Abstract
Otitis media with effusion (OME), a term also encompassing serous otitis media, is a condition in which fluid accumulates in the middle ear, but there are no indications of an acute infection [1]. There is also a subgroup of cases of OME where the fluid is viscous and yellow in colour and has the consistency of glue when viewed during tympanic surgery [2]. This condition is termed ‘glue ear’. Although OME and glue ear are sometimes used interchangeably, glue ear in fact only occurs in a portion of cases of OME [3].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Otitis media with effusion (OME), a term also encompassing serous otitis media, is a condition in which fluid accumulates in the middle ear, but there are no indications of an acute infection [1]. There is also a subgroup of cases of OME where the fluid is viscous and yellow in colour and has the consistency of glue when viewed during tympanic surgery [2]. This condition is termed ‘glue ear’. Although OME and glue ear are sometimes used interchangeably, glue ear in fact only occurs in a portion of cases of OME [3].
The effusion in OME does not contain pus. It is mucoid or serous. The usual presenting symptoms are auditory impairment or feeling the ear is full, whilst pyrexia or otalgia rarely occur. In paediatric cases, auditory impairment is usually no more than mild, being revealed by audiogram rather than spontaneously reported.
Serous otitis media involves the formation of a watery and clear effusion as a result of transudation caused by negative pressure developing in the middle ear space [4]. It is a subtype of OME.
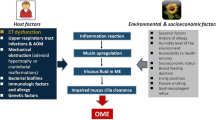
It is important for clinicians to appreciate that there are significant differences between OME and other infective conditions affecting the middle ear cavity [5]. The term ‘otitis media’ is a generalised term for any inflammatory condition of the middle ear. It does not indicate the particular cause or pathological mechanism involved. The pneumatised regions of the temporal bone (mastoid, perilabyrinthine and petrous apex air cells) are interconnected; hence, otitis media may lead to inflammation of these spaces, too. A range of disorders are all grouped under the umbrella term otitis media, namely, acute otitis media (AOM), recurrent acute otitis media (RAOM), OME and chronic otitis media with effusion (COME) [6].
2 Pathophysiology
OME may develop following AOM, as it begins to resolve and the acute phase has passed. Up to 45% of paediatric cases of AOM still have an effusion present 1 month after onset, with this number falling to 10% at the 3 month mark [6].
2.1 Classical Explanation
There are two principal ways in which the pathogenesis of AOM is said to occur. According to the classical theory, the auditory tube does not function correctly, which is a necessary step in pathogenesis. The auditory tube is generally credited with three functions, namely, equalising the pressure in the middle ear cavity and external auditory meatus, draining secretions and defence of the middle ear. If these functions are impaired, AOM may develop. Accordingly, several events may trigger eventual AOM, ranging from anatomical abnormality causing obstruction, to allergic inflammation, an infection of the upper respiratory tract or traumatic injury [6].
In cases where the auditory tube is dysfunctional for more than a brief period, the pressure in the middle ear cavity will fall, since nitrogen and oxygen diffuse out of this space. If there is a sizeable pressure gradient as a result, transudation develops from the mucosa, with a serous, typically sterile, fluid building up in the space. Since there is no drainage through the auditory tube, this stagnant fluid provides a suitable environment for bacterial species to grow, and AOM develops in response. However, this theory cannot be entirely correct, since it has been shown several times that cases of AOM and OME feature identical bacterial pathogens [4].
2.2 More Recent Explanations
More recently, it has been proposed that the initiating event in AOM is mucosal inflammation within the middle ear in response to pre-existing bacteria in the cavity. Imaging studies were used by Bluestone et al. to demonstrate reflux passing into the auditory tube in paediatric cases of recurrent otitis media [7]. Moreover, pepsin has been identified in the middle ear cavity of some 60% of paediatric OME cases [8], albeit reflux into the auditory tube can also be observed in individuals without any evidence of disease.
Likewise, O’Reilly et al. discovered, in their study of 129 children undergoing myringotomy with grommet insertion for an indication of otitis media, that pepsin A was present in aspirates from 64 cases. The presence of pepsin A indicated that stomach contents must have been refluxed as far as the nasopharyngeal region. These researchers relate this finding to the development of AOM by suggesting reflux triggers an inflammatory response or worsens any inflammatory response that is already occurring [9].
2.3 Middle Ear Effusion
Whatever the causative agent is in cases of AOM, a dysfunctional auditory tube is virtually invariably present in cases of OME. In animal models, when the auditory tubes are experimentally ligated, a long-standing effusion develops in the middle ear. Even when the infection has been eradicated and inflammatory responses cease, the effusion continues to be present due to non-drainage of the middle ear. The inability to drain the effusion may be a result of various pathophysiological changes, such as non-functioning cilia, oedema within the mucosa, excessive viscosity of the fluid and, potentially, pressure changes in the middle ear [6].
Not all cases of AOM proceed to become OME. There have been proposed a variety of mechanisms to account for how an effusion develops in the cavity. One is that inflammation of the mucosal lining causes fluid to be secreted. According to this explanation, the mucosa undergoes sensitisation following the presence of bacterial pathogens, and when reflux occurs from time to time, bacterial antigens are present to trigger an inflammatory response. However, as explained earlier, the bacterial species detected in cases of OME do not differ from those present in AOM. The effusion is not completely void of pathogens, as used to be thought [6].
2.4 Cleft Palate
In paediatric cases of cleft palate, OME is always seen. This is explained as occurring due to the incorrect insertion of the tensor veli palatini muscle. Accordingly, when patients swallow or open their mouths wide, this muscle does not perform its usual function of opening the auditory tube. Thus, the tune is functionally blocked [10].
3 Frequently Occurring Pathogens
The bacterial pathogens that are most frequent in cases of AOM are Streptococcus pneumoniae, followed by Haemophilus influenzae and then Moraxella catarrhalis. The same group of organisms are also the most common bacterial causes of sinusitis and pneumonia. These three species collectively are found in 85% of cases of AOM. They occur with the following frequency [6]:
-
S. pneumoniae is present 35% of the time, with no apparent effect of age on prevalence. The most frequently identified serotype is 19, followed by 23, 6, 14 and 3.
-
Some 20% of cases are attributed to H. influenzae. At present, between 25 and 45% of the organisms isolated are capable of expressing a beta-lactamase, and the frequency of antibiotic insensitivity is clearly rising.
-
In cases of AOM, M. catarrhalis is responsible for between 4 and 13%. This figure rises during the winter and autumn months. Beta-lactamase expression occurs in 70–100% of these pathogens.
The other pathogenic bacteria involved are Streptococcus pyogenes, Staphylococcus aureus, enteric bacteria-staining Gram-negative, and anaerobic species. Pseudomonas is the most commonly isolated bacterial genus in cases where an effusion has persisted for more than 3 months.
No pathogen is identified in 30% of aspirates obtained during tympanocentesis. A meta-analysis based on ten studies dating from 30 years ago collated data on 663 patients. In 29 cases (4.4%), a viral pathogen was identified. Data from more recent research indicate that a viral pathogen is identifiable in 15–20% of cases of AOM, co-existing with a bacterial pathogen. The viral pathogens most common in such cases are respiratory syncytial virus (RSV) and influenza virus [6].
4 Risk Factors
A number of risk factors for OME have been identified. These include the environment, age and dysfunction of the auditory tube [6].
4.1 Environment
There are a number of factors in the environment of the patient that increase the risk of OME, besides the presence of specific pathogens. Epidemiological data indicate the following are associated with raised risk: bottle feeding, being fed lying down, having a brother or sister with a middle ear infection, going to a childcare facility, being allergic to frequently encountered environmental allergens, low socioeconomic condition, presence of smokers in the household and a family history of OME in at least one parent [11, 12].
4.2 Age
Age is strongly associated with a heightened risk of OME. During infancy, the auditory tube in the anatomical position is virtually parallel to the ground, with the angle steadily increasing up to adulthood, when it has an angle of 45°. Furthermore, the anatomical configuration of the auditory tube in neonates permits less efficient ventilation of the middle ear than in adults [6, 13].
The results of several Danish studies on paediatric cases show that, up to the age of 1 year, in 24% of ears assessed, a type B (flat) or type C (indicating negative pressure) was found on tympanometry. Auditory function became more normal in springtime or the summer months, but winter witnessed declining function. The peak occurrence of a type B pattern was between the ages of 2 and 4 years. Thereafter, this type of tympanogram is more rarely seen, as could be anticipated from the lower frequency of OME in children after the age of 6 years [6].
It is vital that OME affecting one ear in an adult is identified without delay as the most dangerous underlying diagnosis is a neoplasm of the nasopharynx [6].
4.3 Disrupted Auditory Tube Function
OME is also more common in cases where the nasopharyngeal ostium cannot remain patent. This situation frequently affects individuals with cleft palate or Down Syndrome, as well as other conditions involving the palatal region. It has been proposed that the reason patients with cystic fibrosis are more prone to OME is because the clearance of accumulated mucus by ciliary action is impaired in cystic fibrosis, where mucus is abnormally viscid [6].
4.4 Diet
Research undertaken by Choi et al. concluded that eating a diet overly rich in fats does increase the risk of paediatric OME, although several other aspects of diet examined did not affect the risk, namely, body mass index and classification, dietary protein, water or sodium, and the times at which carbohydrates were consumed [14].
4.5 Other Factors
Kaya and colleagues disagree with the findings of the research by Choi et al. These authors state that OME is indeed linked to the patient’s being overweight or obese, based on a sample of 60 paediatric cases of OME and 86 healthy controls, with an age range of 2–10 years. They examined data linking body mass and height, noting that there was an association between excess body weight (including obesity) and higher frequency of OME. They speculate that being overweight or obese potentially increases the risk of OME, or, conversely, that OME is a risk factor for obesity [15].
Walker and colleagues noted specific characteristics of paediatric cases of OME occurring prior to school age [16]. These patients typically have a blocked nose, snore frequently or invariably, spend an above average length of time in childcare facilities each week, have coryzal illnesses frequently, tend to have siblings in whom grommet placement has already occurred, were born following prolonged labour and began drinking bovine milk relatively early. Being of Asian extraction and having older siblings, in contrast, had an association with less likelihood of developing OME [16].
5 Epidemiological Features
Acute otitis media occurs once or more in the majority of children, that is, in 84–93%. Moreover, around four in five children experience OME at least once by the time they are 10 years old. The cross-sectional prevalence of auditory impairment secondary to OME of at least 3-month duration is 5% in children aged between 2 and 4 years. The peak prevalence of OME occurs up to the third birthday and falls rapidly after the sixth birthday [6].
6 OME and Auditory Impairment
The aims in managing cases of OME are to remove the effusion from the middle ear cavity and normalise the pressure gradient, to restore auditory function. The first-line treatment consists of either watchful waiting or grommet insertion, which may sometimes be accompanied by removal of the adenoids. Myringotomy without grommet placement is not an effective option for treating OME. Initial results from balloon dilatation of the auditory tube have been encouraging, but the method needs further quantification of its benefits and risks before it can be generally recommended [3].
The method chosen and the timing of any intervention may be decided by considering whether there are other co-existent problems that may worsen the effect of conductive-type auditory impairment, such as speech and language disorders or learning disability. It is also important to consider how severely impaired hearing is, how long the effusion has been present and if the lesion is one- or two-sided [1, 2]. The season in which OME occurs also influences the outcome, with summer and autumn episodes less likely to resolve without intervention [1, 17, 18]. With these factors taken into consideration, the two options of observing and intervening only when required, or performing myringotomy with grommet insertion, remain valid for most cases.
Hearing aids, whether external or implanted, are appropriate only in paediatric cases of persistent OME where the insertion of grommets is unfeasible or where it brings no benefit, such as in cases of aural atresia or abnormally functioning ossicles [3].
Children in whom there is a risk of problems with speech and language development, or learning are a special category within OME clinical management. This group encompasses children with pre-existing auditory impairment, inherited disorders (Down Syndrome, 22q11.2 deletion disorder) or neurodevelopmental conditions (such as autism), abnormal craniofacial development (such as cleft palate) and those with visual problems not corrected by appliances, since these children are especially reliant on hearing to compensate for their lack of vision [1, 2, 19].
Thus, assessment of hearing should be carried out on children with OME falling in the ‘at risk’ category [1]. The conductive-type auditory impairment found in OME has a disproportionately negative effect on disabled patients. The modality used to assess auditory function should be selected according to how old the child is and how much he or she has the ability and willingness to co-operate. Where auditory loss is large, operative intervention is indicated. Patients in whom language development is delayed need to be assessed by a speech and language pathologist.
There is consensus amongst various professional bodies that children diagnosed with OME and who have a speech and language or learning disability require surgical consultation at an early stage, namely, no later than 3 months after diagnosis [1, 19]. In the majority of trials where the outcomes of OME were examined, whether immediate or longer term, such cases have been purposely excluded [20]. Thus, there is a lack of definite evidence to quantify the extent to which these individuals suffer more severe consequences. Practical experience, however, suggests that conductive auditory impairment can only worsen the prognosis where a pre-existing linguistic or intellectual disability is present [21].
Children without pre-existing linguistic or intellectual disabilities. For paediatric patients without these risk factors, the usual practice is to assess auditory function only where OME is persistent and the duration exceeds 3 months [1, 19]. The advantage of this delay is that the auditory impairment has often already spontaneously remitted by this point [2]. There may be an additional need for speech and language pathology appraisal of a case, especially if the auditory impairment is evident at a threshold of 21 dB or more.
Managing patients who do not fall into the special risk category depends on judgements about how the eardrum and middle ear cavity appear, the results of auditory testing (including audiometric and tympanometric evaluation), how long an effusion has been present, whether one ear or both is affected, and the wishes of parents or other carers [3].
Structural changes affecting the ear drum or middle ear. The following are signs that an urgent referral for surgical intervention is required: atelectasis of the tympanic membrane (i.e. retraction); partial collapse of the drum/retraction pocket, a perforated eardrum, discharge from the ear, and cholesteatoma [1]. In these cases, surgical intervention may be needed for another indication, besides OME.
The threshold for hearing is 40 dB or higher. Children with OME-induced auditory impairment raising the threshold for hearing to 40 dB or above should receive a referral for surgical assessment.
Despite the paucity of evidence about longer-term outcomes in paediatric patients with this degree of impairment, if the loss becomes longstanding, there may be negative effects on the development of speech and language and scholastic achievement. Thus, an ENT assessment is warranted [22,23,24].
A systematic review has examined the benefits of grommet insertion on auditory perception. The trials reviewed all involved paediatric patients with OME. The procedure seems to offer limited benefit, and by 6–9 months post-surgery, any benefit appears minimal. This is the normal time frame for the condition to resolve spontaneously [20]. No benefit in terms of speech or language acquisition, nor intellectual development, was proven, albeit the evidence base consulted was slender. There is a lack of published evidence regarding paediatric patients in whom a pre-existing deficit of speech, linguistic performance, psychological development or intellect is present [3].
Hearing threshold located between 21 and 39 dB. In children without pre-existing disability who experience auditory impairment sufficient to raise the hearing threshold to between 21 and 39 dB as a result of OME, a more conservative approach is suitable. Since the degree of auditory impairment is mild, decisions may be taken on the basis of a risk-benefit discussion with parents or carers about watchful waiting or grommet insertion [1, 2]. The majority of patients in this situation do not require surgical intervention, and watchful waiting is adequate management. The general recommendation is to rebook the patient and arrange further hearing tests after between 3 and 6 months [3].
The extent to which benefit outweighs the risks of surgery can usually be decided by considering how the child would be affected by having a mild auditory impairment lasting 6–9 months. This is the period for which grommet insertion has been shown to be beneficial. Nonetheless, even when various studies conducted in this area, including some RCTs and other studies using controls, have been systematically reviewed, the evidence base supporting the benefits on speech and linguistic performance of grommet insertion for children with mild auditory deficits appears weak [20, 25,26,27]. Grommet insertion is potentially justified in children with OME affecting one or both ears, which has persisted for at least 3 months (i.e. it qualifies as chronic), and impacts scholastic achievement and creates challenging behaviour or lowers the quality of life [19].
A number of factors need to be taken into consideration when deciding, such as the following [1, 2, 19, 28]
-
Is language abnormal or delayed, if the child is very young? Is scholastic achievement impacted by an older child’s acting out-of-character?
-
Is OME chronic (has lasted at least 3 months) and affecting both ears? Research employing an observational design has determined that OME affecting both ears tends to cause a higher degree of severity in auditory impairment and to resolve more slowly than unilateral cases. After 6 months only around 25% of cases remit spontaneously, whilst this figure is 30% at 1 year [1, 2, 29].
-
Is OME of at least 3 months’ duration (i.e. chronic), whether affecting one or both ears, whilst also being the most probable cause of any of the following issues: difficulty balancing (from vestibular involvement), scholastic underachievement, challenging behaviours, otalgia, and a poorer quality of life than usual, from the point of view of parents or carers? This change in life quality may be manifested as insomnia or acting out-of-character [30,31,32].
-
An extended period for which OME persists, such as OME affecting one ear which persists for at least 6 months, or which has been present for a minimum of 6 months in the preceding year, makes the lesion less likely to resolve spontaneously [33] and entails greater likelihood that the eardrum will be structurally damaged [24].
If there is no auditory impairment present (in other words, any loss of hearing occurs at a level of 20 dB or less), and speech, language and development are all on target, the patient may be followed-up by watchful waiting. Since auditory testing usually occurs 3 months after the onset of OME, this means watchful waiting lasts for 6 months in total [1, 2].
7 Clinical Work-Up
Probably, the diagnostic test most value in assessing a case of OME is tympanometry. Some 43% of tympanometric tests in patients with OME are of type B, with 47% of type C, showing the middle ear pressure is lowered. The effusion may be aspirated by performing tympanocentesis, which may be satisfactorily performed in clinic, even if the patient is very young. Performing tympanocentesis allows culture of the aspirate and is therapeutic in its own right [34].
However, to obtain the gold standard diagnostic evidence to show OME, myringotomy is needed. This procedure benefits from a greater degree of exposure of the lesion and allows more effective suctioning than is possible through tympanocentesis [35].
Imaging using computed tomography (CT) plays a significant role in excluding potential complications of otitis media, such as mastoiditis, thrombus formation in the sigmoid sinus or where the lesion may erode into the bone and spread intracranially. It is also valuable if there is an atypical cause, such as cholesteatoma. CT is especially beneficial in cases of OME affecting one side only and in which a suspected mass may be present in the nasopharynx or auditory tube [6].
Magnetic resonance imaging (MRI) has particular value in cases where a mass lesion within the soft tissues is potentially aggravating a middle ear effusion. MRI is especially good at defining the boundary between different soft tissues and can show how far a mass, in the nasopharynx, for example, has invaded into the cranial cavity. MRI can also easily reveal particular vascular complications, for example, thrombosis within the venous sinuses. For these purposes, the augmented MRI techniques, such as magnetic resonance venography (MRV) and magnetic resonance arteriography (MRA) are helpful. In all cases where the lesion extends into the cranial cavity, nonetheless, CT is necessary to define the osseous anatomy and determine the path the lesion has taken, whether via the nasopharynx or the temporal bone [6].
8 Management
Pharmacotherapy for OME involves the use of antibiotics, corticosteroids, antihistamines, decongestant and mucolytic agents. This approach to treatment has, however, been criticised on the grounds of limited evidence for clinical benefit in the longer term, plus the cost and side effect burden involved. In particular, the International Federation of Otorhinolaryngological Societies Congress in 2017 looked at each class of agent and recommend against their use on the grounds outlined above [7].
In chronic OME, the treatment modality enjoying the broadest acceptance is surgery, for the effectiveness of which there is clear evidence. Surgical options include myringotomy (+/− grommet insertion), removal of the adenoids or a procedure involving all three. As first-line therapy for OME, tonsillectomy has not been demonstrated to offer significant advantages [6].
8.1 Grommets
Possible indications: Before deciding to treat OME by grommet insertion, there should be a discussion of the benefits and risks involving the patient, parents or carers, GP and ENT specialist.
The following are possible situations where grommet placement is indicated [1, 2, 19]
-
OME in a child who has risk factors for speech or language delay or learning disability. This applies regardless of the degree of auditory impairment. It is recommended such cases are seen by an ENT specialist within 3 months.
-
Alteration to the eardrum, such as a retraction pocket.
-
Chronic auditory impairment as a result of OME resulting in an auditory threshold of 40 dB or above.
-
OME in both ears that has persisted for at least 3 months, or in one ear for at least 6 months, or OME which has recurred within a year, resulting in a total duration lasting at least 6 months within a year.
References
Rosenfeld RM, Shin JJ, Schwartz SR, et al. Clinical practice guideline: otitis media with effusion (update). Otolaryngol Head Neck Surg. 2016;154:S1.
National Institute for Health and Care Excellence. Otitis media with effusion in under 12s: surgery. www.nice.org.uk/nicemedia/pdf/CG60NICEguideline.pdf. Accessed 8 Nov 2012.
Pelton SI, Marom T. Otitis media with effusion (serous otitis media) in children: management. In: Kaplan SL, Isaacson GC, Torchia MM, editors. . Waltham: UpToDate; 2021.
O'Connor SS, Coggins R, Gagnon L, Rosenfeld RM, Shin JJ, Walsh SA. Plain language summary: otitis media with effusion. Otolaryngol Head Neck Surg. 2016;154(2):215–25.
Minovi A, Dazert S. Diseases of the middle ear in childhood. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2014;13:Doc11.
Higgins TS. Otitis media with effusion. In: Meyers AD, editor. Medscape;2020. https://emedicine.medscape.com/article/858990-overview. Accessed 10 Jan 2022.
Bluestone CD, Beery QC, Andrus WS. Mechanics of the eustachian tube as it influences susceptibility to and persistence of middle ear effusions in children. Ann Otol Rhinol Laryngol. 1974;83(Suppl 11):27–34.
Crapko M, Kerschner JE, Syring M, Johnston N. Role of extra-esophageal reflux in chronic otitis media with effusion. Laryngoscope. 2007;117:1419.
O'Reilly RC, Soundar S, Tonb D, et al. The role of gastric pepsin in the inflammatory cascade of pediatric otitis media. JAMA Otolaryngol Head Neck Surg. 2015;141:350.
Harman NL, Bruce IA, Callery P, Tierney S, Sharif MO, O’Brien K, et al. MOMENT—Management of Otitis Media with Effusion in cleft palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials. 2013;14(1):70.
Siddartha, Bhat V, Bhandary SK, Shenoy V, Rashmi. Otitis media with effusion in relation to socio economic status: a community based study. Indian J Otolaryngol Head Neck Surg. 2012;64(1):56–8.
Erdivanli OC, Coskun ZO, Kazikdas KC, Demirci M. Prevalence of otitis media with effusion among primary school children in eastern Black Sea, in Turkey and the effect of smoking in the development of otitis media with effusion. Indian J Otolaryngol Head Neck Surg. 2012;64(1):17–21.
Mills R, Hathorn I. Aetiology and pathology of otitis media with effusion in adult life. J Laryngol Otol. 2016;130(5):418–24.
Choi HG, Sim S, Kim SY, Lee HJ. A high-fat diet is associated with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2015;79(12):2327–31.
Kaya S, Selimoglu E, Cureoglu S, Selimoglu MA. Relationship between chronic otitis media with effusion and overweight or obesity in children. J Laryngol Otol. 2017;131(10):866–70.
Walker RE, Bartley J, Flint D, Thompson JM, Mitchell EA. Determinants of chronic otitis media with effusion in preschool children: a case-control study. BMC Pediatr. 2017;17(1):4.
Gordon MA, Grunstein E, Burton WB. The effect of the season on otitis media with effusion resolution rates in the New York Metropolitan area. Int J Pediatr Otorhinolaryngol. 2004;68:191.
van Balen FA, de Melker RA. Persistent otitis media with effusion: can it be predicted? A family practice follow-up study in children aged 6 months to 6 years. J Fam Pract. 2000;49:605.
Rosenfeld RM, Schwartz SR, Pynnonen MA, et al. Clinical practice guideline: tympanostomy tubes in children. Otolaryngol Head Neck Surg. 2013;149:S1.
Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;10:CD001801.
Kuo CL, Tsao YH, Cheng HM, et al. Grommets for otitis media with effusion in children with cleft palate: a systematic review. Pediatrics. 2014;134:983.
Davis JM, Elfenbein J, Schum R, Bentler RA. Effects of mild and moderate hearing impairments on language, educational, and psychosocial behavior of children. J Speech Hear Disord. 1986;51:53.
Karchmer MA, Allen TE. The functional assessment of deaf and hard of hearing students. Am Ann Deaf. 1999;144:68.
Carney AE, Moeller MP. Treatment efficacy: hearing loss in children. J Speech Lang Hear Res. 1998;41:S61.
Steele DW, Adam GP, Di M, et al. Effectiveness of tympanostomy tubes for otitis media: a meta-analysis. Pediatrics. 2017;139:e20170125.
https://effectivehealthcare.ahrq.gov/ehc/products/387/1485/otitis-media-executive-130504.pdf. Accessed 1 June 2016.
Wallace IF, Berkman ND, Lohr KN, et al. Surgical treatments for otitis media with effusion: a systematic review. Pediatrics. 2014;133:296.
Bluestone CD, Klein JO. Management. In: Otitis media in infants and children. 4th ed. BC Decker: Hamilton; 2007. p. 213.
Gravel JS, Wallace IF. Effects of otitis media with effusion on hearing in the first 3 years of life. J Speech Lang Hear Res. 2000;43:631.
Richards M, Giannoni C. Quality-of-life outcomes after surgical intervention for otitis media. Arch Otolaryngol Head Neck Surg. 2002;128:776.
Rosenfeld RM, Bhaya MH, Bower CM, et al. Impact of tympanostomy tubes on child quality of life. Arch Otolaryngol Head Neck Surg. 2000;126:585.
Brouwer CN, Maillé AR, Rovers MM, et al. Health-related quality of life in children with otitis media. Int J Pediatr Otorhinolaryngol. 2005;69:1031.
Rosenfeld RM, Kay D. Natural history of untreated otitis media. Laryngoscope. 2003;113:1645.
Pichichero ME, Poole MD. Assessing diagnostic accuracy and tympanocentesis skills in the management of otitis media. Arch Pediatr Adolesc Med. 2001;155(10):1137–42.
Kaleida PH. Evidence assessment of the accuracy of methods of diagnosing middle ear effusion in children with otitis media with effusion. J Pediatr. 2004;145(1):138.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kar, M., Bayar Muluk, N., Negm, H. (2023). Otitis Media with Effusion and Hearing Loss in Children. In: Arısoy, A.E., Arısoy, E.S., Bayar Muluk, N., Cingi, C., Correa, A.G. (eds) Hearing Loss in Congenital, Neonatal and Childhood Infections. Comprehensive ENT. Springer, Cham. https://doi.org/10.1007/978-3-031-38495-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-031-38495-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-38494-3
Online ISBN: 978-3-031-38495-0
eBook Packages: MedicineMedicine (R0)