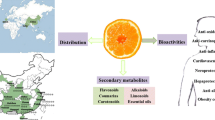
Abstract
Citrus fruits are famous globally because of their pleasant taste and richness in biologically active compounds and nutrients. Citrus fruits confer several health benefits to mankind by strengthening the immune, cardiovascular, and digestive systems. Polyphenols, flavonoids, carotenoids, pectin and essential oils are some of the bioactive phytochemicals found abundantly in citrus fruits. The amount and type of bioactive compounds and their antioxidant capacity widely depend on the fruit, cultivar, or part of the fruit, climate, and growing environment. This chapter aims to highlight bioactive compounds found in citrus fruits and summarize potential extraction techniques that could be utilized to recover these bioactive phytochemicals. The advantage of green extraction methods over traditionally used methods and the influence of extraction parameters on the extract yield along with a summary of the analytical techniques are discussed in this chapter. This chapter bridges the gap between traditional knowledge and modern research on citrus bioactive compounds and green/non-traditional extraction techniques.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Citrus fruits are the widely cultivated and consumed fruits all around the globe. Orange (Citrus sinensis), Grapefruit (Citrus paradisi), Mandarin/Tangerine (Citrus reticulate), Lemon (Citrus limon), as well as Lime (Citrus aurantiifolia) are cultivated worldwide because of their high commercial value (Suri et al. 2022a). The origin and history of the production of citrus fruits are still unknown. It is believed that the tropical, as well as subtropical climates of the Asian Island and the Malaysian Archipelago, started citrus planting at least 4000 years ago, but the exact beginning of citrus farming remains unclear (Berk 2016).
Annual citrus fruit production has ascended rapidly around the world in recent years, increasing from about 51.48 MT in 1975 to 158 MT in 2020. Maximum citrus fruits are produced in Asia (47.7%) then Africa (43.7%), United States of America (8.1%), followed by Europe (0.4%), and Oceania (0.1%). In context to the citrus producing nations, China occupies the first position with 44.63 million tonnes of citrus fruits produced, which is equivalent to 28.16% of the global overall citrus fruit produced in the year 2020. Among other nations, Brazil, India, and Mexico are some important nations, with each nation producing above 5% of the world’s total citrus production. Approximately 10.07 million hectares of land worldwide are used to produce citrus fruits. China, India, Brazil, Nigeria, and Mexico are the world’s largest producers of citrus fruits (FAOSTAT 2022). Besides, these citrus fruits are highly adaptable to a variety of climates and soil diversities, contributing towards their vast production in tropical and subtropical areas and also in mild temperature areas (Suri et al. 2021).
Citrus fruits are recognized throughout the globe for their pleasant taste and abundance of bioactive substances and nutrients. Citrus fruits provide health benefits by stimulation of the immune system, cardiovascular as well as digestive organs. These fruits also possess anti-bacterial, anti-inflammatory, anti-atherosclerosis, and anti-cancer effects. These effects are partly because of the existence of many bioactive compounds (Czech et al. 2021). These citrus fruits are a rich source of bioactive compounds, particularly polyphenols viz; flavonoids & phenolic acids, terpenoids (limonoids & carotenoids), essential oils, dietary fiber, and pectin. Besides, citrus fruits comprise of other nutrients for example vitamins viz.; vitamin B-complex, vitamin C, vitamin E (tocopherols & tocotrienols), and micronutrients (iron, zinc, copper, selenium, and manganese) (Saini et al. 2022). Many of the bioactive components found in citrus fruits possess antioxidative, regulatory, and metabolic-stimulating action that guards the body’s tissues as well as fluids from damage related with the occurrence of reactive oxygen species (Czech et al. 2021). The amount as well as the type of bioactive components, and their antioxidant activity, depend broadly on the type of fruit, its cultivar or part of the fruit, and the climate and growing conditions (Bermejo et al. 2011).
Extraction, segregation/isolation, as well as characterization of biologically active components from fruits/vegetables are the important steps for obtaining bioactive compounds from plant matrix. Appropriate measures should be taken to prevent destruction, loss or deterioration of the potent biologically active ingredients during the extraction process. Furthermore, the adequacy of the extraction technique used to recover exact bioactive components should be verified. The use of non-traditional green extraction techniques has become very important due to increased efficiency, little or no use of organic solvents, and reduced consumption of time, energy, and other resources (Anticona et al. 2021). Microwave-assisted extraction, pressurized liquid extraction, enzyme-assisted extraction, ultrasound-assisted extraction, supercritical fluid extraction, and pulsed electric field extraction are among the green extraction methods used by the scientists (Suri et al. 2021).
14.2 Citrus fruits: Taxonomy
Citrus fruits are the member of Rutaceae family with taxonomical description:
-
Kingdom: Plantae
-
Order: Sapindales
-
Family: Rutaceae
-
Sub-family: Aurantiodeae
-
Genus: Citrus L.
-
Species: Citrus reticulata; Citrus cavaleriei; Citrus maxima; Citrus sinensis; Citrus medica; Citrus micrantha; Citrus japonica; Citrus hystrix
-
Hybrids Species: Citrus limon; Citrus sinensis; Citrus paradisi; Citrus tangerine; Citrus latifolia; Citrus aurantifolia; etc.
Taxonomy of citrus fruits is complex and debatable. The citrus family includes 150 genera and 1600 species including mandarin (Citrus reticulata L.), lemon (Citrus limon L.), sweet orange (Citrus sinensis L.), and grapefruit (Citrus paradisi L.) is the further most marketable species and is favored for its attractive color, aroma and taste (Karn et al. 2021; Ledesma-Escobarand and de Castro 2014).
Cultivated citrus fruits are derivative of several citrus species present in nature. Some are just assortments from the original wild type of citrus fruits, various other citrus fruits are produced through hybridization amongst two or more original citrus fruit species, while other are backcrosses between the cross and one of the parent species of the hybrid. Citrus plants simply cross amongst species with entirely diverse morphology, and citrus fruits that look alike can have pretty different ancestors (Wu et al. 2014; Velasco and Licciardello 2014). Most of the marketable citrus fruits varieties are derived from one or more “core species” such as mandarins, citrons, as well as pomelos, which contributes a composite floral structure that leads to a more complex fruit. These major core citrus fruit species have led to the development of many hybrid citrus species (Curk et al. 2014).
14.3 Nutritional Characterization of Citrus Fruits
Citrus fruits comprise of excellent nutritional value and have many health-promoting effects, for instances their action on aiding the digestion process, averting cardiovascular ailments and lowering inflammation (Maugeri et al. 2019; Yamada et al. 2011). The nutrients as well as bioactive phytochemical found in citrus fruits contributes towards their excellent health benefits. Major portion of citrus fruits (more than 80%) comprise of water, however citrus fruits are also good source of many other nutrients, for instance simple sugars (glucose, fructose, and sucrose), other carbohydrates (fiber, pectin, cellulose, and hemicellulose), fats & essential oils (D-limonene), vitamins (vitamin B-complex, vitamin C and vitamin E), minerals (potassium, phosphorus and calcium) and plant pigments (carotenoids, and xanthophyll) (Putnik et al. 2017). Citrus fruits also different enzymes like pectinase, pectinestarase, phosphatase etc. The composition, nutritional content and bioactive substances in citrus fruits varies widely on the basis of the variety of citrus fruit, fruit part and stage of growth (Albertini et al. 2006). This might be attributable to variances in hereditary genes, expression of gene, as well as ecological conditions.
The nutritional as well as bioactive components also differed amongst diverse portions of citrus fruit. The pulp is considered to be rich in nutrients viz; ascorbic acid and sugars, however majority of biologically active substances (e.g., polyphenols, flavonoids, essential oils and pectin) mainly accrue in the fruit peel and pomace (Tocmo et al. 2020). The seeds of citrus fruits contain high essential oil content in the form of limonoids.
Among nutrients, the carbohydrate present in citrus fruits varies from 6.4 to 13.3 g/100 g. Carbohydrates like simple monosaccharides (glucose, fructose etc.) and disaccharides (sucrose) contribute towards the sweetness of citrus fruits. The protein found in the citrus fruits range from 0.1 to 1.3 g/100 g. Out of all protein found in citrus fruits, most of the proteins consists of enzymes that exhibit wide role in metabolic processes. The lipids present in the citrus fruits vary from 0.07 to 0.42 g/100 g. The lipids content of citrus fruits includes fatty acids, phospholipids and complex fats (USDA 2018). Citrus fruits are rich source of different micronutrients like minerals, water-soluble as well as fat-soluble vitamins. It contains large amounts of minerals like calcium, phosphorus, potassium, sodium, and magnesium. The major water-soluble vitamins found in citrus fruits are ascorbic acid/ vitamin C and vitamin B-complex, whereas the vitamin E and vitamin A are the main fat-soluble vitamins (USDA 2018) (Table 14.1).
14.4 Bioactive Compounds Present in Citrus Fruits
Citrus fruits comprise of beneficial phytochemicals for example polyphenols (phenols and flavonoids- naringin, nobiletin, and hesperidin), carotenoids (α-carotene and β-carotene), essential oils (D-limonene, α-pinene, β-pinene, limonoids, synephrines etc.), pectin, minerals, vitamins viz;vitamins A, E, C, coumarins, and other components. Several biological properties of citrus fruits including antioxidants, anti-carcinogen, anti-mutagenic, anti-inflammatory, and anti-aging properties are attributable to these phytochemicals found in them (Rajendran et al. 2014; Zhang et al. 2015; Ke et al. 2015).
14.4.1 Polyphenols
14.4.1.1 Phenols
Citrus fruits contain many naturally occurring bioactive components such as polyphenols, largely phenolic acids as well as flavonoids (Sharma et al. 2017). The phenol content of citrus fruits differs depending on different sections of citrus fruit. Remarkably, citrus by-products encompass greater amounts of polyphenols than the edible portion of citrus (Balasundram et al. 2006). In a study, the maximum amount of phenol was observed in the albedo portion of unripe sweet orange (10,910 mg/kg) and it reported for about half of the total cumulative phenol content found in albedo, flavedo and juice sacs. However, in orange and lemons, major phenol content was observed in flavedo portion while in pummelo, albedo of unripe fruit possesses highest total phenol content (Multari et al. 2020). Further, the polyphenol content of citrus fruit varies depending on different extraction techniques, extraction conditions, solvent used for extraction etc. Phenol contained in citrus fruits can be roughly divided into 2subcategoriesnamely, (1) hydroxybenzoic acid and (2) hydroxycinnamic acid (Ignat et al. 2011). Figures 14.1 and 14.2 presents the chemical diagram showing the basic structure of flavonoids and polyphenols contained in citrus fruits.
14.4.1.2 Flavonoids
Flavonoids belongs to a group of lower molecular mass naturally occurring phenolic components that are broadly dispersed in the plant domain including their existence in fruits, vegetables, flowers, tea leaves etc. This plant based secondary metabolites which are commonly known as flavonoids are related to a variety of health aids and remain vital ingredients in different dietary supplements, nutraceutical, medicine, pharmaceuticals and cosmetics sector. This is owing to their antioxidant, anti-carcinogenic, anti-mutagenic, and anti-inflammatory effects, in addition to their capacity to control main cellular enzyme activity. As per the chemical structure, flavonoids are made up of fifteen carbon body (C6-C3-C6) with two 6-carbon phenyl rings associated with an embedded oxygen-containing heterocycle. Flavonoids may be further subdivided on the basis of the carbon atom of the C-ring to which the B-ring is joined and based on the degree of unsaturation and the oxidation of the C-ring. Those flavonoids whose B-ring is joined at the 3rd position of the C-ring are termed isoflavones, while flavonoids having B-ring attached at fourth position are referred to as neo-flavonoids, and those with the B-ring attached at 2nd position are further categorized into diverse sub-groups based on the structural characteristics of the C-ring. The sub-groups formed are flavonols, flavanones, flavones, flavanonols/catechins, isoflavonoids anthocyanins, and chalcones (Panche et al. 2016). Citrus fruits comprise of significant amounts of flavonols for example; rutin, quercetin, and kaempferol; flavanones like naringin, hesperidin, narirutin, and eriocitrin; flavones for example vitexin, rhoifolin, and diosmin; polymethoxylated flavones commonly abbreviated as PMFs such as tangeritin, nobiletin, and 5-demethyl nobiletin; as well as anthocyanin such as cyanidin and peonidin glucoside (Saini et al. 2022).
Citrus fruits comprise of good amounts of natural flavonoids particularly PMFs which shows a variety of biological as well as physiological functions that are useful not only for plants but also for humans. Citrus PMFs acts as an important barrier to pathogenic attack (Luo et al. 2015). Eighty-one PMFs were noticed in the Citrus reticulata leaves, some of which are well-defined by specific names or structures. Thirty-Nine PMFs were recognized in 62 citrus germplasm flavedo, sweet orange and mandarin showed the maximum PMF levels, and lemons and pomelo exhibited lower quantities (Wang et al. 2017). High levels of PMF, for example tangeretin, nobiletin, and sinensetin, were spotted in 4 tissues of 11 citrus genotypes (Durand-Hulak et al. 2015).
Flavonoids show an eminent effect in the removal of reactive oxygen species. Amongst the different flavonoids present in citrus fruits, the antioxidant action of the hesperidin, naringin, and naringenin have been extensively studied (Nakao et al. 2011). Naringin in citrus fruits can greatly improve the immune system’s efficiency to prevent organ as well as tissue damage or diseases triggered due to the oxidation by enhancing the action of certain enzymes like glutathione peroxidase, superoxide dismutase, catalase, paraoxonase and further antioxidative enzymes (Mamdouh and Monira 2004). Naringenin is present in major amounts in citrus fruits. Naringenin is usually observed as an aglycone and/or a glycoside (Erlund 2004). Among different flavonoids in citrus fruits, naringin and narirutin are specifically abundant. Naringenin is helpful in inhibiting fatty acid oxidation in the liver by means of regulating fatty acid oxidation enzymes, for example plasma antioxidant enzymes, carnitine palmitoyl transferase, 3-hydroxy3-methylglutaryllCoA reductase, and PON (Jung et al. 2006; Zou et al. 2016).
Hesperidin possess DPPH radical scavenging capacity and may dose-dependently hinder the in-vitroCu2+ induced oxidation of low-density lipoprotein thereby promoting pancreatic B-cell rejuvenation and preventing oxidative stress in pregnant diabetic rats’ embryo (Toumi et al. 2009). The hesperidin present in candied oranges, lemons and grapefruit were 11 mg/100 g, 22 mg/100 g and 4.37 mg/100 g, correspondingly (Zhou 2016). In a study, 11 major PMFs in fruit flavedo or citrus leaves of 116 citrus plants were assessed in combination with UPLC–DAD–ESI-QTOF-MS/MS and HPLC-DAD examination. All studied citrus plants and their natural or else artificial hybrids have been reported to comprise of enough measurable PMF, particularly in the flavedo portion of wild as well as early grown mandarin which are in the initial periods of fruit development (Peng et al. 2021).
Structural characterization of flavonoid present in pulp of Shatianyu (Citrus grandis L. Osbeck) showed that the flavonoids viz; naringin and rhoifolin exhibited maximum oxygen radical absorbance ability while melitidin, naringin, and bergamjuicin were main supplier of the ORAC in fruit extract (Deng et al. 2022). Nevertheless, current research has revealed that in citrus fruit albedo (which is inner layer of citrus peel), flavonoids accounted for 89.34% of the polyphenol portions, subjugated by flavanones viz; hesperidin and eriocitrin as important components, accounting for 52.81% and 31.31% of total flavonoids (Smeriglio et al. 2019).
Flavonoid concentration in the citrus fruits also vary based on the stage of pollination. Citrus fruit contains higher levels of most of the flavonoids in the intermediate stages of development for example; 60–80 days after pollination. Also, the flavonoid content decreases all through full ripening. This is perhaps because of the higher expression of certain rate limiting enzymes carrying out the biosynthesis of flavonoids viz; chalcone synthase-1 and chalcone isomerase (Ledesma-Escobar et al. 2018). In ripened fruit or in the later stages of development, the hesperidin content peaks in the lemon juice sac (Citrus Akragas) with a concentration of 2213 mg/kg while in different orange varieties the hesperidin content of 1957 and 1975 mg/kg were observed. However, the flavanone narirutin was abundantly found in grapefruit (292 mg/kg). A substantial quantity of eriocitrin flavanone was noted in lemons (913 mg/kg). Also, larger numbers of flavonoids are found in albedo and flavedo portion of citrus fruit peel in comparison to the citrus juice (Multari et al. 2020).
14.4.2 Essential Oils
Essential oils are those aromatic liquids that have lack of affinity for water and are composed of volatile substances generally present in oil sacs of peels and cuticle of citrus fruit (El Asbahani et al. 2015). Essential oils recovered primarily from the citrus fruit are economically significant with health-promoting action owing to the occurrence of terpenes especially monoterpene i.e., D-limonene and limonoids, sesquiterpenes along with other bioactive ingredients such as flavonoids, carotenoids and coumarins. Citrus fruit essential oil usually has more than 90% volatile content. Citrus essential oil possesses analgesic, antioxidative, anxiolytic, anti-inflammatory, neuroprotective and anti-microbial action. Owing to the properties of citrus essential oil, this could have applicability in food, cosmetics, pharma and perfumery industry (Suri et al. 2021). Because of the intense antimicrobial activity lately, citrus essential oil, has gotten huge consideration as a preservative for natural products, vegetables, meat, and handled food items. Monoterpenes and sesquiterpenes are usually present in the volatile fractions, with limonene as the main component. Limonene is one of most abundant essential oil with a usual concentration ranging from 73.9% to 97% (W/W essential oils). The US Food and Drug Administration (US-FDA) has regarded limonene as a Generally Recognized as Safe (GRAS) material. D-limonene is the main terpenoid accounting for 45–90% of the total terpenoid in orange, mandarin, lemon, tangerine, and grapefruit. The terpenoids mainly γ-terpinene and β-pinene accounts for about 8–20% and 0.3–11% of the total terpenoid, correspondingly in the essential oil present in lemon and mandarin (Raspo et al. 2020). D-Limonene comes from the citrus fruit peels and seeds. Limonene is a renewable organic compound and has many uses as an active ingredient in flavors and fragrances, and functionalized foodstuffs (Ciriminna et al. 2014). (+) Limonene (also R or D-Limonene) has a pleasing orange-like scent, and (−) Limonene (also S or L-Limonene) has a stimulating turpentine-like scent, so chirality of limonene is important aspect in flavor or fragrance (Jongedijk et al. 2016).
(+) Limonene could be simply attained from citrus peels and seeds in a cold pressed extraction procedure and thereafter obtained through the process of centrifugation or else steam distillation (Ciriminna et al. 2014). Another method for recovery of limonene is the conventional solid-liquid/ soxhlet extraction by means of pure and/or varied organic solvents. Amongst the cold pressed essential oil obtained from lemon, tangerine, clementine, sweet orange, bergamot, blood orange, bitter orange and grapefruit, the maximum limonin content (21.2 mg/L) was found in bergamot, whereas the limonin content of the essential oils of Blood Orange and Clementine was the lowermost (0.5–0.9 mg/L). Also, the green varieties of mandarin reported four-times greater limonin (4.5 mg/L) as compared to yellow and red mandarin variety (1.1 mg/L).
Meanwhile, various green extraction methodologies are utilized for retrieval of essential oil from citrus fruits for example, by use of supercritical fluid, microwave assisted extraction, steam explosion, and ultrasound assisted methods (Negro et al. 2016). A larger amount of limonene was recovered from the orange byproducts in a shorter amount of time via microwave extraction technology as compared to conventional heating method. D-limonene extraction consisted of a preliminary extraction phase from the outside of the cell followed by transmembrane diffusion. Microwave extraction technology is reported to exhibit high transmembrane diffusivity thereby resulting in higher yields in comparison to conventional oil extraction (Attard et al. 2014). Apart from microwave extraction, microwave-assisted hydro-distillation is effectively used for obtaining of essential oils from moist citrus peel waste, thereby lowering the costs, evading the use of additives, and enhancing process yields (Bustamante et al. 2016).
14.4.3 Pectin
Citrus fruits and processing by-products like peels contain significant amounts of soluble sugars (glucose, fructose and sucrose) that can be effectively used to produce bio-alcohol. The cellular components of the citrus peel include cellulose, hemicellulose, pectin, galacturonic acid, galactose, and arabinose. The abundance of soluble as well as insoluble sugars indicates their ability to effectively utilize value-added goods by consequential biological procedures (Rivas et al. 2008).
Pectin is one of the complex polysaccharides located in the cellular walls of higher plants. The major components of pectin include D-galacturonic acid that is also termed as sugar acid and is attained from galactose. Pectin acts as an emulsifying agent, thickening agent, stabilizing agent, fat substitute and gelling compound in dairy industry, jams, jellies as well as fruit juices (Suri et al. 2021). A literature review showed that with the advancement in the green chemistry, several scientists use the non-traditional green extraction methods to extract pectin from citrus fruits. For example, pectin yield of 27.81% was achieved upon microwave assisted extraction from grapefruit peel (Bagherian et al. 2011). In another research, microwave-assisted extraction from Citrus medica peels yielded 23.83% pectin at power output (660 W) and time (9 min) (Quoc et al. 2015). In addition, a pectin yield of 29.16% from the Citrus maxima peel by Bronsted acidic ionic microwave liquid-based extraction (Liu et al. 2017).
14.4.4 Carotenoids
Carotenoids are a ubiquitous assemblage of isoprenoid complexes that take part in photosynthesis as well as signal transduction. As per their chemical configuration, carotenoids can be subcategorized into two chief classes namely,
-
(a)
Hydrocarbon carotenoids involving α-carotene, β-carotene and lycopene.
-
(b)
Oxygenated derivatives of hydrocarbon carotenoids viz; β-cryptoxanthin, lutein, xanthophyll-neoxanthin, and violaxanthin.
However, β-carotene, β-cryptoxanthin, lutein, lycopene, as well as zeaxanthin are the most significant carotenoids present in citrus fruits. Carotenoids are those pigments that impart different colors like red, yellow, and orange to flowers plus fruits of different plant classes. Carotenoids are also known to offer the unique colors to birds, fishes and crustaceans (salmon, trout, lobsters, shrimp, pelicans, etc.). Nevertheless, the carotenoid pigment is not synthesized by plants and their color as well as carotenoid content vary depending on their diet (Fraser and Bramley 2004). Besides imparting the color, carotenoids also possess several important biological functions. They are considered as a vital source of vitamin A and can help in preventing the growth of degenerative illnesses viz; macular degeneration, metabolic disorder, cardiovascular diseases and cancer (Boukroufa et al. 2017). Carotenoids are involved in photoprotection and light harvesting complex of photosynthetic apparatus (Merchant and Sawaya 2005) They also play a role as a light stabilizer and free radical scavenger (Fraser and Bramley 2004).
Carotenoids present in colored citrus fruits viz; yellow and green-colored fruits had gained further importance in food sector as a result of their health promoting action (including richness in provitamin A and anti-cancer effects) (Rao and Rao 2007). Also, the antioxidative action of citrus fruits is mainly attributable to the occurrence of hydrophilic constituents in fruit (Cano et al. 2002). Usually, the carotenoid content of citrus fruits is nearly double that of other fruits. Around 115 different carotenoids are found in citrus fruits and their characteristic color is because of the carotenoid components found in citrus fruits (Tsai et al. 2007). Pink grapefruit contains good amount of β-carotene. Further, citrus fruits also comprise of large amounts of carotenoids for example lutein, zeaxanthin, and β-cryptoxanthin. The red colors of red navel orange and valencia orange are mainly because of high lycopene and cryptoxanthin content, respectively. Pink grapefruit contain high β-carotene content; Other citrus fruits contain high carotenoid content, such as zeaxanthin, lutein, and β-cryptoxanthin (Lee 2001). The carotenoid content in the orange was observed to be 11.25 mg/L when the sample extraction was done using ultrasound technique at power of 208 W/cm2, temperature of 20 °C and time of 5 min. It was revealed that the carotenoid content of orange was increased by 40% through ultrasound-based extraction in comparison to the conventional extraction (Boukroufa et al. 2017). In another research, carotenoid levels were observed to be significantly greater in tangerine species, such as Citrus unshiu and Citrus reticulata, as compared to those in the orange variety, Citrus sinensis and the hybrid, Citrus changshanensis (Abeysinghe et al. 2007). Similarly, cultivar difference was observed among the carotenoid content of different citrus varieties (Fanciullino et al. 2006).
14.5 Extraction & Isolation of Bioactive Compounds
Qualitative and quantitative research on the bioactive components present in plant materials are primarily based on the selection of appropriate extraction methods. The extraction method is sometimes called “sample preparation technique”. In most cases, two-thirds of the effort of analytical chemists is spent on sample preparation techniques, but this part of the study is ignored and carried out by untrained personnel. It is true that the advancement of state-of-the-art chromatographical and spectrophotometric procedures makes the study of bioactive components simpler than before, however the accuracy hinge on the type of extraction techniques, input variables, and the specific properties of the plant. Common parameters that affect the extraction process are the matrix features, solvent utilized for extraction, temperature, pressure, and time of the treatment. As a result of these tremendous technological advancements; different food as well as non-food sectors are increasingly interested in bioactive compounds from natural resources (Azmir et al. 2013).
Extraction of plant material could be carried through several extraction methods. Many scientists have been conducting experiments on innovative, inexpensive and effective ways of extraction. The extraction procedure performed to prepare and process the sample is of some interest to the scientific community. Extraction, separation/isolation, as well as description of bioactive ingredients from fruit/vegetables are the basic phases for obtaining bioactive components. Appropriate measures must be taken to prevent the destruction, loss or deterioration of potent bioactive components all through the extraction. Besides, the choice of solvent must be dependent on the properties of the bioactive compound of interest (Suri et al. 2022a). Over the last few years, environmentally friendly, non-conventional green extraction methods have been developed that use less synthetic plus organic chemicals, reduce treatment time, and improve yield and quality of extract. Ultrasonication, pulsed electric fields, supercritical fluids, enzymatic extraction, microwave extraction, extrusion, ohmic heating, high pressure extraction and accelerating solvents are used to enhance the total extraction yield as well as selectivity of bioactive compounds obtained from sample (Lusas and Watkins 1988; Lakkakula et al. 2004; Gaur et al. 2007; Ghafoor et al. 2009; Azmir et al. 2013).
14.5.1 Traditional/Conventional Extraction Methods
Traditional extraction methods viz; Soxhlet extraction are taken as a standard/control technique for comparing the achievement of afresh established methods. There are quite a few technical reports and data, in which unconventional methods are critically studied (Smith 2003; Wang and Weller 2006). Existing traditional methods for recovering bioactive components from plants, fruits, vegetables etc. are soxhlet extraction, maceration, and steam distillation/hydro-distillation. To date, numerous traditional and innovative extraction methods for extracting phenol from citrus by-products have been described. The most commonly used method is maceration/immersion in a solvent. It is also considered appropriate at scale-up levels. Nevertheless, this extraction method mainly needs high temperatures (50–150 °C), extended extraction periods (up to some hours), and highly polar solvents such as ethanol (Khan and Dangles 2014). Other traditional methods including soxhlet extraction, hydro-distillation etc. are also used to recover bioactive compounds from citrus fruits.
14.5.1.1 Soxhlet Extraction
A German scientist named Franz Ritter von Soxhlet was the first person who studied and researched soxhlet extractor in 1879. It was developed primarily for the recovery of lipids, yet with advancement it is used for isolation of many different components. Soxhlet extraction method is utilized to extract important bioactive components from a number of natural materials. Besides, it also serves as a model for comparing new/innovative extraction techniques. Usually, for extraction of compounds in soxhlet extractor, a minute quantity of dry sample is positioned in the thimble. The thimbles are thereafter kept in a distillation flask comprising of the appropriate solvent. Later when the solvent reaches to the overflow stage, the thimble holder solution is sucked out through the siphon. The siphon returns the solution to the distillation unit. This solution transports the recovered solute to a bulk liquid. The solute particles persist in the distillation unit and the solvent is returned to the permanent plant bed. This procedure repeats till the complete recovery happens (Azmir et al. 2013).
14.5.1.2 Maceration
Maceration has long been employed in the preparation of home-based tonics. It has become a common and reasonable method to acquire essential oils as well as bioactive components. For small use or laboratory type extractions, maceration usually comprise of different stages. Initially, the plant material is crushed to enhance its surface area for appropriate mixing of solvent. Second, during maceration, a suitable solvent known as menstruum is added to the bolted container. Third, the straining of liquid is done, however the solid filtrate left after this extraction procedure, ex-citrus pomace is pressed for recovery of huge amounts of trapped solution. The sieve obtained and the squeezed liquid are grouped together, and impurities are parted through filtration process. Infrequent shaking during the process of maceration facilitates recovery of bioactive compounds in two ways. (A) It leads to an increase in process of diffusion, (b) It eliminate concentrated solution out of the surface of sample and bring new solvent into menstruum to increase extract yield (Azmir et al. 2013).
14.5.1.3 Steam Distillation/Hydro-Distillation
It is a conventional technique of extracting bioactive components, essential oils etc. from plant sources. It did not contain organic solvents and this procedure can be conducted prior to dehydration of plant material. Hydro-distillation can be done through 3 different methods namely water-based distillation process, direct steam distillation and water coupled steam distillation.
In the hydro-distillation process, the plant material is first sealed in a booth, thereafter water is added, and plant material is boiled. Sequentially, steam is injected directly into the plant material. The major reason behind the release of bioactive components from plant tissues is hot water and steam treatment. The indirect cooling with water leads to condensation of the vapor blend of water plus oil. The mix obtained after condensation moves from the condenser to the separator, at that place the oil as well as bioactive components are involuntary separated from the water. Hydro-distillation process encompasses three major physicochemical processes, firstly hydro-diffusion, secondly hydrolysis and thirdly disintegration through heat. At higher temperatures, few volatile compounds can be destroyed. This limitation of hydro-distillation restricts the use of this method for the recovery of thermo-labile components (Azmir et al. 2013).
14.5.2 Non-traditional/Green Extraction Methods
14.5.2.1 Microwave Assisted Extraction
This type of extraction technology uses electromagnetic waves with frequencies between 0.3 and 300 GHz. This is a frequently used process for recovering bioactive compounds from citrus fruits. Microwave based extraction treatment is said to include 3 consecutive stages namely (a) the disassociation of solutes from the active sites of food material at higher temperature as well as pressure (b) the diffusion of the solvent through the food material (c) the liberation of solutes from the food material into the solvent (Alupului et al. 2012). The main benefits of using microwaves over different traditional used methods are it requires low energy consumption, short time of extraction and low solvent usage (Suri et al. 2021). This recovery technique can extract bioactive components faster than traditional extraction methods and has a higher recovery rate. This is a selective method for recovering the intact organic plus organometallic complexes. It is likewise well-known green extraction technology to lessen the utilization of organic solvents (Alupului et al. 2012). Interestingly, researchers have been working on recovery of pectin, polyphenols, flavonoids, carotenoids, and dietary fibre from citrus fruit peels in a non-conventional way.
Pectin extraction from Citrullus lanatus peels using microwaves at different power (160–480 W), irradiation time (60–180 s) and liquid-solid ratio (1: 10–1:30 g/mL). A total yield of pectin (25.79%) was achieved with power (477 W), time (128 s), and solid-liquid ratio (1:20) (Maran et al. 2014). Besides, the recovery of pectin from the skin of citrus sinensis by surfactant-microwave-assisted extraction exhibited 28% pectin yield at time (7 min), power (400 W), and pH (1.2) (Su et al. 2019). In addition to the recovery of pectin from citrus fruits, polyphenols were also recovered utilizing microwave extraction technique. Phenolic components found in the peel of Citrus inshiu was extracted by using microwaves. The study showed hesperidin content of 5860 mg/100 g and narirutin content of 1310 mg/100 g in skin extract of citrus fruit (Inoue et al. 2010). Citrus limon peels reported total phenol (1574 mg GAE/100 g) by extraction using microwaves (Dahmoune et al. 2013).
14.5.2.2 Ultrasound Assisted Extraction
Ultrasound is a distinct form of sound wave that goes over and beyond the human hearing. In the field of chemistry, ultrasound usually range from 20 kHz to 100 MHz. Like the other wave types, it penetrates the medium through producing firmness as well as expanding. The ultrasound treatment causes cavitation, which means the formation, development along with collapse of bubbles. By converting kinetic energy into heating of the bladder contents, a large amount of energy can be generated (Azmir et al. 2013). Advantages of the ultrasound method is the reduction in time of extraction, energy and solvent consumption. Ultrasonic energy for recovery of bioactive compounds provides further efficient mixing, quicker transference of energy, condensed temperature gradient and temperature of extraction, extraction of selective compounds, compact equipment size, faster start-up, promote improved production and eliminate processing stages (Chemat et al. 2008). The possible mechanism behind ultrasound assisted extraction is enhanced ultrasonic mass transfer along with accelerated solvent access to cellular components of the plant. The mechanistic action of ultrasonic extraction encompasses two physical phenomena’s, (a) diffusion through the cellular walls and (b) releasing of intracellular substances subsequently after rupturing of wall (Mason et al. 1996). Sample moisture, sample particle size, degree of milling, as well as solvent are central features for an effective and efficient extraction process. In addition, the extraction temperature, frequency, pressure, and duration of ultrasonic waves are the determining aspects of ultrasonic wave performance.
Ultrasound extraction has also been utilized with many classical systems as they are believed to improve the effectiveness of a traditional scheme. In the solvent extraction, an ultrasonic device is positioned in a suitable place to improve the effectiveness of extraction (Vinatoru et al. 1998). The ultrasound extraction was lately used to recover hesperidin from peels of Penggan (Citrus reticulata) fruit (Ma et al. 2008) polyphenols and flavonone glycosides of Citrus unshiu Marc peels and total phenol content of Penggan peels (Ma et al. 2009). Researchers recovered polyphenols from the skin of mandarin and sweet orange by maceration and ultrasound technology, and the efficacy of ultrasonically assisted extraction was greater to that of the previously used techniques. The extraction yields obtained by ultrasonic treatment of mandarin and sweet orange were 5.85% and 12.95%, correspondingly, whereas the extract yields of 5.20% and 12.20% were obtained by the maceration technique (Saini et al. 2019). Recovery of polyphenols from fresh sweet lime (Citrus sinensis) peels by utilizing ultrasound treatment at power (200 W), frequency (40 kHz) and treatment time (20 min) exhibited the total phenol of 25.60 mg GAE/g and total flavonoid content of 18.85 mg QE/g (Suri et al. 2022b). Polyphenols obtained from three hybrid varieties of mandarin fruit (Clemenvilla, Ortanique, and Nadorcott) by means of ultrasonic assisted extraction techniques at power (400 W), temperature (40 °C) plus duty cycle (80%) was conducted. Ultrasound treatment for a shorter period of time (in between the first 5 & 15 min) caused the improved physicochemical characteristics, bioactive compounds, along with the antioxidative action of mandarin peel extract (Anticona et al. 2021). This depicts that ultrasonic extraction is an effective as well as sustainable extraction technology.
14.5.2.3 Pulsed Electric Field Extraction
This extraction technique depends on inducing electroporation of cell membranes, ensuing in an improved in extraction yield. In this method, the electric potential passes by the cellular membrane at a short period of time (1–2500 ls) and the particles separate on the basis of the inherent charge. Therefore, repulsive action leads to the establishment of pores and increases their porosity (Azmir et al. 2013). Normally, pulsed electric fields are used to preserve food and extract intracellular components from plant matrices, agro-food waste and their by-products (Suri et al. 2021).
The influence of pulsed electric field on extraction of total phenols as well as flavonoids from orange peels were evaluated. The maximum disintegration index was observed when the time of processing was 60ls. Besides, an increase in the yield of extraction of total phenols by 20%, 129%, 153% and 159% from orange peel was observed following the pulsed electric field treatment at 1, 3, 5 and 7 kV/cm, respectively. Also, in comparison with the untreated material, the pulsed electric field treated orange peels exhibited enhanced flavonoids namely hesperidin and naringin, and total antioxidant property (Luengo et al. 2013).
14.5.2.4 Supercritical Fluid Extraction
All matter has 3 fundamental states viz; solid, liquid, and gas. Supercritical state is definite and could only be reached when a material is exposed to temperatures and pressures above the critical point. The critical point is explained as the distinctive temperature as well as pressure beyond which the characteristic gas and liquid phases are absent (Inczedy et al. 1998). In supercritical conditions, certain attributes of gases and liquids are lost. In other words, supercritical fluids cannot be liquefied even if the temperature or pressure is changed. Supercritical fluids have gas-like features such as diffusion, surface tension, viscosity, along with liquid-like density plus solvation (Sihvonen et al. 1999) These features make it appropriate for extracting components in high yields in a quick span of time.
Supercritical extraction technique is considered as an environmental friendly substitution to traditional techniques of extraction. This supercritical method of extraction synthesizes fluids near the critical point at higher temperatures as well as pressures (Diaz-Reinoso et al. 2006). A number of scientists examined the effect of supercritical fluid on extract yield of bioactive components from the citrus fruits. The supercritical fluid process for extracting essential oil from citrus lemon zest led to higher yields of D-limonene (4.5%) (Lopresto et al. 2019). In analogous research, the process of extraction of D-limonene from tangerine peel (Citrus unshiu Kuno) using supercritical CO2was carried out, where a high yield of D-limonene (30.65%) at a pressure of 300 bar, however 13.16% yield was reported at a pressure of 100 bar (Safranko et al. 2021). Therefore, although the supercritical extraction technique was known to be advantageous for the extracting oil, the main problems in supercritical fluid extraction were recognized as high maintenance and equipment cost (Suri et al. 2022a).
14.5.2.5 Enzyme Assisted Extraction
Usually some of the bioactive components and plant-based chemicals in the plant matrix are disseminated in the cytoplasm of the cell and few remained in the polysaccharide lignin arrangement by the help of hydrogen bonding or hydrophobicity, that are inaccessible to solvents in the extraction process. Several elements for example; concentration and composition of enzyme, plant particle size, solid-to-liquid ratio, time of hydrolysis, etc. are considered important aspects for the extraction process (Niranjan and Hanmoungjai 2004). Enzymatic pre-treatment step is observed as an innovative and efficient technique for extracting bound compounds thereby improve total yield (Rosenthal et al. 1996). In this process, the utilization of specific enzymes for example cellulase, pectinase, α-amylase etc. in the extraction process improves retrieval by disrupting cell walls and hydrolysing structural plus lipid-soluble polysaccharides. There are 2 methods of enzyme-assisted extraction: (1) enzyme-assisted aqueous based extraction method, (2) enzyme-assisted cold pressed extraction method (Latif and Anwar 2009; Azmir et al. 2013). Traditionally, the enzyme-assisted aqueous extraction functions primarily to extract oil from varied seeds. Enzyme-assisted cold pressing extraction technology uses enzymes to hydrolyze seed cellular walls as polysaccharide-protein colloids are not accessible in this system (Concha et al. 2004).
14.5.2.6 Pressurized Liquid Extraction
This extraction technique is termed through different titles viz; pressurized fluid extraction, improved solvent extraction, accelerated fluid extraction, and high-pressure solvent extraction process (Nieto et al. 2010). The idea behind pressurized fluid extraction is to use high pressure to keep the solvent liquid above its usual boiling point. The peculiarity of pressurized liquid extraction is that its elevated pressure carries forward the process of extraction. Automated approach is the major reason for the further expansion of pressure based liquid extraction process, in addition to its benefits like reduced extraction times plus solvent requirements. This extraction technology requires a minimum quantity of solvent due to its high temperature and pressure, which speeds up the recovery of plant materials. Higher extraction temperatures can increase the solubility of the analyte by enhancing both the solvability the mass transfer rate, reducing the viscosity plus surface tension of the solvent, thus increasing the extraction rate (Ibañez et al. 2012). Several studies have explained the applications of pressurized water-based extraction method to obtain bioactive components from citrus fruit peels, pomace etc. High-pressure based extraction of polyphenols from peels of lemon and orange was performed. Testing of pressure-treated citrus peels (300 MPa, 10 min, & 500 MPa, 3 min) showed higher levels of phenol and antioxidants than control. The total phenol present in the extract obtained from the fresh peels of lemon at 300 MPa and 500 MPa was 265.95 mg GAE/100 g and 344.53 mg GAE/100 g, correspondingly, whereas for orange it was 364.57 and 378.90 mg GAE/100 g, correspondingly (Casquete et al. 2014).
14.6 Factors Influencing the Extraction of Bioactive Compounds from Citrus Fruits
14.6.1 Solvent
Solvents utilized for recovering plant bioactive components have a direct influence on the efficiency of phytochemicals like polyphenols, flavonoids, carotenoids etc. With a wide series of solvents from polar to non-polar, different components with respective polarities could be recovered into solvents based on the polarity of the solvent and the target plant material (Dailey and Vuong 2015). In general, coupling of polar as well as less polar solvents is further effective in the recovery of biologically active components from plant matrix (Vuong et al. 2013). Environmental friendly, non-toxic food grade organic solvents such as n-butanol, ethanol, and isopropanol are suggested by the United States Food and Drug Administration for recovery of bioactive constituents (Bartnick et al. 2006). The usage of ethanol in the ultrasound-based process has been known to be more efficient in recovering polyphenols from citrus fruit viz; orange peel waste than traditional solvent-based method (Khan et al. 2010). In addition, the non-conventional methods like ultrasound, microwave etc. provided higher yields in natural product extraction than conventional methods, not only on a laboratory scale however also on a pilot plant scale (Boonkird et al. 2008).
14.6.2 Solid to Solvent Ration
Solvent volume or the plant material to solvent ratio have been studied to enhance the effectiveness of bioactive components from plant matrix. Hypothetically, the lesser volume of solvent utilized, the lower is the extraction efficiency that occurs due to saturation. Nevertheless, an appropriate plant material (solid)/solvent ratio must be useful for cost-effective purpose as further energy is required to heat the bigger mass. Moreover, greater energy is needed to eliminate the water for more concentrated or powdered production (AL Ubeed et al. 2022).
14.6.3 Extraction Conditions: Treatment Time, Temperature, Agitation etc.
Extraction temperature and time greatly influences the efficiency of extraction of bioactive complexes from plant materials (Fig. 14.3). Higher temperature with elongated recovery time generally results in greater extraction efficiency. Yet, the stability of bioactive compounds may decline with prolonged exposure to high temperature since major plant-based compounds are susceptible to heat. Hence, it is vital to regulate the extraction temperature and time to recover high concentrations of bioactive compounds with minimal deterioration.
Process agitation and pressure were observed to impact the proficiency of extraction of phytochemicals compounds from plant matrix. Research has proven that agitation prominently enhances the extraction competence of phytochemicals in comparison to processes where no agitation is used (Ahmed et al. 2020). The treatment time or number of extractions has been shown to influence the effectiveness of extraction process. The larger the extraction time given to the same amount of sample, the greater bioactive compounds can be recovered (Vuong et al. 2011).
14.7 Conclusion
The present chapter gives an in-depth view of different bioactive compounds found in citrus fruits, their methods of extraction and important considerations during extraction processes. It sums up reports on the taxonomy, nutritional, and bioactive content of different varieties of citrus fruits. It can be proposed from the chapter that citrus fruits contain excellent repository of biologically active compounds viz; phenols, flavonoids, carotenoids, essential oils, pectin, minerals, vitamins, coumarins, and other compounds that offers several health benefits to mankind. Extraction, isolation and characterization are the basic stages for efficient retrieval of bioactive compounds. Therefore, selection of a potential extraction techniques that can be used to effectively recover these bioactive compounds is utmost important. With the advent of green technology, certain non-conventional energy saving methods are extensively utilized for extraction of bioactive components from citrus fruits. These green extraction methods exhibit various benefits over traditionally used methods. Also, the chapter discusses the influence of extraction condition on the extract yield.
References
Abeysinghe DC, Li X, Sun C, Zhang W, Zhou C, Chen K (2007) Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem 104:1338–1344
Ahmed M, Ramachandraiah K, Jiang GH, Eun JB (2020) Effects of ultra-sonication and agitation on bioactive compounds and structure of amaranth extract. Foods 9:11–16
Al Ubeed HMS, Bhuyan DJ, Alsherbiny MA, BasuA VQV (2022) A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 27:604
Albertini MV, Carcouet E, Pailly O, Gambotti C, Luro F, Berti L (2006) Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. J Agric Food Chem 54:8335–8339
Alupului A, Calinescu I, Lavric V (2012) Microwave extraction of active principles from medicinal plants. UPB Sci Bul Ser B 74:129–142
Anticona M, Blesa J, Lopez-Malo D, Frigola A, Esteve MJ (2021) Effects of ultrasound-assisted extraction on physicochemical properties, bioactive compounds, and antioxidant capacity for the valorization of hybrid mandarin peels. Food Biosci 42:101185
Attard TM, Watterson B, Budarin VL, Clark JH, Hunt AJ (2014) Microwave assisted extraction as an important technology for valorising orange waste. New J Chem 38:2278–2283
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F et al (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117:426–436
Bagherian H, Ashtiani FZ, Fouladitajar A, Mohtashamy M (2011) Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem Eng Pro Process Intensif 50:1237–1243
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Bartnick D, Mohler C, Houlihan M (2006) U.S. Patent Application No. 10/972,751
Berk Z (2016) Chapter 1: Introduction: history, production, trade, and utilization. In: Berk Z (ed) Citrus fruit processing. Academic, San Diego, pp 1–8
Bermejo A, Llosa MJ, Cano A (2011) Analysis of bioactive compounds in seven citrus cultivars. Food Sci Tech Int 17:55–62
Boonkird S, Phisalaphong C, Phisalaphong M (2008) Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a lab-and pilot-plant scale. Ultra Sonochem 15:1075–1079
Boukroufa M, Boutekedjiret C, Chemat F (2017) Development of a green procedure of citrus fruits waste processing to recover carotenoids. Resour Eff Technol 3:252–262
Bustamante J, van Stempvoort S, Garcia-Gallarreta M, Houghton JA, Briers HK, Budarin VL, Clark JH (2016) Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J Clean Prod 137:598–605
Cano A, Alcaraz O, Acosta M, Arnao MB (2002) On-line antioxidant activity determination: comparison of hydrophilic and lipophilic antioxidant activity using the ABTS•+ assay. Redox Rep 7:103–109
Casquete R, Castro SM, Villalobos MC, Serradilla MJ, Queirós RP, Saraiva JA, ... Teixeira P (2014) High pressure extraction of phenolic compounds from citrus peels. High Pres Res 34(4):447–451
Chemat F, Tomao V, Virot M (2008) Ultrasound-assisted extraction in food analysis. In: Handbook of food analysis instruments, pp 85–103
Ciriminna R, Lomeli-Rodriguez M, Cara PD, Lopez-Sanchez JA, Pagliaro M (2014) Limonene: a versatile chemical of the bioeconomy. Chem Comm 50:15288–15296
Concha J, Soto C, Chamy R, Zuniga ME (2004) Enzymatic pretreatment on rose-hip oil extraction: hydrolysis and pressing conditions. J Am Oil Chem Soc 81:549–552
Curk F, Ancillo G, Garcia-Lor A, Luro F, Perrier X, Jacquemoud-Collet JP, Navarro L, Ollitrault P (2014) Next generation haplotyping to decipher nuclear genomic interspecific admixture in citrusspecies: analysis of chromosome 2. BMC Genet 15:1–19
Czech A, Malik A, Sosnowska B, Domaradzki P (2021) Bioactive substances, heavy metals, and antioxidant activity in whole fruit, peel, and pulp of citrus fruits. Int J Food Sci 2021:6662259
Dahmoune F, Boulekbache L, Moussi K, Aoun O, Spigno G, Madani K (2013) Valorization of Citrus limon residues for the recovery of antioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Indust Crop Prod 50:77–87
Dailey A, Vuong QV (2015) Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric 1:1115646
Deng M, Jia X, Dong L, Liu L, Huang F, Chi J et al (2022) Structural elucidation of flavonoids from Shatianyu (Citrus grandis L. Osbeck) pulp and screening of key antioxidant components. Food Chem 366:130605
Díaz-Reinoso B, Moure A, Domínguez H, Parajó JC (2006) Supercritical CO2 extraction and purification of compounds with antioxidant activity. J Agril Food Chem 54:2441–2469
Durand-Hulak M, Dugrand A, Duval T, Bidel LP, Jay-Allemand C, Froelicher Y, Bourgaud F, Fanciullino AL (2015) Mapping the genetic and tissular diversity of 64 phenolic compounds in citrus species using a UPLC–MS approach. Ann Bot 115:861–877
El Asbahani A, Miladi K, Badri W, Sala M, Addi EA, Casabianca H et al (2015) Essential oils: from extraction to encapsulation. Int J Pharm 483:220–243
Erlund I (2004) Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res 24:851–874
Fanciullino AL, Dhuique-Mayer C, Luro F, Casanova J, Morillon R, Ollitrault P (2006) Carotenoid diversity in cultivated citrus is highly influenced by genetic factors. J Agric Food Chem 54:4397–4406
FAOSTAT (2022) Food and agriculture data. Retrieved February 21, 2022, from https://www.fao.org/faostat/en/#data/QC
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43(3):228–265
Gaur R, Sharma A, Khare SK, Gupta MN (2007) A novel process for extraction of edible oils: enzyme assisted three phase partitioning (EATPP). Bioresour Technol 98:696–699
Ghafoor K, Choi YH, Jeon JY, Jo IH (2009) Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J Agric Food Chem 57:4988–4994
Ibañez E, Herrero M, Mendiola JA, Castro-Puyana M (2012) Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates. In: Marine bioactive compounds. Springer, Boston, pp 55–98
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835
Inczédy J, Lengyel T, Ure AM, Gelencsér A, Hulanicki A (1998) Compendium of analytical nomenclature. Blackwell Science, Hoboken
Inoue T, Tsubaki S, Ogawa K, Onishi K, Azuma JI (2010) Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem 123(2):542–547
Jongedijk E, Cankar K, Buchhaupt M, Schrader J, Bouwmeester H, Beekwilder J (2016) Biotechnological production of limonene in microorganisms. Appl Microbiol Biotechnol 100:2927–2938
Jung UJ, Lee MK, Park YB, Kang MA, Choi MS (2006) Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol 38:1134–1145
Karn A, Zhao C, Yang F, Cui J, GaoZ WM et al (2021) In-vivo biotransformation of citrus functional components and their effects on health. Crit Rev Food Sci Nutr 61:756–776
Ke Z, Xu X, Nie C, Zhou Z (2015) Citrus flavonoids and human cancers. J Food Nutr Res 3:341–351
Khan MK, Dangles O (2014) A comprehensive review on flavanones, the major citrus polyphenols. J Food Compos Anal 33:85–104
Khan MK, Abert-Vian M, Fabiano-Tixier AS, Dangles O, Chemat F (2010) Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem 119:851–858
Lakkakula NR, Lima M, Walker T (2004) Rice bran stabilization and rice bran oil extraction using ohmic heating. Bioresour Technol 92:157–161
Latif S, Anwar F (2009) Physicochemical studies of hemp (Cannabis sativa) seed oil using enzyme-assisted cold-pressing. Eur J Lipid Sci Technol 111:1042–1048
Ledesma-Escobar CA, de Castro MDL (2014) Towards a comprehensive exploitation of citrus. Trends Food Sci Technol 39:63–75
Ledesma-Escobar CA, Priego-Capote F, Robles Olvera VJ, Luque de Castro MD (2018) Targeted analysis of the concentration changes of phenolic compounds in Persian lime (Citrus latifolia) during fruit growth. J Agric Food Chem 66:1813–1820
Lee HS (2001) Characterization of carotenoids in juice of red navel orange (Cara Cara). J Agric Food Chem 49:2563–2568
Liu Z, Qiao L, Gu H, Yang F, Yang L (2017) Development of Brönsted acidic ionic liquid based microwave assisted method for simultaneous extraction of pectin and naringin from pomelo peels. Sep Purif Technol 172:326–337
Lopresto CG, Meluso A, Di Sanzo G, Chakraborty S, Calabrò V (2019) Process-intensified waste valorization and environmentally friendly d-limonene extraction. Eur Mediterranean J Env Integr 4:1–12
Luengo E, Álvarez I, Raso J (2013) Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov Food Sci Emerg Technol 17:79–84
Luo T, Xu K, Luo Y, Chen J, Sheng L, Wang J, Han J, Zeng Y, Xu J, Chen J, Deng X (2015) Distinct carotenoid and flavonoid accumulation in a spontaneous mutant of ponkan (Citrus reticulata Blanco) results in yellowish fruit and enhanced postharvest resistance. J Agric Food Chem 63:8601–8614
Lusas EW, Watkins LR (1988) Oilseeds: extrusion for solvent extraction. J Am Oil Chem Soc 65:1109–1114
Ma Y, Ye X, Hao Y, Xu G, Xu G, Liu D (2008) Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticulata) peel. Ultrason Sonochem 15:227–232
Ma YQ, Chen JC, Liu DH, Ye XQ (2009) Simultaneous extraction of phenolic compounds of citrus peel extracts: effect of ultrasound. Ultrason Sonochem 16:57–62
Mamdouh MA, Monira AAEK (2004) The influence of naringin on the oxidative state of rats with streptozotocin-induced acute hyperglycaemia. Zeitschrift für Naturforschung C 59:726–733
Maran JP, Sivakumar V, Thirugnanasambandham K, Sridhar R (2014) Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydr Polym 101:786–791
Mason TJ, Paniwnyk L, Lorimer JP (1996) The uses of ultrasound in food technology. Ultrason Sonochem 3:S253–S260
Maugeri A, Cirmi S, Minciullo PL, Gangemi S, Calapai G, Mollace V, Navarra M (2019) Citrus fruits and inflammaging: a systematic review. Phytochem Rev 18:1025–1049
Merchant S, Sawaya MR (2005) The light reactions: a guide to recent acquisitions for the picture gallery. Plant Cell 17:648–663
Multari S, Licciardello C, Caruso M, Martens S (2020) Monitoring the changes in phenolic compounds and carotenoids occurring during fruit development in the tissues of four citrus fruits. Food Res Int 134:109228
Nakao K, Murata K, Itoh K, Hanamoto Y, Masuda M, Moriyama K et al (2011) Anti-hyperuricemia effects of extracts of immature Citrus unshiu fruit. J Trad Med 28:10–15
Negro V, Mancini G, Ruggeri B, Fino D (2016) Citrus waste as feedstock for bio-based products recovery: review on limonene case study and energy valorization. Bioresource Technol 214:806–815
Nieto A, Borrull F, Pocurull E, Marcé RM (2010) Pressurized liquid extraction: a useful technique to extract pharmaceuticals and personal-care products from sewage sludge. Trends Anal Chem 29:752–764
Niranjan K, Hanmoungjai P (2004) Enzyme-aided aquous extraction. In: Nutritionally enhanced edible oil processing. AOCS Publishing
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47
Peng Z, Zhang H, Li W, Yuan Z, Xie Z, Zhang H et al (2021) Comparative profiling and natural variation of polymethoxylated flavones in various citrus germplasms. Food Chem 354:129499
Putnik P, Barba FJ, Lorenzo JM, Gabrić D, Shpigelman A, Cravotto G, Bursać Kovačević D (2017) An integrated approach to mandarin processing: food safety and nutritional quality, consumer preference, and nutrient bioaccessibility. Compr Rev Food Sci Food Saf 16:1345–1358
Quoc LPT, Huyen VTN, Hue LTN, Hue NTH, Thuan NHD, Tam NTT, Thuan NN, Duy TH (2015) Extraction of pectin from pomelo (Citrus maxima) peels with the assistance of microwave and tartaric acid. Int Food Res J 22:1637
Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U et al (2014) Antioxidants and human diseases. Clin Chim Acta 436:332–347
Rao AV, Rao LG (2007) Carotenoids and human health. Pharmacol Res 55:207–216
Raspo MA, Vignola MB, Andreatta AE, Juliani HR (2020) Antioxidant and antimicrobial activities of citrus essential oils from Argentina and the United States. Food Biosci 36:100651
Rivas B, Torrado A, Torre P, Converti A, Domínguez JM (2008) Submerged citric acid fermentation on orange peel autohydrolysate. J Agric Food Chem 56:2380–2387
Rosenthal A, Pyle DL, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb Technol 19:402–420
Šafranko S, Ćorković I, Jerković I, Jakovljević M, Aladić K, Šubarić D, Jokić S (2021) Green extraction techniques for obtaining bioactive compounds from mandarin peel (Citrus unshiu var. Kuno): Phytochemical analysis and process optimization. Foods 10:1043
Saini A, Panesar PS, Bera MB (2019) Comparative study on the extraction and quantification of polyphenols from citrus peels using maceration and ultrasonic technique. Curr Res Nutr Food Sci 7:678
Saini RK, Ranjit A, Sharma K, Prasad P, Shang X, Gowda KGM, Keum YS (2022) Bioactive compounds of citrus fruits: a review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 11:239
Sharma K, Mahato N, Cho MH, Lee YR (2017) Converting citrus wastes into value-added products: economic and environmently friendly approaches. Nutrition 34:29–46
Sihvonen M, Järvenpää E, Hietaniemi V, Huopalahti R (1999) Advances in supercritical carbon dioxide technologies. Trends Food Sci Technol 10:217–222
Smeriglio A, Cornara L, Denaro M, Barreca D, Burlando B, Xiao J, Trombetta D (2019) Antioxidant and cytoprotective activities of an ancient Mediterranean citrus (Citrus lumia Risso) albedo extract: microscopic observations and polyphenol characterization. Food Chem 279:347–355
Smith RM (2003) Before the injection—modern methods of sample preparation for separation techniques. J Chromatogr 1000:3–27
Su DL, Li PJ, Quek SY, Huang ZQ, Yuan YJ, Li GY, Shan Y (2019) Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chem 286:1–7
Suri S, Singh A, Nema PK (2021) Recent advances in valorization of citrus fruits processing waste: a way forward towards environmental sustainability. Food Sci Biotechnol 30:1601–1626
Suri S, Singh A, Nema PK (2022a) Current applications of citrus fruit processing waste: a scientific outlook. Appl Food Res 2:100050
Suri S, Singh A, Nema PK, Malakar S, Arora VK (2022b) Sweet lime (Citrus limetta) peel waste drying approaches and effect on quality attributes, phytochemical and functional properties. Food Biosci 48:101789
Tocmo R, Pena-Fronteras J, Calumba KF, Mendoza M, Johnson JJ (2020) Valorization of pomelo (Citrus grandis Osbeck) peel: a review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr Rev Food Sci Food Saf 19:1969–2012
Toumi ML, Merzoug S, Boutefnouchet A, Tahraoui A, Ouali K, Guellati MA (2009) Hesperidin, a natural citrus flavanone, alleviates hyperglycaemic state and attenuates embryopathies in pregnant diabetic mice. J Med Plant Res 3:862–869
Tsai HL, Chang SK, Chang SJ (2007) Antioxidant content and free radical scavenging ability of fresh red pummelo [Citrus grandis (L.) Osbeck] juice and freeze-dried products. J Agric Food Chem 55:2867–2872
USDA (2018) National Nutrient Database for Standard Reference (All Nutrients). Nutrient data for 2018, Citrus fruit. https://fdc.nal.usda.gov/fdc-app.html#/. Accessed 29 May 2022
Velasco R, Licciardello C (2014) A genealogy of the citrus family. Nat Biotechnol 32:640–642
Vinatoru M, Toma M, Filip P, Achim T, Stan N, Mason TJ, … Lazurca D (1998) Ultrasonic reactor dedicated to the extraction of active principles from plants. Romanian Patent 98-01014
Vuong QV, Golding JB, Stathopoulos CE, Nguyen MH, Roach PD (2011) Optimizing conditions for the extraction of catechins from green tea using hot water. J Sep Sci 34:3099–3106
Vuong QV, Golding JB, Stathopoulos CE, Roach PD (2013) Effects of aqueous brewing solution pH on the extraction of the major green tea constituents. Food Res Int 53:713–719
Wang L, Weller CL (2006) Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol 17:300–312
Wang S, Yang C, Tu H, Zhou J, Liu X, Cheng Y, Luo J, Deng X, Zhang H, Xu J (2017) Characterization and metabolic diversity of flavonoids in citrus species. Sci Rep 7:1–10
Wu GA, Prochnik S, Jenkins J, Salse J, Hellsten U, Murat F, Perrier X, Ruiz M, Scalabrin S, Terol J, Rokhsar D (2014) Complex history of admixture during citrus domestication revealed by genome analysis. Nat Biotechnol 32:LBNL-7054E
Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K (2011) Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol 21:1103010218
Zhang H, Xi W, Yang Y, Zhou X, Liu X, Yin S et al (2015) An on-line HPLC-FRSD system for rapid evaluation of the total antioxidant capacity of Citrus fruits. Food Chem 172:622–629
Zou Z, Xi W, Hu Y, Nie C, Zhou Z (2016) Antioxidant activity of Citrus fruits. Food Chem 196:885–896
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Suri, S., Singh, A., Nema, P.K. (2023). Bioactive Compounds in Citrus Fruits: Extraction and Identification. In: Singh Purewal, S., Punia Bangar, S., Kaur, P. (eds) Recent Advances in Citrus Fruits. Springer, Cham. https://doi.org/10.1007/978-3-031-37534-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-37534-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-37533-0
Online ISBN: 978-3-031-37534-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)