Abstract
Sherritt developed the Dilute Pressure Oxidation (Dilute POX) process for the recovery of base and/or precious metals from high impurity or refractory sulphide concentrates. In this process, the autoclave is operated at a lower slurry solids content than what is required for autothermal operation to control the acidity of the pressure leach solution, with heat recovery used to maintain the autoclave temperature. This study presents the results from treating high-silver refractory gold feeds. Operating under Dilute POX conditions suppressed the formation of argentojarosite, which increased the silver extractions in the direct cyanidation of the POX residue to over 90%, from 0% to 30% under autothermal conditions, while maintaining high gold extractions in cyanidation (>98%) and high sulphide oxidation (>97%) in POX. The relationships between free acid in solution and silver extraction, and between magnesium in solution and effective free acid, were consistent with previous Dilute POX studies with arsenical copper concentrates. With lower sulphate in the solids under these conditions, the formation of basic ferric sulphate was reduced or eliminated, iron and arsenic in solution were reduced, and the majority of the sulphide in the feed was converted to free acid. Based on these results, operating under Dilute POX conditions may eliminate the need for hot curing in POX flowsheets for refractory sulphide feeds, and/or allow for treating feeds with higher carbonate contents.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Refractory
- Sulphides
- Gold
- Silver
- Pressure oxidation
- Cyanide amenability tests
- MgSO4
- Basic ferric sulphate
- Hot curing
- Dilute POX
1 Introduction
Sherritt developed and patented the Dilute POX process for treating arsenical copperconcentrates and refractorygold concentrates [1]. In Dilute POX, the autoclave is operated at a lower slurry solids content than what is required for autothermal operation (i.e., 100% autothermal solids) to control the acidity of the resulting pressure leach solution. To maintain the autoclave temperature, heat is recovered from the autoclave discharge slurry and recycled back to the autoclave (e.g., through flashing and the recycle of flash steam or by preheating the quench water added to the autoclave with an indirect heat exchanger).
Decreasing the free acid during pressure oxidation improves base metal extractions, iron and arsenic deportment, and residue stability [1, 2]. The solids produced at lower acidity are amenable to the recovery of both gold and silver by direct cyanidation of the pressure leach solids [1, 2], with silver extractions of over 90% reported [1, 2].
Further development of the Dilute POX process for the treatment of arsenical copper concentrates [3, 4] showed that magnesium in the solution can buffer free acid in the autoclave leach slurry, resulting in lower effective acid concentrations at temperature. This buffering allows the above benefits of operating at lower acidity to be achieved at higher autoclave solids contents.
This paper presents the treatment of refractory gold feeds containing silver under Dilute POX conditions, including the effect on the rate and extent of sulphide oxidation, basic ferric sulphate formation, free acid generation during pressure oxidation, and gold and silver extractions in cyanidation of the pressure leach solids.
2 Conventional Pressure Oxidation of Refractory Sulphides
In pressure oxidation of refractory sulphide feeds, gold trapped in metal sulphides, typically as metallic gold, is liberated by oxidizing the sulphide minerals to metal sulphates with oxygen [5]. This gold is then dissolved during a subsequent cyanidation step. Pressure oxidationautoclaves typically operate with an oxygen overpressure in the temperature range of 180–225 °C, with a higher tendency toward elemental sulphur formation at temperatures less than 200 °C [5].
During pressure oxidation, iron in sulphides is leached, with the majority of that iron oxidized to ferric sulphate and then hydrolyzed and precipitated as one or more iron compounds depending on the slurry composition, pH, and temperature. Hematite is favored at lower free acid concentrations [6], with jarosites and basic ferric sulphate (FeOHSO4; BFS) forming at higher free acid concentrations [6]. In the presence of arsenic, ferric arsenates precipitate [6, 7]; basic ferric arsenate sulphate (Fe(AsO4)1−x(SO4)x(OH)x•(1−x)H2O; BFAS) is typically favored at 220 °C [7].
The iron compounds formed can significantly impact the downstream processing and recovery of precious metals. Silver tends to form cyanide-insoluble argentojarosite (AgFe3(SO4)2(OH)6), leading to low silver recoveries in direct cyanidation, or increased costs by treating this material in a lime boil process to break down the jarosites. Basic ferric sulphate (BFS) breaks down in cyanidation to consume lime and/or increase cyanide losses. If BFS is formed in the autoclave, “hot curing” of the autoclave discharge slurry is commonly used in the industry to remove BFS ahead of cyanidation. In “hot curing,” the flashed slurry is held between 60 and 100 °C to destroy BFS using the acid contained in the pressure leach discharge solution [6].
Refractory sulphide feeds commonly contain carbonate minerals, which, if added directly to the autoclave, will generate carbon dioxide (CO2) and reduce oxygen utilization. Destruction of the carbonate minerals outside of the autoclave by reaction with acidic solutions (acidulation) is one method that is used commercially to reduce the impact of CO2. Acid solutions generated in POX are often recycled to supply acid to this process step.
3 Feed Materials
For this testwork, two refractory gold feeds, one ore and one concentrate, were obtained from two operating mines. The received ore was crushed and ground before testing, while the concentrate was tested as-received. The compositions of the two feeds are shown in Table 1. Gold and silver recoveries from direct cyanide leaching of the feed samples were 58.3% Au and 36.0% Ag for the ore, and 73.1% Au and 88.2% Ag for the concentrate.
Reagent grade silver sulphide (Ag2S) was added to all of the performed POX tests to ensure a minimum of 1000 g/t silver was present in the charge. Reagent grade magnesium sulphate (MgSO4•7H2O) was used in specific tests to study the effect on buffering of free acid at temperature.
4 Experimental
Batch pressure oxidation tests were conducted in a 4 L batch autoclave at 220 °C with 500 kPa of oxygen partial pressure (2720 kPa(g) total pressure), with rate samples taken at defined intervals. For each feed, a preliminary mass and energybalance was completed to determine the autoclave solids content to simulate for autothermal conditions (i.e., 100% autothermal solids) in a commercial autoclave. Tests were then performed at different percentages of autothermal solids content to simulate Dilute POX conditions.
After a set oxidation time, the autoclave was depressurized, cooled to 95 °C, and held for 40 minutes (i.e., conditioning) to simulate the conditions observed in a commercial flash tank and/or hot curing circuit. The autoclave slurry was then cooled, filtered and washed, and cyanide amenability (CNA) testing (carbon in leach (CIL)) was conducted on the final solids to determine the gold and silver extraction.
5 Results and Discussion
5.1 Effect of Lower Autoclave Solids Content on Recoveries in Direct Cyanidation
Table 2 shows the results of the POX tests at different autoclave solids contents and of the cyanide amenability tests on the final washed residue from each test.
Sulphide oxidation was rapid for all tests, with greater than 97% oxidation in 20 minutes.
Under conventional POX conditions (i.e., 100% autothermal solids), silver extractions in cyanidation were low, indicating that the majority of the silver in the feed and the silver added as silver sulphide was converted to cyanide-insoluble compounds (e.g., argentojarosite) during pressure oxidation.
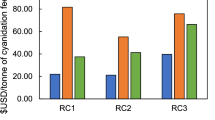
Silver extraction in cyanidation increased as the acid concentrations in the solution decreased with decreasing solids (Fig. 1). Over 90% silver extraction was achieved below 31 g/L H2SO4 for the ore, and over 80% for the concentrate below 21 g/L H2SO4.
Gold extractions in cyanidation were consistently over 97% from the concentrate. For the ore, gold extractions went from over 90% under conventional POX conditions (100% autothermal solids) to over 97% as the autoclave solids content decreased. It is possible that a portion of the fine gold particles was trapped in the sulphate-containing iron precipitates produced at higher autoclave solids contents.
5.2 Effect of Lower Autoclave Solids on Sulphate in Solids
The amount of sulphate in solids decreased significantly as the autoclave solids content decreased for both the ore and the concentrate (Table 2). Figure 2 plots the relationship between free acid in solution and the sulphate sulphur in the conditioned POX residues.
Both feeds show a significant drop in sulphate in solids as the acid concentration decreased (i.e., at less than 43 g/L for the ore and less than 30 g/L for the concentrate), followed by a gradual decrease at lower acid concentrations. High silver extractions in cyanidation were achieved from solids with low sulphate in solids (Table 2).
Sulphate in solids at the lowest acid concentrations was lower for the low arsenic ore (0.23 wt% sulphate sulphur) compared to the high arsenic concentrate (0.69 wt% sulphate sulphur). As indicated previously, lower acid concentrations are expected to limit the formation of BFS and jarosite, but may also encourage the formation of ferric arsenate compounds with less sulphate (e.g., basic ferric arsenate sulphate (Fe(AsO4)1−x(SO4)x(OH)x•(1−x)H2O) with lower values of x).
5.3 Effect of Lower Autoclave Solids on Basic Ferric Sulphate Formation and Free Acid Generation
Table 3 shows the conversion of sulphide sulphur to free acid in the POX solution or sulphate in solids at pressure oxidative conditions.
In conventional POX (100% autothermal solids), only 32–40% of the sulphide sulphur in the feed is converted to free acid, while a large portion of the sulphur reports as sulphate in solids. A significant portion of the sulphate in solids is present as BFS.
Under Dilute POX conditions, the amount of sulphide converted to free acid increases dramatically to between 55% and 75% at lower autoclave solids contents. This increase would provide more acid to destroy carbonate in the POX feed before the autoclave (acidulation), which would give more flexibility in POX to treat feeds with higher levels of carbonate.
The additional acid generated under Dilute POX conditions comes from generating solids with lower levels of sulphate, including lower concentrations of BFS. Thus, operating at Dilute POX conditions would also limit the formation of BFS, and reduce or remove the need for a hot curing step. Eliminating hot curing would reduce capital costs for the plant, and reduce the amount of dissolved iron and arsenic that would need to be removed during neutralization.
5.4 Effect of Magnesium in Solution on Dilute POX Performance
Development of the Dilute POX process for the treatment of arsenical copper concentrates [3, 4] showed that magnesium in solution can buffer free acid in the autoclave leach slurry. This buffering is due to the formation of bisulphate ions, which results in lower effective acid concentrations at temperature. This work [3, 4] indicated a buffering of 1 g/L H2SO4 per 1 g/L Mg at 220 °C. For the rest of the discussion, the term “effective free acid” is used to describe the net acidity including this buffering effect by Eq. 1 below:
Pressure oxidation plants typically operate with a high level of water recycle, which could make it possible to add magnesium easily to the autoclave with the recycle of process water. Operating with elevated levels of magnesium in solution at higher solids contents could produce the benefits of lower effective acidity described earlier in Sects. 5.1, 5.2, and 5.3, but with a lower capital cost for the autoclave flash tanks and/or heat recovery system.
A series of tests were performed to investigate the effect of magnesium in solution under Dilute POX conditions with the same refractory gold feeds tested in previous sections. Table 4 contains the results of these POX tests, which were performed with magnesium sulphate added to the autoclave charge.
The sulphide oxidation kinetics were similar to those described in Sect. 5.1, with greater than 96% in 20 minutes, and no observed impact of magnesium sulphate addition on sulphide oxidation kinetics. Gold extractions in cyanidation were also unchanged with the addition of magnesium sulphate.
Figure 3 shows the effective free acid concentration versus the corresponding silver extractions in cyanidation for each of these tests, along with the data from the tests without magnesium addition from Sect. 5.1. The results from these tests are consistent with the trends observed between silver extraction and effective free acid concentrations for the tests without magnesium sulphate addition. The magnitude of the relationship between magnesium and effective acid (i.e., 1 g/L H2SO4 per 1 g/L Mg) was also consistent with what was established in previous studies [3, 4].
For the ore, silver extractions in the cyanidation of over 80% were possible at 50% of the autothermal solids content. For the concentrate, silver extractions were achieved at 40% of the autothermal solids content that was only possible at 30% (i.e., >80% Ag) or 20% (i.e., >90% Ag) without the magnesium added in the autoclave solution.
The sulphate sulphur values in solids from the tests with magnesium addition were also consistent with the trends, with respect to effective free acid concentration, without magnesium addition. Those values are replotted below in Fig. 4 with the addition of the data points from the tests with magnesium addition. The sulphate sulphur in solids with magnesium addition is similar or lower for a given effective free acid concentration without magnesium addition.
These findings confirm the results from previous testing with arsenical copper concentrates [3, 4]. Moreover, these findings show that, with magnesium in solution, increased silver recovery in cyanidation and lower sulphate in solids are possible at higher solids content, closer to autothermal operation, than would be otherwise possible without magnesium present in the solution.
6 Conclusions
This study presents the results of treating silver-containing refractory gold feeds under Dilute POX conditions.
Over 90% of the silver could be extracted during cyanidation of the POX residues by lowering the effective free acid at temperature in the pressure leach slurry by either reducing the solids content of the autoclave (i.e., percent of autothermal solids) and/or by adding magnesium to the solution, for buffering. Gold extractions in cyanidation were either similar or higher at lower solids and/or lower effective acid concentrations.
Dilute POX conditions also resulted in a higher conversion of sulphide sulphur to free H2SO4 in solution (65–75%), compared to conventional pressure oxidation under autothermal conditions (30–40%). This feature may provide more flexibility for treating high carbonate feeds in a refractory gold flowsheet by providing additional free acid for carbonate destruction before the autoclave (acidulation).
Dilute POX conditions also produced solids with notably less sulphate sulphur than conventional POX at autothermal conditions. In addition to less jarosite formation (i.e., higher silver extractions in cyanidation), this limits the formation of basic ferric sulphate. It may be possible to reduce or eliminate the need for a hot curing step when operating under Dilute POX conditions.
This study also confirmed that adding magnesium in the solution reduced the effective free acid during pressure oxidation, and confirmed the magnitude of the relationship between magnesium and effective acid that was established in previous studies (i.e., 1 g/L H2SO4 per 1 g/L Mg). Thus, operating with higher solids contents with magnesium in solution could allow similar benefits to be achieved to those observed at lower solids contents, but with significant savings in capital costs.
References
Smit JT, Holloway PC (2021) Low acidity, low solids pressure oxidative leaching of sulphidic feeds. U.S. Patent No. 11,118,244 B2. Washington, DC: U.S. Patent and Trademark Office
Smit JT, Buban KR, Collins MJ, Holloway PC (2019) Environmentally responsible processing of copper-arsenic concentrates. In: Copper 2019, proceedings of the 58th conference of metallurgists (COM) hosting the 10th international copper conference 2019
Holloway PC, Molaei A, Smit JT (2022) The integration of Sherritt’s Dilute POX process with copper heap leaching. In: Instituto de Ingenieros de Minas de Chile (IIMCh) (ed) Copper 2022, vol 4: Hydrometallurgy, p 200–210
Holloway PC, Smit JT, Molaei A (2023) Integrated pressure oxidative leach of copper sulphidic feed with copper heap leach. U.S. Patent No. 11584975. Washington, DC: U.S. Patent and Trademark Office
Marsden JO, House CI (2009) The chemistry of gold extraction, 2nd edn. Society for Mining, Metallurgy, and Exploration (SME) Inc
Fleming CA (2010) Basic iron sulphate – a potential killer in the processing of refractory gold concentrates by pressure oxidation. Miner Metall Process 27(2):81–88. https://doi.org/10.1007/BF03402383
Gomez MA et al (2011) Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3-As2O5-H2SO4 solutions. Hydrometallurgy 107:74–90. https://doi.org/10.1016/j.hydromet.2011.01.007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Pavelich, J.S., Holloway, P.C., Smit, J.T. (2023). Silver Recovery from Refractory Gold Materials with Sherritt’s Dilute POX Process. In: Proceedings of the 62nd Conference of Metallurgists, COM 2023. COM 2023. Springer, Cham. https://doi.org/10.1007/978-3-031-38141-6_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-38141-6_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-38140-9
Online ISBN: 978-3-031-38141-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)