2022) The Canadian women’s heart health alliance atlas on the epidemiology, diagnosis, and management of cardiovascular disease in women—Chapter 5: sex- and gender-unique manifestations of cardiovascular disease. CJC Open 4:243–262
Vallabhajosyula S, Verghese D, Desai VK, Sundaragiri PR, Miller V (2021) Sex differences in acute cardiovascular care: a review and needs assessment. Cardiovasc Res 118:667–685
Koopman C, Vaartjes I, Heintjes EM, Spiering W, van Dis I, Herings RMC, Bots M (2013) Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998–2010. Eur Heart J 34:3198–3205
Peters SAE, Colantonio LD, Zhao H, Bittner V, Dai Y, Farkouh ME, Monda KL, Safford MM, Muntner P, Woodward M (2018) Sex Differences in High-Intensity Statin Use Following Myocardial Infarction in the United States. J Am Coll Cardiol 71(16):1729–1737
Wilson R (1966) Feminine forever. M. Evans and Company, Inc.
Houck JA (2003) ‘What do these women want?’: feminist responses to feminine forever, 1963–1980. Bull Hist Med 77:103–132+233
Ziel HK, Finkle WD (1975) Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med 293:1167–1170
Smith DC, Prentice R, Thompson DJ, Herrmann WL (1975) Association of exogenous estrogen and endometrial carcinoma. N Engl J Med 293:1164–1167
Woodruff JD, Pickar JH (1994) Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. The menopause study group. Am J Obstet Gynecol 170:1213–1223
Hulley S et al (1998) Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 280:605–613
Rossouw JE et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Manson JAE et al (2019) Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med 171:406–414
Boardman H, Hartley L, Eisinga A, Main C, Figuls MRI (2016) Cochrane corner: oral hormone therapy and cardiovascular outcomes in post-menopausal women. Heart 102:9–11
Hodis HN et al (2016) Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 374:1221–1231
Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, Gabriel Sanchez R, Knight B (2015) Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev (3):CD002229
Harper-Harrison G, Shanahan MM (2023) Hormone Replacement Therapy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
Pinkerton JAV et al (2017) The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 24:728–753
Trémollieres FA et al (2022) Management of postmenopausal women: Collège National des Gynécologues et Obstétriciens Français (CNGOF) and Groupe d’Etude sur la Ménopause et le Vieillissement (GEMVi) Clinical Practice Guidelines. Maturitas 163:62–81
Lee SR et al (2020) The 2020 menopausal hormone therapy guidelines. J Menopausal Med 26:69
Arnal JF et al (2017) Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev 97:1045–1087
Pare G et al (2002) Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res 90:1087–1092
Usselman CW, Stachenfeld NS, Bender JR (2016) The molecular actions of oestrogen in the regulation of vascular health. Exp Physiol 101:356–361
Störk S, Von Schacky C, Angerer P (2002) The effect of 17beta-estradiol on endothelial and inflammatory markers in postmenopausal women: a randomized, controlled trial. Atherosclerosis 165:301–307
Sumino H et al (2006) Different effects of oral conjugated estrogen and transdermal estradiol on arterial stiffness and vascular inflammatory markers in postmenopausal women. Atherosclerosis 189:436–442
Kip KE et al (2005) Global inflammation predicts cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Am Heart J 150:900–906
Novella S, Heras M, Hermenegildo C, Dantas AP (2012) Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol 32:2035–2042
Cutolo M et al (2006) Estrogens and autoimmune diseases. Ann NY Acad Sci 1089:538–547
Arnal JF et al (2004) Estrogens and atherosclerosis. Eur J Endocrinol 150:113–117
Shwaery GT, Vita JA, Keaney JF (1997) Antioxidant protection of LDL by physiological concentrations of 17 beta-estradiol. Requirement for estradiol modification. Circ 95:1378–1385
Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6:508–519
Kuohung W, Shwaery GT, Keaney JF (2001) Tamoxifen, esterified estradiol, and physiologic concentrations of estradiol inhibit oxidation of low-density lipoprotein by endothelial cells. Am J Obstet Gynecol 184:1060–1063
Novensà L et al (2011) Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERα/ERβ balance in female mice. PLoS One 6:e25335
Alm-Roijer C, Stagmo M, Udén G, Erhardt L (2004) Better knowledge improves adherence to lifestyle changes and medication in patients with coronary heart disease. Eur J Cardiovasc Nurs 3:321–330
Bairey Merz CN et al (2017) Knowledge, attitudes, and beliefs regarding cardiovascular disease in women: the women’s heart alliance. J Am Coll Cardiol 70:123–132
McDonnell LA et al (2014) Perceived vs actual knowledge and risk of heart disease in women: findings from a Canadian survey on heart health awareness, attitudes, and lifestyle. Can J Cardiol 30:827–834
Hoare E, Stavreski B, Kingwell BA, Jennings GL (2017) Australian adults’ behaviours, knowledge and perceptions of risk factors for heart disease: a cross-sectional study. Prev Med Rep 8:204–209
Miller VM et al (2013) Embedding concepts of sex and gender health differences into medical curricula. J Womens Health (Larchmt) 22:194–202
Anderson NN, Gagliardi AR (2021) Medical student exposure to women’s health concepts and practices: a content analysis of curriculum at Canadian medical schools. BMC Med Educ 21
Rusiecki J, Rojas J, Oyler J, Pincavage A (2022) An expanded primary care-based women's health clinic to improve resident education and patient care in resident continuity clinic. J Gen Intern Med 37(9):2314–2317
Brooks HL, Pollow DP, Hoyer PB (2016) The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiol (Bethesda) 31:250–257
Konhilas JP et al (2020) Using 4-vinylcyclohexene diepoxide as a model of menopause for cardiovascular disease. Am J Physiol Heart Circ Physiol 318:H1461–H1473
Adreak N et al (2021) Incorporating a women’s cardiovascular health curriculum into medical education. CJC Open 3:S187–S191
Acknowledgements
Glen Pyle is a Heart and Stroke Foundation of Canada Senior Career Investigator for Improving the Heart and Brain Health for Women in Canada. His research is supported with funding from the Heart and Stroke Foundation of Canada, Health Canada, Canadian Institutes of Health Research, and the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rouhana, S., Glen Pyle, W. (2023). Menopause and the Bridge to Cardiovascular Disease. In: Kirshenbaum, L., Rabinovich-Nikitin, I. (eds) Biology of Women’s Heart Health. Advances in Biochemistry in Health and Disease, vol 26. Springer, Cham. https://doi.org/10.1007/978-3-031-39928-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-39928-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-39927-5
Online ISBN: 978-3-031-39928-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)